Abstract
Cognitive deficits, including impaired verbal memory, are prominent in schizophrenia and lead to increased disability. Functional neuroimaging of patients with schizophrenia performing memory tasks has revealed abnormal activation patterns in prefrontal cortex and temporo-limbic regions. Aberrant fronto-temporal interactions thus represent a potential pathophysiological mechanism underlying verbal memory deficits, yet this hypothesis of disturbed connectivity is not tested directly with standard activation studies. We performed within-subject correlations of frontal and temporal timeseries to measure functional connectivity during verbal encoding. Our results confirm earlier findings of aberrant fronto-temporal connectivity in schizophrenia, and extend them by identifying distinct alterations within dorsal and ventral prefrontal cortex. Relative to healthy controls, patients with schizophrenia had reduced connectivity between the dorsolateral prefrontal cortex (DLPFC) and temporal lobe areas including parahippocampus and superior temporal gyrus. In contrast, patients showed increased connectivity between a region of ventrolateral prefrontal cortex (VLPFC) and these same temporal lobe regions. Higher temporal-DLPFC connectivity during encoding was associated with better subsequent recognition accuracy in controls, but not patients. Temporal-VLPFC connectivity was uncorrelated with recognition accuracy in either group. The results suggest that reduced temporal-DLPFC connectivity in schizophrenia could underlie encoding deficits, and increased temporal-VLPFC connectivity may represent an ineffective compensatory effort.
Keywords: Schizophrenia, episodic memory, verbal encoding, fronto-temporal, fMRI, functional connectivity
1. Introduction
Schizophrenia is associated with pervasive cognitive deficits (Heinrichs and Zakzanis, 1998). Verbal episodic memory is particularly affected, with impairment relating in part to use of inefficient organizational strategies (Koh and Peterson, 1978; Paulsen et al., 1995; Iddon et al., 1998). Providing a semantic organizational strategy during word encoding normalizes subsequent recognition accuracy in patients with schizophrenia (Ragland et al., 2003). This suggests that deficits in verbal memory may result in part from aberrant fronto-temporal interactions, a hypothesis that fits well with the prevailing view of schizophrenia as a disorder of disturbed neural and psychological integration (Friston and Frith, 1995; Andreasen et al., 1998).
Supporting this hypothesis, functional neuroimaging studies in schizophrenia have revealed abnormalities in both frontal and temporal activity. In a related series of PET studies (Frith et al., 1995; Fletcher et al., 1996; Friston et al., 1996; Fletcher et al., 1999), healthy controls performing verbal tasks activated prefrontal regions (left DLPFC, anterior cingulate) and deactivated superior temporal gyrus (STG). In contrast, schizophrenia patients showed less STG deactivation (relative overactivation) and a positive prefrontal-STG correlation. Further evidence of reciprocal changes in left frontal and left temporal regions was provided by Yurgelen-Todd et al. (1996) in an fMRI study of word fluency; by Andreasen et al. (1997) in a resting-state PET study; and by Ragland and colleagues in a series of studies of word encoding and recognition (Ragland et al., 2001; 2004; 2005).
In the absence of a specified encoding strategy, schizophrenia patients exhibited reduced DLPFC activation during encoding (Ragland et al., 2004). In contrast, there was no difference between DLPFC activity in patients and healthy controls when encoding strategy was constrained (Ragland et al., 2005). In the latter study, left VLPFC was also activated during deep versus shallow encoding, without group differences. In both studies, significant activation was observed within the left STG and left parahippocampal gyrus (PHIP) in the schizophrenia group, but not in the control group. Neither study directly assessed fronto-temporal connectivity, and abnormalities in connectivity may go undetected in standard analyses of regional activation (Lenartowicz and McIntosh, 2005).
Defined as the temporal correlations in neural activity among brain regions (Friston, 1994), functional connectivity provides a conceptual and methodological framework for identifying spatially distributed patterns of brain activity, and for quantifying inter-regional interactions. Several specific approaches have been implemented (Horwitz, 2003), and their relative merits have not been established. The timeseries correlation method (Biswal et al., 1995; Lowe et al., 1998) examines interregional correlations within individual subjects over the timecourse of an experiment, and has been effectively applied to measure functional connectivity across a wide range of cognitive and physiological states (Li et al., 2000; Lowe et al., 2002; Greicius et al., 2003; Honey et al., 2003; Anand et al., 2005; Bartels and Zeki, 2005; Fox et al., 2005; Koshino et al., 2005; Menon and Levitin, 2005; Peltier et al., 2005).
The goal of the present study was to use this timeseries correlation approach to assess fronto-temporal functional connectivity in healthy people and patients with schizophrenia performing constrained-strategy verbal encoding (Ragland et al., 2005). Previous functional connectivity studies in healthy subjects (see Discussion) have consistently reported significant fronto-temporal correlations. However, the sign of the correlation and sublocalization within the frontal cortex has varied, likely due to differences in populations, tasks, and analysis. We hypothesized that schizophrenia is associated with reduced functional connectivity between left temporal and left prefrontal regions involved in verbal memory processing. We expected that within both groups, stronger fronto-temporal connectivity during encoding would be associated with better performance during recognition.
2. Methods
Raw imaging data and behavioral measures were obtained from a previously reported study of regional activation during verbal encoding and recognition in schizophrenia (Ragland et al., 2005).
2.1 Participants
The sample comprised 14 outpatients with stable schizophrenia (2 female), and 14 healthy comparison subjects (1 female). Groups did not differ significantly on age (control 31.1 ± 6.7, patient 35.2 ± 8.2), education (control 14.1 ± 1.9, patient 13.5 ± 2.8), parental education (control 14.5 ±2.6, patient 13.4 ± 3.0), reading level (National Adult Reading Test scores: control 31.8 ± 6.4, patient 28.5 ± 7.4), or handedness (all controls and all but one patient were right-handed). All participants were assessed with standardized procedures including medical, neurological, psychiatric, neurocognitive and laboratory evaluations. Psychiatric evaluation of patients included clinical assessment, structured interview, collateral history, and symptom rating scales administered by trained investigators (reliability criterion 0.90, intraclass correlation). All patients were 18-45 years old, with a DSM-IV diagnosis of schizophrenia established in consensus conference based on all available information. Exclusion criteria for patients were other Axis I disorders (including substance-related disorders); prior electroconvulsive therapy; history of a neurological event or disease; medical diseases affecting brain function or interfering with participation; non-proficiency in English; mental retardation or learning disorders. Additional exclusion criteria for controls were any history of psychiatric illness, and any major psychiatric illness in a first-degree relative.
Patients were mildly to moderately ill according to the Scale for Assessment of Positive Symptoms (SAPS, 18.1 ± 13.4, range 0-48), Scale for Assessment of Negative Symptoms (SANS, 25.4 ± 15.6, range 2-62), and Brief Psychiatric Rating Scale (BPRS, 30.5 ± 8.0, range 18-43). Patients were all taking antipsychotic medications (3 first generation, 10 second generation, 1 combined; chlorpromazine equivalents 386.7 ± 196, range 160-500). None were receiving anticholinergic medication. Age of illness onset was 20.6 ± 3.1, with duration 14.6 ± 9.4 years. The study was explained verbally and in writing to all participants, and written informed consent obtained.
