Abstract
Synapses onto dendritic spines in the lateral amygdala formed by afferents from the auditory thalamus represent a site of plasticity in Pavlovian fear conditioning. Previous work has demonstrated that thalamic afferents synapse onto LA spines expressing glutamate receptor (GluR) subunits, but the GluR subunit distribution at the synapse and within the cytoplasm has not been characterized. Therefore, we performed a quantitative analysis for ∝-amino-3-hydroxy-5-methyl-4-isoxazole proprionate (AMPA) receptor subunits GluR2 and GluR3 and N-methyl-D-aspartate (NMDA) receptor subunits NR1 and NR2B by combining anterograde labeling of thalamo-amygdala afferents with postembedding immunoelectron microscopy for the GluRs in adult rats. A high percentage of thalamo-amygdala spines was immunoreactive for GluR2 (80%), GluR3 (83%), and NR1 (83%), while a smaller proportion of spines expressed NR2B (59%). To compare across the various subunits, the cytoplasmic to synaptic ratios of GluRs were measured within thalamo-amygdala spines. Analyses revealed that the cytoplasmic pool of GluR2 receptors was twice as large compared to the GluR3, NR1 and NR2B subunits. Our data also show that in adult brain, the NR2B subunit is expressed in the majority of in thalamo-amygdala spines and that within these spines, the various GluRs are differentially distributed between synaptic and non-synaptic sites. The prevalence of the NR2B subunit in thalamo-amygdala spines provides morphological evidence supporting its role in the fear conditioning circuit while the differential distribution of the GluR subtypes may reflect distinct roles for their involvement in this circuitry and synaptic plasticity.
Indexing terms: GluR2, GluR3, excitatory amino acids, immunogold, NR1, NR2B, postembedding, immunohistochemistry, tracing, electron microscopy
INTRODUCTION
In recent years, considerable progress has been made in elucidating the neural circuits that underlie Pavlovian fear conditioning, a behavioral task in which a neutral conditioned stimulus (CS), often an auditory tone, acquires the ability to elicit fear responses following its association with an aversive unconditioned stimulus (US), typically footshock. Evidence from different kinds of studies points to the lateral amygdala (LA) as an important site of plasticity in auditory fear conditioning (LeDoux, 2000; Maren 2002; Sah et al., 2003; Tsvetkov et al., 2004). The LA is the primary recipient of convergent inputs from auditory thalamic and neocortical areas that process the CS. Lesions of the LA, or pharmacological disruption of neural activity in this region, prevent fear conditioning (LeDoux et al, 1990; Nader et al., 2001; Wilensky et al., 1999, 2000; Bailey et al., 1999). Neurons in LA are responsive to both CS and US (Romanksi et al., 1993) and their responses to the CS changes after CS-US pairing (Quirk et al., 1995; Rogan et al., 1997; McKernan and Shinnick Gallagher, 1997; Repa et al., 2000; Pare and Collins, 2000; Goosens and Maren, 2002). Moreover, induction of long-term potentiation by electrical stimulation of auditory thalamus enhances CS-evoked responses in LA (Rogan and LeDoux, 1995). Together, these findings suggest that thalamo-amygdala synapses are facilitated in response to Pavlovian fear conditioning (LeDoux 2000; Maren, 2001).
Auditory thalamic inputs into LA exert their effects through the postsynaptic glutamate N-methyl-D-aspartate (NMDA) and ∝-amino-3-hydroxy-5-methyl-4-isoxazole proprionate (AMPA) receptors (Li et al., 1995; Farb and LeDoux, 1997; Humeau et al., 2003). Blockade of NMDA receptors in LA prevents fear conditioning (Miserindino et al. 1990; Walker and Davis, 2000; Rodrigues et al., 2001), while facilitation of AMPA receptors, and in turn NMDA receptors, enhances fear conditioning (Rogan et al., 1997).
Increasing evidence suggests that NMDA and AMPA receptors are capable of modulating synaptic plasticity through shifts in their receptor subunit stoichiometry (Shi et al., 2001; Rumpel et al., 2005; Barria and Malinow, 2006). While some information has been gleaned regarding the glutamate receptor subunit composition in identified auditory pathways to LA (Farb and LeDoux, 1997; 1999; Rodrigues et al., 2001; Mead et al., 2006), the subcellular organization of glutamate receptors at thalamo-amygdala synapses is not known. Therefore, the aim of the present study was to characterize and quantify the synaptic and non-synaptic distribution of the GluR2, GluR3, NR1, and NR2B glutamate receptor (GluR) subunits within dendritic spines that receive synapses from the auditory thalamus. Since receptor trafficking is a candidate mechanism underlying memory formation (Shi et al 2001; Baudry et al., 1980), differences between the cytoplasmic and synaptic distribution of these GluR subtypes may reflect distinct roles in synaptic plasticity. Thalamic afferents to the LA were identified by anterograde transport of biotinylated dextran amine (BDA) following injections in the auditory thalamus and the GluRs were immunolabeled using postembedding gold.
MATERIALS AND METHODS
Animals
All procedures related to the care and treatment of animals were conducted in accordance with the National Institutes of Health Guidelines for the Care and Use of Experimental Animals using protocols approved by the Institutional Animal Care and Use Committee at Mount Sinai School of Medicine and New York University. Male Sprague-Dawley rats (Hilltop Laboratories, Scottdale, PA) weighing 300–400 g were housed on an alternating 12 hour light/dark cycle (lights on 7 AM, lights off 7 PM).
BDA injections
Animals (n=4) were anesthetized with a combination of ketamine (Ketaset, 120 mg/kg, i.p.), xylazine (Xyla-Jet, 6.0 mg/kg, i.p.) and medetomidine hydrochloride (Domitor, 0.5mg/kg, i.p) and placed in a stereotaxic apparatus. Lysine-fixable biotinylated dextran-amine (BDA) (10%, dissolved in 10 mM phosphate buffer, pH 7.25; Molecular Probes, Eugene, OR) or BDA conjugated to tetramethylrhodamine (micro-ruby, Molecular Probes) was iontophoretically delivered (5 μA pulsed DC current, 7 s on, 7 s off) for 15–20 minutes to the medial subdivision of the medial geniculate nucleus (MGm) and posterior intralaminar nucleus (PIN) through glass micropipettes (tip diameter 12–15 μm). Following BDA injections, the wound was closed and animals recovered in the surgery area before returning to the animal facility.
Fixation and BDA histochemistry
After a 3–5 day survival period, animals were deeply anesthetized with sodium pentobarbital (120 mg/kg) and transcardially perfused with 1% paraformaldehyde (PFA) made in phosphate buffer (PB; pH 7.4), followed by a mixture of 0.125% glutaraldehyde and 4% PFA made in PB. Brains were removed, blocked, and postfixed in the same fixative overnight at 4°C. Blocks were sectioned on a Vibratome (50 μm), freeze-thawed as previously described (Farb and LeDoux, 1997), and incubated in avidin-biotin horseradish peroxidase substrate (ABC Elite Kit; Vector Laboratories, Burlingame, CA) overnight. The following day, sections were rinsed and reacted with 3,3′-diaminobenzidine tetrachloride (DAB; Sigma-Aldrich, St. Louis, MO) for 10–15 minutes. To optimize BDA staining, sections were incubated a second time in ABC for 2 hours and then reacted with DAB a second time. The LA was identified at low magnification on the light microscope, dissected, and processed for postembedding immunogold.
Postembedding immunogold
The freeze substitution and low-temperature embedding of the specimens were performed as described previously with several modifications (Chaudhry et al., 1995). Briefly, sections were cryoprotected by immersion in 4% D-glucose, followed by incubations of increasing concentrations of glycerol in PB (10, 20, and 30%) and were plunged rapidly into liquid propane (−180°C) cooled by liquid nitrogen in a Universal Cryofixation System KF80 (Reichert-Jung, Vienna, Austria). The samples were immersed in 1.5% uranyl acetate (for en bloc fixation) made in anhydrous methanol (−90°C, 24 h) in a cryosubstitution Automated Freeze Substitution unit (Leica, Vienna, Austria). The temperature was increased in steps of 4°C/hour from −90°C to −45°C. The samples were washed with anhydrous methanol and infiltrated with Lowicryl HM20 resin (Electron Microscopy Sciences, Fort Washington, PA) at −45°C, with a progressive increase in the ratio of resin to methanol for 1 hour each, followed with pure Lowicryl overnight. Polymerization was performed with ultraviolet light (360 nm) at −45°C for 48 hours, followed by 24 hours at room temperature. Ultrathin sections of the LA were cut by diamond knife on a Reichert-Jung ultramicrotome (Vienna, Austria) and collected on nickel mesh grids. Grids containing the ultrathin sections were initially treated with a saturated solution of sodium hydroxide in absolute ethanol, rinsed, treated in 0.1% sodium borohydrate and 50 mM glycine for 5 minutes, followed by treatment in Tris-buffered saline containing 2% normal human serum for 10 minutes. The immunogold procedure was carried out by incubating ultrathin sections in primary antibodies to GluR2 (mouse monoclonal, 5 μg/ml; Pharmingen, San Diego, CA; Vissavajjhala et al., 1996), GluR3 (mouse monoclonal, 1.0 μg/ml; Chemicon, Temecula, CA; Moga et al., 2003), NR1 (rabbit polyclonal, 2 μg/ml; Chemicon, Temecula, CA; Huntley et al., 1997) and NR2B (rabbit polyclonal,10 μg/ml; Novus, Littleton, CO; Adams et al., 2004) overnight. On the following day, sections were incubated in 1:40 dilution of either goat anti-mouse IgG conjugated to 10 nm gold particles (GluR2 and GluR3; Electron Microscopy Sciences, Fort, Washington, PA) or goat anti-rabbit IgG conjugated to 10 nm gold particles (NR1 and NR2B; Electron Microscopy Sciences). Ultrathin sections were counterstained with uranyl acetate and Reynolds lead citrate, and viewed at 80 kV on a Jeol 1200EX electron microscope (Tokyo, Japan). Images were captured at 10,000X using the Advantage CCD camera (Advanced Microscopy Techniques Corporation, Danvers, MA). The specificities for these primary antibodies have been previously demonstrated in our laboratory (GluR2, Vissavajjhala et al., 1996; GluR3, Moga et al., 2003; NR1, Siegel et al., 1994; NR2B, Adams et al., 2004). In summary, Western blot analyses across a panel of different glutamate receptor subunit rat cDNAs transfected into HEK cells showed that each antibody recognized only a single band corresponding to its predicted molecular weight. When each antibody was preadsorbed to its corresponding protein, staining in brain tissue was abolished. Secondary antibody specificity was established by verifying that no immunogold labeling was observed when the primary antisera were omitted from the immunocytochemical protocol.
Data Analysis
The immunogold particle density and distribution were analyzed using software developed in our laboratory (SynBin; Adams et al., 2001), and is based upon the concept of particle localization relative to its proximity to the pre- and postsynaptic membrane structures (Blackstad et al., 1990; Ruud and Blackstad, 1999). The program analyzes the resulting data map and objectively assigns each gold particle to a specific bin, with bin sizes and synaptic domains established prospectively. The designated bin width was 60 nm from the PSD since this width readily accommodates the theoretical limit of lateral resolution for gold particle localization at the ultrastructural level (He et al., 2000). Thus, for pre- and postsynaptic structures, particles located within 60nm bin of the cleft were considered “synaptic” and those outside the 60nm bin width were considered “cytoplasmic”. The information from each labeled spine was stored in a database from which the average number of gold particles per bin can be determined.
LA spines were quantified when there was clear visualization and delineation of the classic synaptic structures such as pre- and postsynaptic membranes, a synaptic cleft, presynaptic vesicles, and a postsynaptic density. Thalamo-amygdala spines without these features or those that were obliquely cut were excluded from the quantitative analysis. The cytoplasmic to synaptic gold particle ratios within thalamo-amygdala spines were computed to allow for the comparison across subunits, since this ratio minimizes qualitative differences that may arise from different antibodies (e.g., specificity, epitope location). Statistical comparisons were performed with a one-way analysis of variance followed by a post-hoc tests (Bonferroni), and data expressed as mean ± SEM.