2.2 Tasks
The experimental paradigm included four conditions performed separately in the following fixed sequence: word encoding, letter N-back, word recognition, and source monitoring. The current fronto-temporal connectivity analysis focused on fMRI data acquired during the encoding condition, in a single 12.5 minute scanning run. During encoding, subjects were visually presented with 80 words over 4 “shallow” and 4 “deep” blocks. Words were randomly assigned to blocks, and blocks were presented in pseudorandom order. Each block contained an equal number of concrete and abstract words, with their order counterbalanced across blocks. During shallow blocks subjects were instructed to make a left button press for uppercase, and right button press for lowercase letters. During deep encoding a left button press was made for concrete and right button press for abstract words. Each word was presented for 3 seconds, separated by “jittered” interstimulus intervals of 0-9 seconds of crosshair fixation. The entire sequence of trials lasting 12 minutes (240 3sec scans) was analyzed as a single timeseries.
To relate functional connectivity during encoding to subsequent memory performance, behavioral data (recognition accuracy) from the recognition condition were examined. During recognition, 40 previously seen words (20 deep, 20 shallow) and 20 novel words (matched on length, frequency, and concreteness) were visually presented in the scanner and subjects were required to indicate by button press whether each word was new or had been previously seen during the encoding condition. A filled delay of approximately 10 minutes (during which subjects performed the N-back task) elapsed between the end of encoding and the beginning of the recognition task.
2.3 Image acquisition
Images were acquired with a clinically approved 3T Siemens Trio Scanner (Siemens USA, Malvern PA). Structural images for spatial normalization were obtained using a 5 minute magnetization-prepared, rapid acquisition gradient echo sequence. fMRI blood-oxygen-level-dependent (BOLD) axial images were obtained with a 36-slice whole-brain, single-shot gradient-echo, echo-planar sequence (TR 3000 msec, TE 30 msec, FOV 240mm, matrix 64×64, slice thickness/gap=4/0 mm).
2.4 Image preprocessing
SPM2 (Wellcome Department of Cognitive Neurology, University College, London) was used in the initial preprocessing steps, including slice-time correction; motion correction (to median image using b-spline interpolation); coregistration of the median image to the structural image; transformation into standard anatomical space (Montreal Neurological Institute, MNI) using tri-linear interpolation; and spatial smoothing (7.5 × 7.5 × 8 mm full-width at half maximum gaussian kernel). Further preprocessing steps were adapted from previously published timeseries correlation studies (Lowe et al., 1998; Greicius et al., 2003), and implemented in Matlab (Mathworks, Sherborn MA, USA). Non-brain voxels were removed using an intensity threshold. Each voxel's timeseries was then converted to percent change from the mean. Although no subject had more than minimal head motion (< 1 voxel translation in any direction) and images were motion-corrected, residual motion artifacts are known to occur (Grootoonk et al., 2000) and can affect correlational analyses. Therefore, artifactual outliers were replaced with the mean for the timeseries (Christoff et al., 2001), using absolute percent change thresholds empirically determined by visual inspection of 4D “movies” of whole brain timeseries (Culham, 2006). Timeseries were then bandpass filtered to remove low frequency drift and high-frequency noise, while retaining the range of frequencies (0.008 Hz <f <0.15 Hz) previously shown to contain most of the signal relevant to functional connectivity (Cordes et al., 2001; Greicius et al., 2003).
2.5 Anatomical regions of interest
Two reference, or “seed” regions of interest (ROI), were delineated in the left temporal lobe in MNI space: the parahippocampal gyrus (PHIP) and superior temporal gyrus (STG). Two target ROIs in left prefrontal cortex were defined: dorsolateral prefrontal cortex (DLPFC), and ventrolateral prefrontal cortex (VLPFC). These anatomical ROIs were derived from publicly available digital atlases: PHIP from the Automated Anatomical Labeling (AAL) atlas provided with WFU_Pickatlas (Tzourio-Mazoyer et al., 2002); STG (BA 22), DLPFC (BAs 9 and 46), and VLPFC (BA 47) from the Brodmann Area atlas provided with MRIcro (Rorden and Brett, 2000). Individual ROI masks were extracted from the atlases (after resampling into the same voxel dimensions as the functional data) by selecting voxels with the appropriate numerical atlas label, and inspected to confirm their correct alignment with the standard MNI brain image. ROI voxel number/ volume in cubic mm were: PHIP, 156/8775; STG 222/12488; DLPFC 610/34313, VLPFC 268/15075. Our a priori ROIs were limited to the left hemisphere, given the predominantly left-sided brain involvement in verbal encoding and the reduction in statistical power that occurs with increasing numbers of comparisons. While hippocampus would also be interesting to study, we selected temporal seed ROIs based on the consistent PHIP and STG abnormalities found in this and our previous memory studies. Hippocampal involvement was not consistent, possibly due to the nature of the task that did not require much relational processing.
2.6 Functional connectivity maps
Whole brain correlation maps were generated. Within each subject, the average timeseries of each temporal seed ROI was correlated with the timeseries of each voxel in the brain (Bartels and Zeki, 2005; Fox et al., 2005). To correct for nonspecific global differences in correlation values, each subject's distribution was mean-centered at zero (Lowe et al., 1998). Fisher's r-to-Z transformation (Z = 0.5*[ln(1+r)-ln(1−r)]) was used to normalize the distribution of correlation coefficients prior to statistical analysis. For each subject, this produced two whole-brain correlation maps, one from the STG seed ROI and one from the PHIP seed ROI. Group-average maps were generated for visual display and qualitative assessment of whole-brain connectivity patterns.
Group differences in functional connectivity were identified using individual subject maps and voxelwise two-sample t-tests. Given the anatomical restriction of our a priori hypothesis to DLPFC and VLPFC target ROIs, we used a statistical threshold at each voxel of P < 0.05, and applied a cluster-size cutoff of 33 contiguous voxels for a final threshold of P < 0.05 corrected for multiple comparisons (i.e., “cluster-corrected”). The cluster-size cutoff was determined using AlphaSim (B. D. Ward), a component of AFNI image analysis software (R. W. Cox, NIH), using a mask containing the 878 voxels of the combined DLPFC and VLPFC ROIs. AlphaSim implements a nonparametric permutation test (via Monte Carlo simulation) within a user-defined space in order to determine the probability of false positive detection based on the combination of the individual voxel probability threshold and the minimum cluster size threshold.
2.7 Connectivity-performance correlations
To assess the functional significance of individual differences in fronto-temporal connectivity, correlation analyses were performed across subjects within each group. Each subject had four fronto-temporal connectivity measures: PHIP seed- DLPFC target, STG seed- DLPFC target, PHIP seed – VLPFC target, STG seed- VLPFC target. For each seed-target pair, the measure of connectivity was the average of all positive Fisher's Z coefficients within the target ROI. For each subject, connectivity measures were correlated with that subject's recognition accuracy, defined as the d' discriminability score according to the Two-High Threshold Theory (Snodgrass and Corwin, 1988). There were no significant group differences in recognition accuracy, and both groups had a similar range of scores across subjects (control 0.43 ± 0.17, patient 0.38 ± 0.14; for a detailed performance summary see Ragland et al., 2005). Pearson's r values > 0.53 (P < 0.05 for n = 14) were considered significant. Group differences in performance-connectivity correlations were also tested and considered significant at P < 0.05.
3. Results
Qualitative assessment of group-average whole-brain functional connectivity maps for the STG and PHIP revealed distinct connectivity patterns for the two seed regions. As expected (Biswal et al., 1995; Lowe et al., 1998; Bartels and Zeki, 2005) the strongest correlations were localized to the seed region and its contralateral homolog.
3.1 Group differences in connectivity
Quantitative assessment of functional connectivity was limited to the two a priori target ROIs in the left PFC. Voxel-based t-tests were used to identify regions within the DLPFC and VLPFC ROIs that exhibited significant group differences (P < 0.05, cluster-corrected) in connectivity with the temporal lobe seed ROIs. As seen in Figs. 1 and 2, a single region within DLPFC exhibited reduced connectivity with both STG and PHIP in the schizophrenia group (STG peak Z 2.95, P = 0.002; PHIP peak Z 2.54, P = 0.006). This region is also easily identified by visual inspection of group-average connectivity maps. In contrast, a region within VLPFC exhibited the reverse pattern, with increased temporal lobe connectivity in the schizophrenia group (STG peak Z 2.67, P = 0.004; PHIP peak Z 2.93, P = 0.002). We repeated the analysis using multiple regression to remove the variance associated with task effects (Whalley et al., 2005), and the results were essentially unchanged. We also repeated the analysis after dividing deep and shallow conditions into separate timeseries. The pattern of results was unchanged, with no differences between the two encoding conditions.
Fig. 1.

Group differences in left STG-DLPFC and STG-VLPFC connectivity. Panel A: Group-averaged STG timeseries correlation maps for control group (left) and schizophrenia group (right), from a representative coronal plane (y = 30). A region of DLPFC shows greater connectivity (measured as average Fisher's Z-transformed correlation coefficients) with STG in controls, while a region in VLPFC shows greater connectivity with STG in patients. Panel B: Results of voxel-by voxel t-tests are overlaid on the two prefrontal ROIs and a standard reference brain (Colin, MNI), in the same coronal plane. The group differences seen within DLPFC and VLPFC are statistically significant (P < 0.05 corrected, cluster threshold 33 voxels). The DLPFC cluster contains 39 contiguous voxels, peak Z 2.95 at MNI x = −38, y = 34, z = 36); the VLPFC cluster contains 65 voxels, peak Z 2.67 at MNI x = −41, y = 26, z = 0).
Fig. 2.
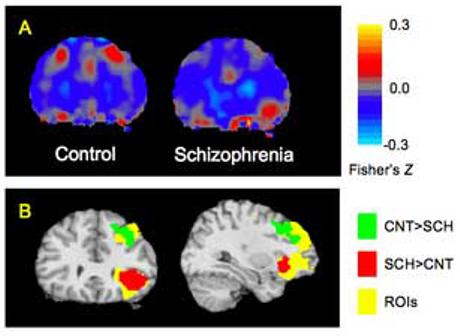
Group differences in left PHIP-DLPFC and PHIP-VLPFC connectivity. Panel A: Group-averaged PHIP timeseries correlation maps reveal a region within DLPFC showing greater connectivity with PHIP in controls, while a region within VLPFC shows greater connectivity with PHIP in patients. Panel B: Group differences seen within DLPFC and VLPFC are statistically significant. The DLPFC cluster contains 42 contiguous voxels, peak Z 2.54 at MNI x = −30, y = 11, z = 48); the VLPFC cluster contains 36 voxels, peak Z 2.93 at MNI x = −34, y = 26, z = −8). Thresholding and display format are as in Fig. 1.
Although these group differences in prefrontal connectivity were identified using correlations to specific temporal lobe seed regions, it is possible that the abnormalities driving these differences lie entirely within the DLPFC and VLPFC themselves, rather than within the fronto-temporal connection per se. For example, if the DLPFC were altered in such a way that its correlations were reduced non-specifically with all brain regions, we would expect to see the same pattern we observed here. The same concern arises with regard to a possible non-specific increase in VLPFC correlations. In order to assess whether the observed group differences in connectivity were indeed selective for temporal-prefrontal circuits, we generated “reverse direction” correlation maps (see also Meyer-Lindenberg et al., 2005), taking as seed ROIs the two prefrontal clusters exhibiting significant group differences, and examining correlations with all voxels in the brain. Connectivity with the DLPFC region was not diffusely increased in controls, but as expected showed greater connectivity primarily in left temporal lobe, with significant (P < 0.05 cluster-corrected) group differences in regions overlapping the STG and the PHIP. As previously, the VLPFC region showed reverse group differences primarily in the temporal lobe, with significantly greater connectivity in schizophrenia patients in left temporal regions overlapping STG and PHIP. This supports the selectivity of prefrontal connectivity changes to circuits involving left temporo-limbic regions.
3.2 Performance-Connectivity correlations
For control subjects, greater connectivity of both temporal ROIs with DLPFC during encoding was correlated (P < 0.01) with better performance in the recognition task (Fig. 3). In contrast, within the schizophrenia group there was no correlation between performance and temporal-DLPFC connectivity. This group difference in correlation strength was statistically significant (P < 0.05) for both STG and PHIP. Neither group had any significant correlation of recognition performance with functional connectivity between temporal regions and VLPFC.
Fig. 3.

Bar graph showing the correlation of recognition performance with the measures of fronto-temporal connectivity, for each of the four seed-target connectivity pairs. Among controls, STG and PHIP connectivity with DLPFC during encoding was positively correlated with subsequent recognition accuracy (STG: P = 0.006, PHIP: P < 0.001). Performance was not correlated with temporal-DLPFC connectivity in the patients (STG: P = 0.98, PHIP: P = 0.75). Neither group showed significant correlations of performance with temporal-VLPFC connectivity (STG-VLPFC: Controls P = 0.25, Patients P = 0.30; PHIP-VLPFC: Controls P = 0.30, Patients P = 0.56).
Scatterplots were used to ensure that performance correlations with temporal-DLPFC connectivity were not driven by outliers. As can be seen in Fig. 4 the significant correlations in controls were consistent across subjects and did not appear to be influenced by extreme values. A single statistical outlier in the PHIP-DLPFC data from the patient group can be seen. Performance remained uncorrelated after excluding that subject's data (PHIP-DLPFC: r = −0.22, P = 0.47; STG-DLPFC: r = 0.04, P = 0.90; PHIP-VLPFC: r = 0.17, P = 0.58; STG-VLPFC: r = −0.26, P = 0.39).
Fig. 4.
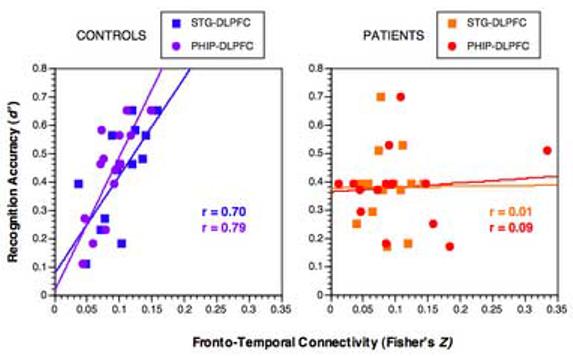
Scatterplots relating each subject's STG-DLPFC AND PHIP-DLPFC connectivity measure (Fisher's Z-transformed correlation coefficients) during encoding (x-axis) to the discriminability (d') measure of subsequent recognition memory (y-axis). Controls (left) exhibit a positive correlation of STG-DLPFC connectivity with performance, while patients (right) have no significant correlation. These observations are not due to statistical outliers.