RESULTS
Thalamic inputs to LA
Injections of BDA into MGm/PIN produced labeling of cell bodies, axons, and processes at the injection site and in adjacent locally-projecting regions (Fig. 1A, B). A dense plexus of anterogradely-labeled BDA fibers was evident throughout the LA, particularly within the dorsal LA, and in the striatum and amygdala-striatum transition areas, regions immediately dorsal and medial to the amygdaloid complex. (Fig.1C). Higher magnification (Fig. 1D) revealed the presence of many labeled fibers and boutons. No labeled cell bodies or dendrites in the LA were observed. Electron microscopic verification of BDA-labeling was performed on ultrathin sections prior to the immunogold analysis. Thalamo-amygdala labeled terminals were comprised of loosely packed clear, round vesicles and sometimes contained dense-core vesicles. Some BDA-labeled terminals did not have any identifiable postsynaptic contacts or made synapses that were not obviously asymmetric (i.e., thin postsynaptic density), due to the plane of sectioning. Since serial section analysis (unpublished observations) revealed that thalamo-amygdala terminals with these characteristics often form asymmetric synapses in other sections, our analysis was restricted to those labeled terminals that formed asymmetric synapses. We analyzed 526 BDA-labeled terminals and found that the vast majority (74%; 391/526) of these terminals formed synapses onto spines. The remaining labeled terminals (26%; 135/526) formed asymmetric synapses onto small and large dendritic shafts. These findings are consistent with previous observations about thalamo-amygdala synapses (LeDoux et al., 1991).
Figure 1.
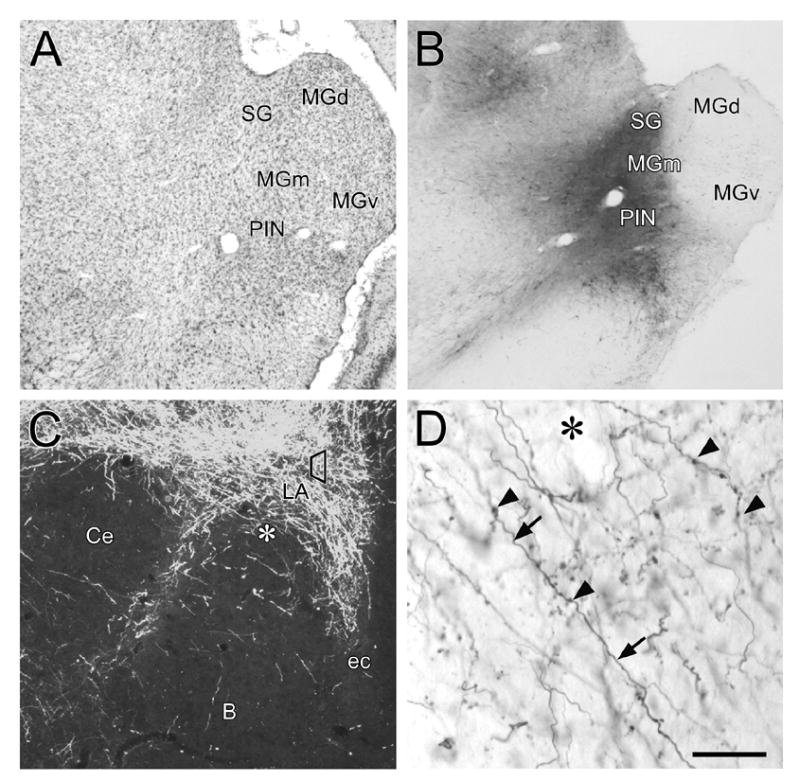
Low-power photomicrographs showing BDA injections in the auditory thalamus and corresponding anterograde transport to the amygdala. A: Nissl-stained coronal section, approximately 6.0 mm caudal to bregma, containing the subregions of the auditory thalamus. B: Representative BDA injection site into the MGm/PIN. Local anterograde and retrograde transport in nearby regions is also shown. C: Dark field photomicrograph of anterograde transport to LA following a BDA injection in MGm/PIN. The trapezoid in the LA represents the approximate region sampled for electron microscopic examination. The asterisk corresponds to the region of higher-magnification shown in D. D: High-power magnification using Nomarski optics reveals the presence of BDA-labeled axons (arrows) and boutons (arrowheads) in the LA. Cell bodies and dendrites are not seen in the LA. Asterisk denotes region shown at lower power in C. Scale bar = 500 μm in A and B, 100 μm in C, and 25 μm in D.
Distribution of glutamate receptors at thalamic auditory spines in LA
Since the vast majority of thalamic afferents to the LA target dendritic spines, the quantitative analysis for GluR subunits in this study was limited to thalamo-amygdala spines with identifiable synaptic structures, as previously defined. Thus, within a randomly-selected grid square, every BDA-labeled terminal forming an asymmetric synapse (Gray’s Type I) onto a spine was analyzed. Spines were considered to be GluR-labeled if they contained two or more gold particles within its cytoplasm or within 60 nm of the PSD or synaptic cleft. The pattern of immunogold localization within thalamo-amygdala spines was similar for NR1, NR2B, and GluR3, with the most abundant labeling in compartments within or subjacent to the synaptic cleft and postsynaptic density (Fig. 2A) and less labeling within the cytoplasmic compartment. By contrast, GluR2 had a higher proportion of immunogold labeling in the cytoplasmic compartment than in the synaptic compartment. Though GluR subunits are usually associated with endomembranous structures, damage caused by the postembedding immunogold processing and the high sensitivity of endomembranes to such treatment prevented us from observing this relationship.
Figure 2.