To rule out confounds due to illness severity or chronicity, we assessed whether fronto-temporal connectivity measures correlated with SANS, SAPS, or duration of illness. There were no significant correlations (all P > 0.10). Factoring out the influence of these measures using partial correlations did not alter the connectivity-performance results.
4. Discussion
Our study is the first to apply the within-subject timeseries correlation method to functional connectivity during verbal encoding in schizophrenia. Results confirm the existence of aberrant fronto-temporal connectivity in schizophrenia, and identify new aspects of this disturbance. Specifically, we identified reduced connectivity in schizophrenia between temporal lobe regions important in memory and language functions, and the DLPFC, which is required for executive control. In contrast, we found increased connectivity between these same temporal lobe regions and the VLPFC, a region strongly recruited during verbal semantic processing. Temporal-DLPFC connectivity was correlated with subsequent recognition accuracy in controls, but not patients, and temporal-VLPFC connectivity was uncorrelated with performance in either group. This effect highlights the heterogeneity of prefrontal function in both healthy subjects and in schizophrenia, and underscores the power of functional connectivity studies to complement standard analyses of task-related activation.
Most studies explicitly measuring fronto-temporal correlations have confirmed the presence of abnormalities in schizophrenia. In a PET study of verbal fluency, Friston et al. (1996) found an abnormal positive correlation between left STG and left DLPFC in schizophrenia, as did Fletcher et al. (1999) using a verbal memory task. Jennings et al. (1998) used structural equation modeling in a PET study of semantic categorization and found that the influence of left ventral PFC (BA 45) on left STG activity was positive in controls but negative in patients, in the context of equal task-related prefrontal and temporal activation. Boksman et al. (2005) used a timeseries covariation approach to examine anterior cingulate (AC) connectivity in an fMRI study of word fluency and observed significant AC-left STG connectivity in patients, but not in controls. In a canonical variates analysis of group PET data during an n-back working memory task, Meyer-Lindenberg et al. (2001) found high intra-temporal and low intra-frontal functional correlations in patients, with the reverse finding in controls. In a more recent PET n-back study, Meyer-Lindenberg et al. (2005) used a within-subject timeseries covariation approach to explore hippocampal formation connectivity, and found task-dependent negative correlations of the left hippocampal formation with right DLPFC in control subjects that were persistent (task-independent) in patients.
In contrast to these positive findings, one group performed two PET studies of word fluency and did not find significant left fronto-temporal abnormalities in minimally-symptomatic schizophrenia patients (Dye et al., 1999; Spence et al., 2000). Taken together with the results of other studies that have directly assessed the relationship of fronto-temporal dysfunction to illness severity (Andreasen et al., 1997; Lawrie et al., 2002; Fu et al., 2005), these findings indicate that the degree of left STG overactivation and abnormally positive STG-prefrontal interactions may be state-dependent, correlating with the severity of active illness, and tending to normalize during remission. However, patients in the current study were clinically stable and had mild to moderate symptoms indicating that abnormal fronto-temporal connectivity may be a trait-like variable. The lack of correlation between fronto-temporal connectivity and measures of positive symptoms (SAPS), negative symptoms (SANS), and duration of illness also supports this conclusion. The similar results between deep versus shallow encoding conditions likewise buttress the notion that the observed abnormalities are relatively insensitive to cognitive state.
Further evidence of fronto-temporal dysconnectivity comes from EEG studies reporting reduced fronto-temporal coherence in schizophrenia (Norman et al., 1997; Kissler et al., 2000; Ford et al., 2002; Winterer et al., 2003). There is also abundant evidence of structural abnormalities in fronto-temporal regions, which may contribute to functional dysconnectivity. This includes both reductions in gray matter, particularly prominent in fronto-temporal regions (Mathalon et al., 2001; Davatzikos et al., 2005; McDonald et al., 2005), and reduced integrity of white matter tracts that mediate fronto-temporal interactions (Kubicki et al., 2002; Burns et al., 2003; Szeszko et al., 2005; Shin et al., 2006).
Our finding of positive temporal-DLPFC connectivity in controls, with less-positive connectivity in schizophrenia, stands in contrast to some of the earlier findings and deserves comment. First, the positive connectivity we observed lies only in a subregion of DLPFC, and thus could be missed in studies focusing on DLPFC as a whole. Second, most PET functional connectivity studies measured connectivity as a correlation of activity across subjects within a group. Within-subject connectivity analysis assesses a different source of variance and may give quite different results from across-subject analyses. Only a few studies have examined temporal-DLPFC connectivity using within-subject timeseries correlations. Meyer-Lindenberg et al. (2005) found negative correlations for temporal to contralateral, but not ipsilateral, DLPFC. This negative correlation was stronger in patients than controls at high task loads. Whalley et al. (2005), studying a population at high genetic risk for schizophrenia, also found contralateral but not ipsilateral temporal-DLPFC negative correlations. In the study most directly comparable to ours, Lawrie et al. (2002) found, as we did, positive left STG-left DLPFC connectivity in controls that was reduced in patients.
Our results reveal a dissociation between DLPFC and VLPFC connectivity changes in schizophrenia. The increase in temporal-VLPFC connectivity constitutes an exception to the usual finding of reduced fronto-temporal connectivity in schizophrenia. Relative to the DLPFC, the possible role of VLPFC abnormalities in schizophrenia has received little attention. We are not aware of any studies reporting increased temporal-VLPFC connectivity, although it occurred at subthreshold levels in one study described above (Meyer-Lindenberg et al., 2005, supplementary online data). While both groups in the current study activated VLPFC equally (Ragland et al., 2005), as in Jennings et al. (1998) and Barch et al. (2001), other studies have found increased task-related activity of VLPFC in schizophrenia (Kim et al., 2003; Bonner-Jackson et al., 2005; Tan et al., 2005). Overactivation of left inferior frontal cortex has also been observed in a resting PET study (Andreasen et al., 1997), and intriguingly, treatment with either haldoperidol or clozapine reduced VLPFC activity (Lahti et al., 2003).
The schizophrenia subjects in previous studies showing VLPFC overactivation also exhibited hypoactivation of DLPFC, leading some investigators to suggest a compensatory role for the VLPFC (Andreasen et al., 1997; Kim et al., 2003; Tan et al., 2005). Our connectivity-performance analysis does not support the idea that increased temporal-VLPFC connectivity is an effective compensatory mechanism, as there was no correlation with performance in either group. A compensatory role would also suggest that subjects with lower temporal-DLPFC connectivity should exhibit higher temporal-VLPFC connectivity, however this relationship was not seen. It is possible that any compensatory effects were masked by other deficits, or that both inhibition of VLPFC connectivity and enhancement of DLPFC connectivity are necessary to restore performance correlations. However it is also possible that increased temporal-VLPFC connectivity is an abnormality without benefit.
In the present study, an effort was made to ensure that performance levels were equated for patients and controls. This improves interpretability of activation differences, but precludes distinguishing among these alternatives, which may have implications for guiding behavioral and pharmacologic intervention. Studies varying task difficulty within groups may help resolve these questions. It will be important to determine whether effective treatment of encoding deficits in schizophrenia is associated with both reduced VLPFC and increased DLPFC connectivity, restoring the robust correlation observed in healthy controls.
We observed differential changes in the connectivity of DLPFC and VLPFC to temporal regions in schizophrenia, while traditional SPM analysis of this data (Ragland et al., 2005) did not reveal group differences in DLPFC or VLPFC regional activation. The dissociation between activation and connectivity highlights the possibility that activation in a particular region may be equal in magnitude between groups or conditions, but still reflect differential involvement in distinct neural circuits (Jennings et al., 1998; Lenartowicz and Mcintosh, 2005). For example, VLPFC activity may be disproportionately related to temporal-VLPFC connections in patients with schizophrenia, while in control subjects a greater proportion of VLPFC activity may involve intra-frontal connections. Furthermore, our measure of functional connectivity (timeseries correlation) is sensitive both to changes in BOLD signal driven by experimental stimuli and to physiological low-frequency BOLD fluctuations that are not directly related to task events (Biswal et al., 1995; Lowe et al., 1998; Raichle and Gusnard, 2005). However, our results were unchanged when the connectivity analysis was performed after statistically removing task-related variance, suggesting they do not simply reflect stimulus-induced changes in activity. Given the limitations of statistical methods in removing all task-related activity, it will be interesting to see if the observed fronto-temporal connectivity abnormalities are as prominent in the resting state.
A distinction between the roles of DLPFC and VLPFC during verbal memory tasks has been noted in studies of healthy subjects, although both regions are strongly activated during such tasks (Wagner et al., 2001; Ranganath, 2006). Functional connectivity studies in healthy subjects performing verbal memory tasks have found positive temporal-VLPFC correlations (Bokde et al., 2001; Sperling et al., 2003). Notably, Grady et al. (2003) found that in healthy young adults performing a verbal encoding task, temporal-VLPFC connectivity predominated and correlated positively with recognition accuracy, while in older adults temporal-DLPFC connectivity predominated and correlated with performance. Menon et al. (2001) also showed an increase in temporal-DLPFC functional connectivity with age in a group of children and adolescents performing a non-verbal encoding task. No age-related changes in temporal-VLPFC connectivity were observed. These findings indicate that temporal-VLPFC and temporal-DLPFC connectivity vary along with normal neurodevelopmental changes, suggesting that observed alterations in connectivity may relate to the abnormal neurodevelopment of schizophrenia.
Although fronto-temporal deficits are prominent in schizophrenia, connectivity abnormalities exist in other parts of the brain as well. A recent functional connectivity study examined fMRI timeseries correlations between all combinations of 116 brain regions and found that, except for increased cerebellar connectivity, schizophrenia subjects in a resting state exhibited global reductions in functional connectivity (Liang et al., 2006). Other studies using timeseries correlations have focused on different specific connections, and identified abnormalities in the connectivity of the cerebellum (Stephan et al., 2001) and anterior cingulate (Honey et al., 2005). Our own results (Fig. 1) suggest that group differences in left temporal lobe connectivity are not restricted to the ipsilateral DLPFC and VLPFC, and occur elsewhere in the brain (note effects in anterior cingulate and contralateral prefrontal areas). Given the anatomically specific hypotheses that motivated this study, and the greatly increased type I error involved in exploratory whole-brain studies, we restricted our formal analysis to the two a priori prefrontal regions.
The approach that we employed uses within-subject correlations across time, rather than within-group correlations across subjects. This presents a statistical advantage, as it is easier to obtain large numbers of scans (in fMRI) than large numbers of subjects. Another advantage is that functional connectivity becomes an individual rather than a group trait, which helps examine links between connectivity measures and other individual differences such as cognitive performance (as we have shown), pharmacological response, or illness phenotype. Furthermore, although no functional connectivity method can be assumed to relate directly to anatomical connectivity, within-subject correlations are more likely to relate to individual anatomy, enhancing the prospect of relating functional connectivity to measures of structural connectivity such as DTI. This prediction is borne out by studies that have shown correspondence between known anatomical connectivity patterns and functional connectivity measured by fMRI timeseries correlations (Koch et al., 2002; Quigley et al., 2003; Johansen-Berg et al., 2004; Bartels and Zeki, 2005).
All patients were taking antipsychotic medications and we cannot exclude a possible medication effect contributing to group differences in connectivity. However, available evidence suggests that antipsychotic treatment generally reduces functional abnormalities (Davis et al., 2005), including normalization of fronto-temporal activation (Lahti et al., 2003), and functional connectivity in the prefrontal-thalamic-cerebellar circuit (Stephan et al., 2001). It is thus likely that fronto-temporal connectivity abnormalities occur in schizophrenia in spite of medications rather than because of them. The regional specificity of our functional connectivity results is also difficult to attribute solely to medication. Nonetheless, confirmatory studies in unmedicated patients will be important, as will studies directly examining the effect of antipsychotic agents and other therapeutics on functional connectivity.
It is important to acknowledge the limitations of functional connectivity studies and exercise care in interpreting their results (Horwitz, 2003). Nonetheless, timeseries correlation analysis has been utilized in studies of normal cognitive and emotional states (Greicius et al., 2003; Bartels and Zeki, 2005; Fox et al., 2005; Menon and Levitin, 2005), disease states (Lowe et al., 2002; Anand et al., 2005; Koshino et al., 2005;), and pharmacological interventions (Li et al., 2000; Honey et al., 2003; Peltier et al., 2005). Our study contributes to a rapidly growing body of knowledge regarding normal and abnormal functional neural circuitry. Further studies relating fronto-temporal connectivity in schizophrenia to specific symptoms, endophenotypes, pharmacological effects, structural abnormalities and genetic variation will be illuminating. Used in conjunction with other functional and anatomical methods, functional connectivity provides a powerful tool in the effort to understand the pathophysiology of schizophrenia.