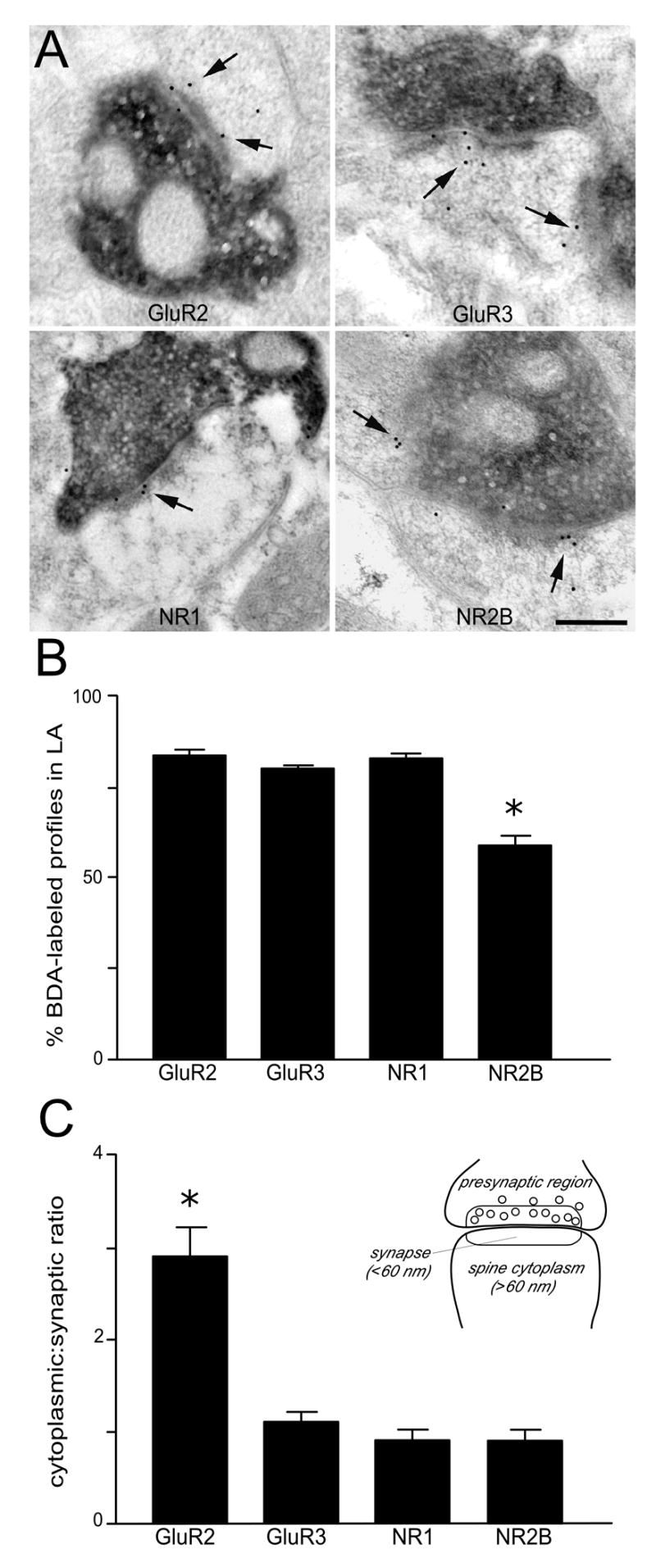
A: Electron micrographs show thalamic afferents labeled with BDA form asymmetric synapses onto LA spines that contain immunogold labeling (arrows) for glutamate receptor (GluR) subunits. Scale bar = 0.25 μm. B: Histogram showing the percentages of thalamo-amygdala spines that contained immunogold labeling for each GluR subunit examined. The vast majority of these spines were immunopositive for the glutamate receptors GluR2, GluR3 and NMDAR1 (>80%), while fewer spines expressed NR2B (59%). C: Histogram showing the cytoplasmic: synaptic ratios for each subunit in thalamo-amygdala spines. The ratio of cytoplasmic: synaptic labeling was 2.2 fold higher for GluR2 than for GluR3, NR1, and NR2B. The inset illustrates how the synaptic bins were established for the assignment of gold particle locations in the spines analyzed. Asterisks in C and B denotes a significant difference from the other 3 subunits; P < 0.001.
The compartmental distribution of immunogold labeling for each subunit in thalamo-amygdala spines is shown in Table 1. Across four animals, a total of 701 spines were quantified, such that, an average of 175 spines per subunit was counted. For each animal, approximately 44 thalamo-amygdala spines per subunit were examined. The proportion of thalamo-amygdala spines in LA expressing immunogold-labeling was 82.9 ± 1.3% (136/164) for GluR2, 80.1 ± 0.6% (157/196) for GluR3, and 82.8 ± 1.0% for NR1 (101/122). By contrast, a significantly smaller proportion of spines expressed the NR2B subunit, 58.9 ± 2.5% (128/219) as compared to the other GluR subunits examined (F(3,12) = 58.3, P < 0.0001; Fig. 2B). Examination of the cytoplasmic: synaptic gold particle ratios within thalamo-amygdala spines revealed the largest difference with the GluR2 subunit, 2.2: 1 (2.2 ± 0.2; F(3,12) = 13.1; P < 0.0005), compared to GluR3, 1.1: 1 (1.1 ± 0.1; P < 0.001), NR1 0.9: 1 (0.9 ± 0.1; P < 0.001), and NR2B 0.9: 1 (0.9 ± 0.1; P < 0.001; Fig 2C). In all cases, immunogold particles were also occasionally located presynaptically (Table 1).
Table 1.
Mean values for each compartment analyzed in thalamo-amygdata axospinous synapses
| Bin Description | GluR2 | GluR3 | NR1 | NR12B |
|---|---|---|---|---|
| Percentage of BDA + immunogold labeling | 83.5 | 80.1 | 82.3 | 58.9* |
| Postsynaptic | ||||
| Synaptic (cleft + 0–60 nm) | 1.76 | 2.38 | 1.93 | 2.29 |
| Spine Cytoplasmic (>60 nm) | 3.86 | 2.68 | 1.79 | 2.00 |
| Cytoplasmic: synaptic ratio | 2.2* | 1.1 | 0.9 | 0.9 |
| Presynaptic | ||||
| 0–60 nm | 0.17 | 0.34 | 0.10 | 0.20 |
| >60nn | 0.46 | 0.11 | 0.46 | 0.36 |
Asterisk indicates significant difference (P < 0.01); comparisons made across subunits.