Acknowledgment
The authors thank members of the Schizophrenia Research Center for subject accrual, and Kosha Ruparel and Ramapriyan Pratiwadi for assistance with image analysis. The research was supported by National Institutes of Health grants (MH-62103, MH060722, T32-MH019112, M01RR0040), Scottish Rite Foundation (D.H.W.), and a NARSAD Young Investigator Award (D.H.W., sponsored by the Sidney R. Baer, Jr. Foundation).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anand A, Li Y, Wang Y, Wu J, Gao S, Bukhari L, Mathews VP, Kalnin A, Lowe MJ. Antidepressant effect on connectivity of the mood-regulating circuit: an fMRI study. Neuropsychopharmacology. 2005;30:1334–1344. doi: 10.1038/sj.npp.1300725. [DOI] [PubMed] [Google Scholar]
- Andreasen NC, O'Leary DS, Flaum M, Nopoulos P, Watkins GL, Boles Ponto LL, Hichwa RD. Hypofrontality in schizophrenia: distributed dysfunctional circuits in neuroleptic-naive patients. Lancet. 1997;349:1730–1734. doi: 10.1016/s0140-6736(96)08258-x. [DOI] [PubMed] [Google Scholar]
- Andreasen NC, Paradiso S, O'Leary DS. “Cognitive dysmetria” as an integrative theory of schizophrenia: a dysfunction in cortical-subcortical-cerebellar circuitry? Schizophrenia Bulletin. 1998;24:203–218. doi: 10.1093/oxfordjournals.schbul.a033321. [DOI] [PubMed] [Google Scholar]
- Barch DM, Carter CS, Braver TS, Sabb FW, MacDonald A, 3rd, Noll DC, Cohen JD. Selective deficits in prefrontal cortex function in medication-naive patients with schizophrenia. Archives of General Psychiatry. 2001;58:280–288. doi: 10.1001/archpsyc.58.3.280. [DOI] [PubMed] [Google Scholar]
- Bartels A, Zeki S. Brain dynamics during natural viewing conditions--a new guide for mapping connectivity in vivo. NeuroImage. 2005;24:339–349. doi: 10.1016/j.neuroimage.2004.08.044. [DOI] [PubMed] [Google Scholar]
- Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magnetic Resonance in Medicine. 1995;34:537–541. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- Bokde AL, Tagamets MA, Friedman RB, Horwitz B. Functional interactions of the inferior frontal cortex during the processing of words and word-like stimuli. Neuron. 2001;30:609–617. doi: 10.1016/s0896-6273(01)00288-4. [DOI] [PubMed] [Google Scholar]
- Boksman K, Theberge J, Williamson P, Drost DJ, Malla A, Densmore M, Takhar J, Pavlosky W, Menon RS, Neufeld RW. A 4.0-T fMRI study of brain connectivity during word fluency in first-episode schizophrenia. Schizophrenia Research. 2005;75:247–263. doi: 10.1016/j.schres.2004.09.025. [DOI] [PubMed] [Google Scholar]
- Bonner-Jackson A, Haut K, Csernansky JG, Barch DM. The influence of encoding strategy on episodic memory and cortical activity in schizophrenia. Biological Psychiatry. 2005;58:47–55. doi: 10.1016/j.biopsych.2005.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns J, Job D, Bastin ME, Whalley H, Macgillivray T, Johnstone EC, Lawrie SM. Structural disconnectivity in schizophrenia: a diffusion tensor magnetic resonance imaging study. British Journal of Psychiatry. 2003;182:439–443. [PubMed] [Google Scholar]
- Christoff K, Prabhakaran V, Dorfman J, Zhao Z, Kroger JK, Holyoak KJ, Gabrieli JD. Rostrolateral prefrontal cortex involvement in relational integration during reasoning. NeuroImage. 2001;14:1136–1149. doi: 10.1006/nimg.2001.0922. [DOI] [PubMed] [Google Scholar]
- Cordes D, Haughton VM, Arfanakis K, Carew JD, Turski PA, Moritz CH, Quigley MA, Meyerand ME. Frequencies contributing to functional connectivity in the cerebral cortex in “resting-state” data. American Journal of Neuroradiology. 2001;22:1326–1333. [PMC free article] [PubMed] [Google Scholar]
- Culham JC. Functional neuroimaging: experimental design and analysis. In: Cabeza R, Kingstone A, editors. Handbook of Functional Neuroimaging of Cognition. 2nd edition MIT Press; Cambridge, MA: 2006. [Google Scholar]
- Davatzikos C, Shen D, Gur RC, Wu X, Liu D, Fan Y, Hughett P, Turetsky BI, Gur RE. Whole-brain morphometric study of schizophrenia revealing a spatially complex set of focal abnormalities. Archives of General Psychiatry. 2005;62:1218–1227. doi: 10.1001/archpsyc.62.11.1218. [DOI] [PubMed] [Google Scholar]
- Davis CE, Jeste DV, Eyler LT. Review of longitudinal functional neuroimaging studies of drug treatments in patients with schizophrenia. Schizophrenia Research. 2005;78:45–60. doi: 10.1016/j.schres.2005.05.009. [DOI] [PubMed] [Google Scholar]
- Dye SM, Spence SA, Bench CJ, Hirsch SR, Stefan MD, Sharma T, Grasby PM. No evidence for left superior temporal dysfunction in asymptomatic schizophrenia and bipolar disorder. PET study of verbal fluency. British Journal of Psychiatry. 1999;175:367–374. doi: 10.1192/bjp.175.4.367. [DOI] [PubMed] [Google Scholar]
- Fletcher P, McKenna PJ, Friston KJ, Frith CD, Dolan RJ. Abnormal cingulate modulation of fronto-temporal connectivity in schizophrenia. NeuroImage. 1999;9:337–342. doi: 10.1006/nimg.1998.0411. [DOI] [PubMed] [Google Scholar]
- Fletcher PC, Frith CD, Grasby PM, Friston KJ, Dolan RJ. Local and distributed effects of apomorphine on fronto-temporal function in acute unmedicated schizophrenia. Journal of Neuroscience. 1996;16:7055–7062. doi: 10.1523/JNEUROSCI.16-21-07055.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford JM, Mathalon DH, Whitfield S, Faustman WO, Roth WT. Reduced communication between frontal and temporal lobes during talking in schizophrenia. Biological Psychiatry. 2002;51:485–492. doi: 10.1016/s0006-3223(01)01335-x. [DOI] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proceedings of the National Academy of Sciences USA. 2005;102:9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston K. Functional and effective connectivity in neuroimaging: a synthesis. Human Brain Mapping. 1994;2:56–78. [Google Scholar]
- Friston KJ, Frith CD. Schizophrenia: a disconnection syndrome? Clinical Neuroscience. 1995;3:89–97. [PubMed] [Google Scholar]
- Friston KJ, Frith CD, Fletcher P, Liddle PF, Frackowiak RS. Functional topography: multidimensional scaling and functional connectivity in the brain. Cerebral Cortex. 1996;6:156–164. doi: 10.1093/cercor/6.2.156. [DOI] [PubMed] [Google Scholar]
- Frith CD, Friston KJ, Herold S, Silbersweig D, Fletcher P, Cahill C, Dolan RJ, Frackowiak RS, Liddle PF. Regional brain activity in chronic schizophrenic patients during the performance of a verbal fluency task. British Journal of Psychiatry. 1995;167:343–349. doi: 10.1192/bjp.167.3.343. [DOI] [PubMed] [Google Scholar]
- Fu CH, Suckling J, Williams SC, Andrew CM, Vythelingum GN, McGuire PK. Effects of psychotic state and task demand on prefrontal function in schizophrenia: an fMRI study of overt verbal fluency. American Journal of Psychiatry. 2005;162:485–494. doi: 10.1176/appi.ajp.162.3.485. [DOI] [PubMed] [Google Scholar]
- Grady CL, McIntosh AR, Craik FI. Age-related differences in the functional connectivity of the hippocampus during memory encoding. Hippocampus. 2003;13:572–586. doi: 10.1002/hipo.10114. [DOI] [PubMed] [Google Scholar]