DISCUSSION
This is the first study to date that characterizes the ultrastructural distribution of the glutamate receptor subunits GluR2, GluR3, NR1 and NR2B within thalamo-amygdala spines. Our results show that within the LA: 1) the GluR subunits GluR2, GluR3, NR1 and NR2B are present in the majority spines that are post-synaptic to auditory thalamic afferents; 2) NR2B was present in 59% of thalamo-amygdala spines, a value that is considerably larger than expected based on studies in the rodent forebrain (Monyer et al., 1994; Lopez de Armentia and Sah, 2003); 3) twice as much GluR2 is expressed in the spine cytoplasm as compared to the synapse. This ratio is two times greater than that of the GluR3, NR1, and NR2B subunits, where approximately the same number of receptors is present in the cytoplasm as compared to the synapse. As discussed below, the prevalence of NMDA receptor subunits in synapses is consistent with past anatomical and physiological studies demonstrating the involvement of both AMPA and NMDA receptors in synaptic transmission in the thalamo-amygdala pathway. Further, the prevalence of the NR2B subunit within thalamo-amygdala spines in adult rats is consistent with the emerging view that NR2B subunits are expressed in adult as well as young animals. These findings also support the idea that cytoplasmic pools of AMPA receptors retained in thalamo-amygdala spines may be available for insertion into the synapse relative to NMDA receptors.
NMDA receptors are heteromeric assemblies composed of NR1 subunits in combination with at least one form of NR2 (A–D). Our observation that NR1 is present in a larger proportion of thalamo-amygdala spines (83%) than NR2B (59%) is consistent with its role as an obligatory subunit in the receptor heteromer. The role of NR2B is of particular interest in the thalamo-amygdala circuit, since the infusion of its antagonist into LA has been shown to block the acquisition of fear conditioning (Rodrigues et al., 2001) and synaptic plasticity (Bauer et al., 2002). The NR2B subunit has a smaller time constant than NR2A and NR2C subunits (Vicini et al., 1998), resulting in a slower attenuation of excitatory postsynaptic currents (Monyer et al.,1994). In addition, the NR2A-NR2B complex is characterized by a slower activation and deactivation and longer rise and decay time course than the NR1-NR2A complex (Chen et al., 1999). This conductive property of NR2B might provide a means for coincidence detection in the enhancement of synaptic efficacy (Tsien, 2000) and may play an integral role in learning and plasticity.
A number of reports have shown that the NR2B subunit mRNA and protein are expressed at peak levels neonatally in the forebrain, and then decline throughout development (Monyer et al., 1994; Lopez de Armentia and Sah, 2003), presumably replaced by NR2A subunit (Vicini et al., 1998). Our results, showing that 59% of thalamo-amygdala spines are positive for NR2B, are in agreement with past studies showing that the NR2B subunit is maintained into adulthood in LA (Rodrigues et al., 2000) and other brain regions (Jin et al., 1997; Charton et al., 1999; Khan et al., 2000) and provide anatomical evidence that is consistent with behavioral and physiological findings for the role of NR2B in fear learning. Direct infusion of an NR2B antagonist into LA disrupts the acquisition of fear conditioning in vivo (Rodrigues et al., 2001) and blocks LTP produced by prolonged tetanic stimulation in vitro (Bauer et al, 2002). Blocking NR2B receptors in vitro also decreases the response properties of LA pyramidal neurons and reduces EPSCs in lateral amygdala but not hippocampus (Lopez de Armentia and Sah, 2003). Collectively these data support the possibility that the role of the NR2B subunit in the LA of the mature brain has been hitherto underestimated.
The receptor subunit stoichiometry and composition of AMPA receptors is more heterogeneous than for NMDA receptors, giving rise to a more complex set of functional properties that may also be relevant for LA plasticity and fear conditioning. One prominent feature of AMPA receptors is that they are dynamically regulated, undergoing frequent insertion into, and removal from synapses (Luscher et al., 1999; Shi et al., 2001; Kauer and Malenka, 2006). Several studies suggest that a maximum of two receptor subunits may be present in the AMPA receptor tetrameric complex (Ayalon and Stern-Bach, 2001; Mansour et al., 2001; Greger et al., 2003). These are comprised either of GluR2/3 or GluR1/2, together with a small proportion of GluR1 homomeric receptors (Wenthold, et al., 1996; Shi et al., 2001). Whereas GluR2/3-containing AMPA receptors are important for their constitutive expression and cycling (Shi et al., 2001), trafficking of GluR1-containing AMPA receptors into activated synapses has been shown to underlie increases in synaptic efficacy (Shi et al., 1999; 2001). Recent evidence suggests that GluR1 receptor trafficking into LA synapses may underlie auditory fear conditioning (Rumpel et al., 2005). Nonetheless, these findings do not necessarily preclude a role of GluR2 in plasticity in learning, as studies using GluR2-knockout mice demonstrated an essential role for the GluR2 subunit in several different operant conditioning tasks (Mead et al., 2003; 2006). In this context, our data showing that GluR2 and GluR3 have a spine cytoplasmic to synaptic ratio of 2:1 and 1:1, respectively, suggest that GluR3 may form a complex with a subpopulation of total GluR2, leaving the remaining pool of spine cytoplasmic GluR2 unassembled and ER-bound. Thus, while the entire population of GluR2 may be representative of AMPA receptors that are inserted in an activity-dependent and constitutive manner, the GluR3 immunogold profile may represent GluR2/3 AMPA receptors that are constitutively cycled. Related ongoing studies investigating the synaptic distribution of GluR1 in thalamo-amygdala spines will help to further clarify this issue.
Conclusions
Our anatomical findings provide a framework with which to understand the role the different glutamate receptor subunits play in synaptic plasticity and learning. Our results are consistent with the ideas that the NR2B subunit is involved in learning and differences between the cytoplasmic and synaptic distribution of these GluR subtypes may reflect distinct roles for their involvement in synaptic plasticity and fear conditioning.
Acknowledgments
We thank Robert A. Townley for editorial comments and general criticism, and Kris W. Trulock for help in the preparation of illustrations. This work was supported by NIH grant MH58911.