- Greicius MD, Krasnow B, Reiss AL, Menon V. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proceedings of the National Academy of Sciences USA. 2003;100:253–258. doi: 10.1073/pnas.0135058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grootoonk S, Hutton C, Ashburner J, Howseman AM, Josephs O, Rees G, Friston KJ, Turner R. Characterization and correction of interpolation effects in the realignment of fMRI time series. NeuroImage. 2000;11:49–57. doi: 10.1006/nimg.1999.0515. [DOI] [PubMed] [Google Scholar]
- Heinrichs RW, Zakzanis KK. Neurocognitive deficit in schizophrenia: a quantitative review of the evidence. Neuropsychology. 1998;12:426–445. doi: 10.1037//0894-4105.12.3.426. [DOI] [PubMed] [Google Scholar]
- Honey GD, Suckling J, Zelaya F, Long C, Routledge C, Jackson S, Ng V, Fletcher PC, Williams SCR, Brown J, Bullmore ET. Dopaminergic drug effects on physiological connectivity in a human cortico-striato-thalamic system. Brain. 2003;126:1767–1781. doi: 10.1093/brain/awg184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honey GD, Pomarol-Clotet E, Corlett PR, Honey RA, McKenna PJ, Bullmore ET, Fletcher PC. Functional dysconnectivity in schizophrenia associated with attentional modulation of motor function. Brain. 2005;128:2597–2611. doi: 10.1093/brain/awh632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz B. The elusive concept of brain connectivity. NeuroImage. 2003;19:466–470. doi: 10.1016/s1053-8119(03)00112-5. [DOI] [PubMed] [Google Scholar]
- Iddon JL, McKenna PJ, Sahakian BJ, Robbins TW. Impaired generation and use of strategy in schizophrenia: evidence from visuospatial and verbal tasks. Psychological Medicine. 1998;28:1049–1062. doi: 10.1017/s0033291798006758. [DOI] [PubMed] [Google Scholar]
- Jennings JM, McIntosh AR, Kapur S, Zipursky RB, Houle S. Functional network differences in schizophrenia: a rCBF study of semantic processing. NeuroReport. 1998;9:1697–1700. doi: 10.1097/00001756-199806010-00005. [DOI] [PubMed] [Google Scholar]
- Johansen-Berg H, Behrens TE, Robson MD, Drobnjak I, Rushworth MF, Brady JM, Smith SM, Higham DJ, Matthews PM. Changes in connectivity profiles define functionally distinct regions in human medial frontal cortex. Proceedings of the National Academy of Sciences USA. 2004;101:13335–13340. doi: 10.1073/pnas.0403743101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JJ, Kwon JS, Park HJ, Youn T, Kang do H, Kim MS, Lee DS, Lee MC. Functional disconnection between the prefrontal and parietal cortices during working memory processing in schizophrenia: a [15(O)] H2O PET study. American Journal of Psychiatry. 2003;160:919–923. doi: 10.1176/appi.ajp.160.5.919. [DOI] [PubMed] [Google Scholar]
- Kissler J, Muller MM, Fehr T, Rockstroh B, Elbert T. MEG gamma band activity in schizophrenia patients and healthy subjects in a mental arithmetic task and at rest. Clinical Neurophysiology. 2000;111:2079–2087. doi: 10.1016/s1388-2457(00)00425-9. [DOI] [PubMed] [Google Scholar]
- Koch MA, Norris DG, Hund-Georgiadis M. An investigation of functional and anatomical connectivity using magnetic resonance imaging. NeuroImage. 2002;16:241–250. doi: 10.1006/nimg.2001.1052. [DOI] [PubMed] [Google Scholar]
- Koh SD, Peterson RA. Encoding orientation and the remembering of schizophrenic young adults. Journal of Abnormal Psychology. 1978;87:303–313. [PubMed] [Google Scholar]
- Koshino H, Carpenter PA, Minshew NJ, Cherkassky VL, Keller TA, Just MA. Functional connectivity in an fMRI working memory task in high-functioning autism. NeuroImage. 2005;24:810–821. doi: 10.1016/j.neuroimage.2004.09.028. [DOI] [PubMed] [Google Scholar]
- Kubicki M, Westin CF, Maier SE, Frumin M, Nestor PG, Salisbury DF, Kikinis R, Jolesz FA, McCarley RW, Shenton ME. Uncinate fasciculus findings in schizophrenia: a magnetic resonance diffusion tensor imaging study. American Journal of Psychiatry. 2002;159:813–820. doi: 10.1176/appi.ajp.159.5.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahti AC, Holcomb HH, Weiler MA, Medoff DR, Tamminga CA. Functional effects of antipsychotic drugs: comparing clozapine with haloperidol. Biological Psychiatry. 2003;53:601–608. doi: 10.1016/s0006-3223(02)01602-5. [DOI] [PubMed] [Google Scholar]
- Lawrie SM, Buechel C, Whalley HC, Frith CD, Friston KJ, Johnstone EC. Reduced frontotemporal functional connectivity in schizophrenia associated with auditory hallucinations. Biological Psychiatry. 2002;51:1008–1011. doi: 10.1016/s0006-3223(02)01316-1. [DOI] [PubMed] [Google Scholar]
- Lenartowicz A, McIntosh AR. The role of anterior cingulate cortex in working memory is shaped by functional connectivity. Journal of Cognitive Neuroscience. 2005;17:1026–1042. doi: 10.1162/0898929054475127. [DOI] [PubMed] [Google Scholar]
- Li SJ, Biswal B, Li Z, Risinger R, Rainey C, Cho JK, Salmeron BJ, Stein EA. Cocaine administration decreases functional connectivity in human primary visual and motor cortex as detected by functional MRI. Magnetic Resonance in Medicine. 2000;43:45–51. doi: 10.1002/(sici)1522-2594(200001)43:1<45::aid-mrm6>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Liang M, Zhou Y, Jiang T, Liu Z, Tian L, Liu H, Hao Y. Widespread functional disconnectivity in schizophrenia with resting-state functional magnetic resonance imaging. NeuroReport. 2006;17:209–213. doi: 10.1097/01.wnr.0000198434.06518.b8. [DOI] [PubMed] [Google Scholar]
- Lowe MJ, Mock BJ, Sorenson JA. Functional connectivity in single and multislice echoplanar imaging using resting-state fluctuations. NeuroImage. 1998;7:119–132. doi: 10.1006/nimg.1997.0315. [DOI] [PubMed] [Google Scholar]
- Lowe MJ, Phillips MD, Lurito JT, Mattson D, Dzemidzic M, Mathews VP. Multiple sclerosis: low-frequency temporal blood oxygen level-dependent fluctuations indicate reduced functional connectivity - initial results. Radiology. 2002;224:184–192. doi: 10.1148/radiol.2241011005. [DOI] [PubMed] [Google Scholar]
- Mathalon DH, Sullivan EV, Lim KO, Pfefferbaum A. Progressive brain volume changes and the clinical course of schizophrenia in men: a longitudinal magnetic resonance imaging study. Archives of General Psychiatry. 2001;58:148–157. doi: 10.1001/archpsyc.58.2.148. [DOI] [PubMed] [Google Scholar]
- McDonald C, Bullmore E, Sham P, Chitnis X, Suckling J, MacCabe J, Walshe M, Murray RM. Regional volume deviations of brain structure in schizophrenia and psychotic bipolar disorder: computational morphometry study. British Journal of Psychiatry. 2005;186:369–377. doi: 10.1192/bjp.186.5.369. [DOI] [PubMed] [Google Scholar]
- Menon V, Anagnoson RT, Glover GH, Pfefferbaum A. Functional magnetic resonance imaging evidence for disrupted basal ganglia function in schizophrenia. American Journal of Psychiatry. 2001;158:646–649. doi: 10.1176/appi.ajp.158.4.646. [DOI] [PubMed] [Google Scholar]