ABBREVIATIONS
- B
basal nucleus of the amygdala
- Ce
central nucleus of the amygdala
- ec
external capsule
- LA
lateral nucleus of the amygdala
- MGd
medial geniculate, dorsal division
- MGm
medial geniculate, medial division
- MGv
medial geniculate, ventral division
- PIN
posterior intralaminar nucleus
- SG
suprageniculate body
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adams MM, Shah RA, Janssen WG, Morrison JH. Different modes of hippocampal plasticity in response to estrogen in young and aged female rats. Proc Natl Acad Sci U S A. 2001;98:8071–8076. doi: 10.1073/pnas.141215898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams MM, Fink SE, Janssen WG, Shah RA, Morrison JH. Estrogen modulates synaptic N-methyl-D-aspartate receptor subunit distribution in the aged hippocampus. J Comp Neurol. 2004;474:419–426. doi: 10.1002/cne.20148. [DOI] [PubMed] [Google Scholar]
- Ayalon G, Stern-Bach Y. Functional assembly of AMPA and kainate receptors is mediated by several discrete protein-protein interactions. Neuron. 2001;31:103–113. doi: 10.1016/s0896-6273(01)00333-6. [DOI] [PubMed] [Google Scholar]
- Bailey DJ, Kim JJ, Sun W, Thompson RF, Helmstetter FJ. Acquisition of fear conditioning in rats requires the synthesis of mRNA in the amygdala. Behav Neurosci. 1999;113:276–82. doi: 10.1037//0735-7044.113.2.276. [DOI] [PubMed] [Google Scholar]
- Barria A, Malinow R. NMDA receptor subunit composition controls synaptic plasticity by regulating binding to CaMKII. Neuron. 2005;48:289–301. doi: 10.1016/j.neuron.2005.08.034. [DOI] [PubMed] [Google Scholar]
- Baudry M, Oliver M, Creager R, Wieraszko A, Lynch G. Increase in glutamate receptors following repetitive electrical stimulation in hippocampal slices. Life Sci. 1980;27:325–30. doi: 10.1016/0024-3205(80)90200-3. [DOI] [PubMed] [Google Scholar]
- Bauer EP, Schafe GE, LeDoux JE. NMDA receptors and L-type voltage-gated calcium channels contribute to long-term potentiation and different components of fear memory formation in the lateral amygdala. J Neurosci. 2002;22:5239–5249. doi: 10.1523/JNEUROSCI.22-12-05239.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackstad TW, Karagulle T, Ottersen OP. MORFOREL, a computer program for two-dimensional analysis of micrographs of biological specimens, with emphasis on immunogold preparations. Comput Biol Med. 1990;20:15–34. doi: 10.1016/0010-4825(90)90041-m. [DOI] [PubMed] [Google Scholar]
- Charton JP, Heckert M, Becker CM, Schroder H. Cellular and subcellular localization of the 2B-subunit of the NMDA receptor in the adult rat telencephalon. Brain Res. 1999;816:609–17. doi: 10.1016/s0006-8993(98)01243-8. [DOI] [PubMed] [Google Scholar]
- Chaudhry FA, Lehre KP, van Lookeren Campagne M, Ottersen OP, Danbolt NC, Storm-Mathisen J. Glutamate transporters in glial plasma membranes: highly differentiated localizations revealed by quantitative ultrastructural immunocytochemistry. Neuron. 1995;15:711–720. doi: 10.1016/0896-6273(95)90158-2. [DOI] [PubMed] [Google Scholar]
- Chen N, Luo Raymond LA. Subtype-dependence of NMDA receptor channel open probability. J Neurosci. 1999;19:6844–6854. doi: 10.1523/JNEUROSCI.19-16-06844.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M. Neurobiology of fear responses: the role of the amygdala. J Neuropsychiatry Clin Neurosci. 1997;9:382–402. doi: 10.1176/jnp.9.3.382. [DOI] [PubMed] [Google Scholar]
- Farb CR, LeDoux JE. NMDA and AMPA receptors in the lateral nucleus of the amygdala are postsynaptic to auditory thalamic afferents. Synapse. 1997;27:106–121. doi: 10.1002/(SICI)1098-2396(199710)27:2<106::AID-SYN2>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Farb CR, LeDoux JE. Afferents from rat temporal cortex synapse on lateral amygdala neurons that express NMDA and AMPA receptors. Synapse. 1999;33:218–229. doi: 10.1002/(SICI)1098-2396(19990901)33:3<218::AID-SYN6>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Goosens KA, Maren S. Long-term potentiation as a substrate for memory: Evidence from studies of amygdaloid plasticity and Pavlovian fear conditioning. Hippocampus. 2002;12:592–599. doi: 10.1002/hipo.10099. [DOI] [PubMed] [Google Scholar]
- Greger IH, Khatri L, Ziff EB. RNA editing at arg607 controls AMPA receptor exit from the endoplasmic reticulum. Neuron. 2002;34:759–772. doi: 10.1016/s0896-6273(02)00693-1. [DOI] [PubMed] [Google Scholar]
- Greger IH, Khatri L, Kong X, Ziff EB. AMPA receptor tetramerization is mediated by Q/R editing. Neuron. 2003;40:763–774. doi: 10.1016/s0896-6273(03)00668-8. [DOI] [PubMed] [Google Scholar]
- He Y, Janssen WG, Rothstein JD, Morrison JH. Differential synaptic localization of the glutamate transporter EAAC1 and glutamate receptor subunit GluR2 in the rat hippocampus. J Comp Neurol. 2000;418:255–269. [PubMed] [Google Scholar]
- Humeau Y, Shaban H, Bissiere S, Luthi A. Presynaptic induction of heterosynaptic associative plasticity in the mammalian brain. Nature. 2003;426:841–845. doi: 10.1038/nature02194. [DOI] [PubMed] [Google Scholar]
- Huntley GW, Vickers JC, Morrison JH. Quantitative localization of NMDAR1 receptor subunit immunoreactivity in inferotemporal and prefrontal association cortices of monkey and human. Brain Res. 1997;749:245–262. doi: 10.1016/S0006-8993(96)00847-5. [DOI] [PubMed] [Google Scholar]
- Jin DH, Jung YW, KO BH, Moon IS. Immunoblot analyses on the differential distribution of NR2A and NR2B subunits in the adult rat brain. Mol Cells. 1997;7:749–754. [PubMed] [Google Scholar]
- Kauer JA, Malenka RC. LTP: AMPA receptors trading places. Nat Neurosci. 2006;9:593–594. doi: 10.1038/nn0506-593. [DOI] [PubMed] [Google Scholar]
- Khan AM, Stanley BG, Bozzetti L, Chin C, Stivers C, Curras-Collazo MC. N-methyl-D-aspartate receptor subunit NR2B is widely expressed throughout the rat diencephalon: an immunohistochemical study. J Comp Neurol. 2000;428:428–49. [PubMed] [Google Scholar]
- LeDoux JE, Cicchetti P, Xagoraris A, Romanski LM. The lateral amygdaloid nucleus: sensory interface of the amygdala in fear conditioning. J Neurosci. 1990;10:1062–9. doi: 10.1523/JNEUROSCI.10-04-01062.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux JE, Farb CR, Milner TA. Ultrastructure and synaptic associations of auditory thalamo-amygdala projections in the rat. Exp Brain Res. 1991;85:577–586. doi: 10.1007/BF00231742. [DOI] [PubMed] [Google Scholar]
- LeDoux JE. Emotion circuits in the brain. Annu Rev Neurosci. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- Li XF, Phillips R, LeDoux JE. NMDA and non-NMDA receptors contribute to synaptic transmission between the medial geniculate body and the lateral nucleus of the amygdala. Exp Brain Res. 1995;105:87–100. doi: 10.1007/BF00242185. [DOI] [PubMed] [Google Scholar]
- Lin CY, Lynch G, Gall CM. AMPA receptor stimulation increases alpha5beta1 integrin surface expression, adhesive function and signaling. J Neurochem. 2005;94:531–46. doi: 10.1111/j.l471-4159.2005.03203.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez de Armentia M, Sah P. Development and subunit composition of synaptic NMDA receptors in the amygdala: NR2B synapses in the adult central amygdala. J Neurosci. 2003;23:6876–6883. doi: 10.1523/JNEUROSCI.23-17-06876.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luscher C, Xia H, Beattie EC, Carroll RC, von Zastrow M, Malenka RC, Nicoll RA. Role of AMPA receptor cycling in synaptic transmission and plasticity. Neuron. 1999;24:649–658. doi: 10.1016/s0896-6273(00)81119-8. [DOI] [PubMed] [Google Scholar]
- Mansour M, Nagarajan N, Nehring RB, Clements JD, Rosenmund C. Heteromeric AMPA receptors assemble with a preferred subunit stoichiometry and spatial arrangement. Neuron. 2001;32:841–853. doi: 10.1016/s0896-6273(01)00520-7. [DOI] [PubMed] [Google Scholar]
- Maren S. Is there savings for pavlovian fear conditioning after neurotoxic basolateral amygdala lesions in rats? Neurobiol Learn Mem. 2001;76:268–83. doi: 10.1006/nlme.2001.4042. [DOI] [PubMed] [Google Scholar]
- Maren S. Auditory fear conditioning increases CS-elicited spike firing in lateral amygdala neurons even after extensive overtraining. Eur J Neurosci. 2000;12:4047–4054. doi: 10.1046/j.1460-9568.2000.00281.x. [DOI] [PubMed] [Google Scholar]
- McKernan MG, Shinnick-Gallagher P. Fear conditioning induces a lasting potentiation of synaptic currents in vitro. Nature. 1997;390:607–611. doi: 10.1038/37605. [DOI] [PubMed] [Google Scholar]
- Mead AN, Stephens DN. Involvement of AMPA receptor GluR2 subunits in stimulus-reward learning: evidence from glutamate receptor gria2 knock-out mice. J Neurosci. 2003;23:9500–7. doi: 10.1523/JNEUROSCI.23-29-09500.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mead AN, Morris HV, Dixon CI, Rulten SL, Mayne LV, Zamanillo D, Stephens DN. AMPA receptor GluR2, but not GluR1, subunit deletion impairs emotional response conditioning in mice. Behav Neurosci. 2006;120:241–248. doi: 10.1037/0735-7044.120.2.241. [DOI] [PubMed] [Google Scholar]
- Miserendino MJD, Sananes CB, Melia KR, Davis M. Blocking of acquisition but not expression of conditioned fear-potentiated startle by NMDA antagonists in the amygdala. Nature. 1990;345:716–8. doi: 10.1038/345716a0. [DOI] [PubMed] [Google Scholar]
- Moga DE, Janssen WG, Vissavajjhala P, Czelusniak SM, Moran TM, Hof PR, Morrison JH. Glutamate receptor subunit 3 (GluR3) immunoreactivity delineates a subpopulation of parvalbumin-containing interneurons in the rat hippocampus. J Comp Neurol. 2003;462:15–28. doi: 10.1002/cne.10710. [DOI] [PubMed] [Google Scholar]
- Monyer H, Burnashev N, Laurie DJ, Sakmann B, Seeburg PH. Developmental and regional expression in the rat brain and functional properties of four NMDA receptors. Neuron. 1994;12:529–540. doi: 10.1016/0896-6273(94)90210-0. [DOI] [PubMed] [Google Scholar]
- Nader K, Majidishad P, Amorapanth P, LeDoux JE. Damage to the lateral and central, but not other, amygdaloid nuclei prevents the acquisition of auditory fear conditioning. Learn Mem. 2001;8:156–63. doi: 10.1101/lm.38101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pare D, Collins DR. Neuronal correlates of fear in the lateral amygdala: multiple extracellular recordings in conscious cats. J Neurosci. 2000;20:2701–10. doi: 10.1523/JNEUROSCI.20-07-02701.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirk GJ, Repa C, LeDoux JE. Links Fear conditioning enhances short-latency auditory responses of lateral amygdala neurons: parallel recordings in the freely behaving rat. Neuron. 1995;15:1029–39. doi: 10.1016/0896-6273(95)90092-6. [DOI] [PubMed] [Google Scholar]