- Menon V, Levitin DJ. The rewards of music listening: response and physiological connectivity of the mesolimbic system. NeuroImage. 2005;28:175–184. doi: 10.1016/j.neuroimage.2005.05.053. [DOI] [PubMed] [Google Scholar]
- Meyer-Lindenberg A, Poline JB, Kohn PD, Holt JL, Egan MF, Weinberger DR, Berman KF. Evidence for abnormal cortical functional connectivity during working memory in schizophrenia. American Journal of Psychiatry. 2001;158:1809–1817. doi: 10.1176/appi.ajp.158.11.1809. [DOI] [PubMed] [Google Scholar]
- Meyer-Lindenberg AS, Olsen RK, Kohn PD, Brown T, Egan MF, Weinberger DR, Berman KF. Regionally specific disturbance of dorsolateral prefrontal-hippocampal functional connectivity in schizophrenia. Archives of General Psychiatry. 2005;62:379–386. doi: 10.1001/archpsyc.62.4.379. [DOI] [PubMed] [Google Scholar]
- Norman RM, Malla AK, Williamson PC, Morrison-Stewart SL, Helmes E, Cortese L. EEG coherence and syndromes in schizophrenia. British Journal of Psychiatry. 1997;170:411–415. doi: 10.1192/bjp.170.5.411. [DOI] [PubMed] [Google Scholar]
- Paulsen JS, Heaton RK, Sadek JR, Perry W, Delis DC, Braff D, Kuck J, Zisook S, Jeste DV. The nature of learning and memory impairments in schizophrenia. Journal of the International Neuropsychological Society. 1995;1:88–99. doi: 10.1017/s135561770000014x. [DOI] [PubMed] [Google Scholar]
- Peltier SJ, Kerssens C, Hamann SB, Sebel PS, Byas-Smith M, Hu X. Functional connectivity changes with concentration of sevoflurane anesthesia. NeuroReport. 2005;16:285–288. doi: 10.1097/00001756-200502280-00017. [DOI] [PubMed] [Google Scholar]
- Quigley M, Cordes D, Turski P, Moritz C, Haughton V, Seth R, Meyerand ME. Role of the corpus callosum in functional connectivity. American Journal of Neuroradiology. 2003;24:208–212. [PMC free article] [PubMed] [Google Scholar]
- Ragland JD, Gur RC, Raz J, Schroeder L, Kohler CG, Smith RJ, Alavi A, Gur RE. Effect of schizophrenia on frontotemporal activity during word encoding and recognition: a PET cerebral blood flow study. American Journal of Psychiatry. 2001;158:1114–1125. doi: 10.1176/appi.ajp.158.7.1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragland JD, Moelter ST, McGrath C, Hill SK, Gur RE, Bilker WB, Siegel SJ, Gur RC. Levels-of-processing effect on word recognition in schizophrenia. Biological Psychiatry. 2003;54:1154–1161. doi: 10.1016/s0006-3223(03)00235-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragland JD, Gur RC, Valdez J, Turetsky BI, Elliott M, Kohler C, Siegel S, Kanes S, Gur RE. Event-related fMRI of frontotemporal activity during word encoding and recognition in schizophrenia. American Journal of Psychiatry. 2004;161:1004–1015. doi: 10.1176/appi.ajp.161.6.1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragland JD, Gur RC, Valdez JN, Loughead J, Elliott M, Kohler C, Kanes S, Siegel SJ, Moelter ST, Gur RE. Levels-of-processing effect on frontotemporal function in schizophrenia during word encoding and recognition. American Journal of Psychiatry. 2005;162:1840–1848. doi: 10.1176/appi.ajp.162.10.1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle ME, Gusnard DA. Intrinsic brain activity sets the stage for expression of motivated behavior. Journal of Comparative Neurology. 2005;493:167–176. doi: 10.1002/cne.20752. [DOI] [PubMed] [Google Scholar]
- Ranganath C. Working memory for visual objects: complementary roles of inferior temporal, medial temporal, and prefrontal cortex. Neuroscience. 2006;139:277–289. doi: 10.1016/j.neuroscience.2005.06.092. [DOI] [PubMed] [Google Scholar]
- Rorden C, Brett M. Stereotaxic display of brain lesions. Behavioral Neurology. 2000;12:191–200. doi: 10.1155/2000/421719. [DOI] [PubMed] [Google Scholar]
- Shin YW, Kwon JS, Ha TH, Park HJ, Kim DJ, Hong SB, Moon WJ, Lee JM, Kim IY, Kim SI, Chung EC. Increased water diffusivity in the frontal and temporal cortices of schizophrenic patients. NeuroImage. 2006;30:1285–1291. doi: 10.1016/j.neuroimage.2005.11.017. [DOI] [PubMed] [Google Scholar]
- Snodgrass JG, Corwin J. Pragmatics of measuring recognition memory: applications to dementia and amnesia. Journal of Experimental Psychology. General. 1988;117:34–50. doi: 10.1037//0096-3445.117.1.34. [DOI] [PubMed] [Google Scholar]
- Spence SA, Liddle PF, Stefan MD, Hellewell JS, Sharma T, Friston KJ, Hirsch SR, Frith CD, Murray RM, Deakin JF, Grasby PM. Functional anatomy of verbal fluency in people with schizophrenia and those at genetic risk. Focal dysfunction and distributed disconnectivity reappraised. British Journal of Psychiatry. 2000;176:52–60. doi: 10.1192/bjp.176.1.52. [DOI] [PubMed] [Google Scholar]
- Sperling R, Chua E, Cocchiarella A, Rand-Giovannetti E, Poldrack R, Schacter DL, Albert M. Putting names to faces: successful encoding of associative memories activates the anterior hippocampal formation. NeuroImage. 2003;20:1400–1410. doi: 10.1016/S1053-8119(03)00391-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephan KE, Magnotta VA, White T, Arndt S, Flaum M, O'Leary DS, Andreasen NC. Effects of olanzapine on cerebellar functional connectivity in schizophrenia measured by fMRI during a simple motor task. Psychological Medicine. 2001;31:1065–1078. doi: 10.1017/s0033291701004330. [DOI] [PubMed] [Google Scholar]
- Szeszko PR, Ardekani BA, Ashtari M, Kumra S, Robinson DG, Sevy S, Gunduz-Bruce H, Malhotra AK, Kane JM, Bilder RM, Lim KO. White matter abnormalities in first-episode schizophrenia or schizoaffective disorder: a diffusion tensor imaging study. American Journal of Psychiatry. 2005;162:602–605. doi: 10.1176/appi.ajp.162.3.602. [DOI] [PubMed] [Google Scholar]
- Tan HY, Choo WC, Fones CS, Chee MW. fMRI study of maintenance and manipulation processes within working memory in first-episode schizophrenia. American Journal of Psychiatry. 2005;162:1849–1858. doi: 10.1176/appi.ajp.162.10.1849. [DOI] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. NeuroImage. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- Wagner AD, Maril A, Bjork RA, Schacter DL. Prefrontal contributions to executive control: fMRI evidence for functional distinctions within lateral prefrontal cortex. NeuroImage. 2001;14:1337–1347. doi: 10.1006/nimg.2001.0936. [DOI] [PubMed] [Google Scholar]
- Whalley HC, Simonotto E, Marshall I, Owens DG, Goddard NH, Johnstone EC, Lawrie SM. Functional disconnectivity in subjects at high genetic risk of schizophrenia. Brain. 2005;128:2097–2108. doi: 10.1093/brain/awh556. [DOI] [PubMed] [Google Scholar]
- Winterer G, Coppola R, Egan MF, Goldberg TE, Weinberger DR. Functional and effective frontotemporal connectivity and genetic risk for schizophrenia. Biological Psychiatry. 2003;54:1181–1192. doi: 10.1016/s0006-3223(03)00532-8. [DOI] [PubMed] [Google Scholar]
- Yurgelun-Todd DA, Waternaux CM, Cohen BM, Gruber SA, English CD, Renshaw PF. Functional magnetic resonance imaging of schizophrenic patients and comparison subjects during word production. American Journal of Psychiatry. 1996;153:200–205. doi: 10.1176/ajp.153.2.200. [DOI] [PubMed] [Google Scholar]