- Repa JC, Muller J, Apergis J, Desrochers TM, Zhou Y, LeDoux JE. Two different lateral amygdala cell populations contribute to the initiation and storage of memory. Nat Neurosci. 2001;4:724–731. doi: 10.1038/89512. [DOI] [PubMed] [Google Scholar]
- Rodrigues SM, LeDoux JE, Morrison JH. Differential expression of the N-methyl-D-aspartate receptor 2B subunit in the lateral nucleus division of the rat amygdala. Soc Neurosci Abstr. 2000;26:465.11. [Google Scholar]
- Rodrigues SM, Schafe GE, LeDoux JE. Intra-amygdala blockade of the NR2B subunit of the NMDA receptor disrupts the acquisition but not the expression of fear conditioning. J Neurosci. 2001;21:6889–6896. doi: 10.1523/JNEUROSCI.21-17-06889.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogan MT, LeDoux JE. LTP is accompanied by commensurate enhancement of auditory-evoked responses in a fear conditioning circuit. Neuron. 1995;15:127–36. doi: 10.1016/0896-6273(95)90070-5. [DOI] [PubMed] [Google Scholar]
- Rogan MT, Staubli UV, LeDoux JE. AMPA receptor facilitation accelerates fear learning without altering the level of conditioned fear acquired. J Neurosci. 1997;17:5928–5935. doi: 10.1523/JNEUROSCI.17-15-05928.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romanski LM, Clugnet MC, Bordi F, LeDoux JE. Somatosensory and auditory convergence in the lateral nucleus of the amygdala. Behav Neurosci. 1993;107:444–50. doi: 10.1037//0735-7044.107.3.444. [DOI] [PubMed] [Google Scholar]
- Rumpel S, LeDoux J, Zador A, Malinow R. Postsynaptic receptor trafficking underlying a form of associative learning. Science. 2005;308:83–88. doi: 10.1126/science.1103944. [DOI] [PubMed] [Google Scholar]
- Ruud HK, Blackstad TW. PALIREL, a computer program for analyzing particle-to-membrane relations, with emphasis on electron micrographs of immunocytochemical preparations and gold labeled molecules. Comput Biomed Res. 1999;32:93–122. doi: 10.1006/cbmr.1999.1508. [DOI] [PubMed] [Google Scholar]
- Sah P, Faber ES, Lopez De Armentia M, Power J. The amygdaloid complex: anatomy and physiology. Physiol Rev. 2003;83:803–834. doi: 10.1152/physrev.00002.2003. [DOI] [PubMed] [Google Scholar]
- Shi SH, Hayashi Y, Petralia RS, Zaman SH, Wenthold RJ, Svoboda K, Malinow R. Rapid spine delivery and redistribution of AMPA receptors after synaptic NMDA receptor activation. Science. 1999;284:1811–1816. doi: 10.1126/science.284.5421.1811. [DOI] [PubMed] [Google Scholar]
- Shi S, Hayashi Y, Esteban JA, Malinow R. Subunit-specific rules governing AMPA receptor trafficking to synapses in hippocampal pyramidal neurons. Cell. 2001;105:331–343. doi: 10.1016/s0092-8674(01)00321-x. [DOI] [PubMed] [Google Scholar]
- Siegel SJ, Brose N, Janssen WG, Gasic GP, Jahn R, Heinemann SF, Morrison JH. Regional, cellular, and ultrastructural distribution of N-methyl-D-aspartate receptor subunit 1 in monkey hippocampus. Proc Natl Acad Sci. 1994;91:564–568. doi: 10.1073/pnas.91.2.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsien JZ. Linking Hebb's coincidence-detection to memory formation. Curr Opin Neurobiol. 2000;10:266–273. doi: 10.1016/s0959-4388(00)00070-2. [DOI] [PubMed] [Google Scholar]
- Tsvetkov E, Shin RM, Bolshakov VY. Glutamate uptake determines pathway specificity of long-term potentiation in the neural circuitry of fear conditioning. Neuron. 2004;41:139–151. doi: 10.1016/s0896-6273(03)00800-6. [DOI] [PubMed] [Google Scholar]
- Vicini S, Wang JF, Li JH, Zhu WJ, Wang YH, Luo JH, Wolfe BB, Grayson DR. Functional and pharmacological differences between recombinant N-methyl-D-aspartate receptors. J Neurophysiol. 1998;79:555–566. doi: 10.1152/jn.1998.79.2.555. [DOI] [PubMed] [Google Scholar]
- Vissavajjhala P, Janssen WG, Hu Y, Gazzaley AH, Moran T, Hof PR, Morrison JH. Synaptic distribution of the AMPA-GluR2 subunit and its colocalization with calcium-binding proteins in rat cerebral cortex: an immunohistochemical study using a GluR2-specific monoclonal antibody. Exp Neurol. 1996;142:296–312. doi: 10.1006/exnr.1996.0199. + [DOI] [PubMed] [Google Scholar]
- Walker DL, Davis M. Involvement of NMDA receptors within the amygdala in short- versus long-term memory for fear conditioning as assessed with fear-potentiated startle. Behav Neurosci. 2000;114:1019–33. [PubMed] [Google Scholar]
- Wenthold RJ, Petralia RS, Blahos JII, Niedzielski AS. Evidence for multiple AMPA receptor complexes in hippocampal CA1/CA2 neurons. J Neurosci. 1996;16:1982–1989. doi: 10.1523/JNEUROSCI.16-06-01982.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilensky AE, Schafe GE, LeDoux JE. Functional inactivation of the amygdala before but not after auditory fear conditioning prevents memory formation. J Neurosci. 1999;19(RC48):1–5. doi: 10.1523/JNEUROSCI.19-24-j0006.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilensky AE, Schafe GE, LeDoux JE. The amygdala modulates memory consolidation of fear-motivated inhibitory avoidance learning but not classical fear conditioning. 2000;20:7059–7066. doi: 10.1523/JNEUROSCI.20-18-07059.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]


