Abstract
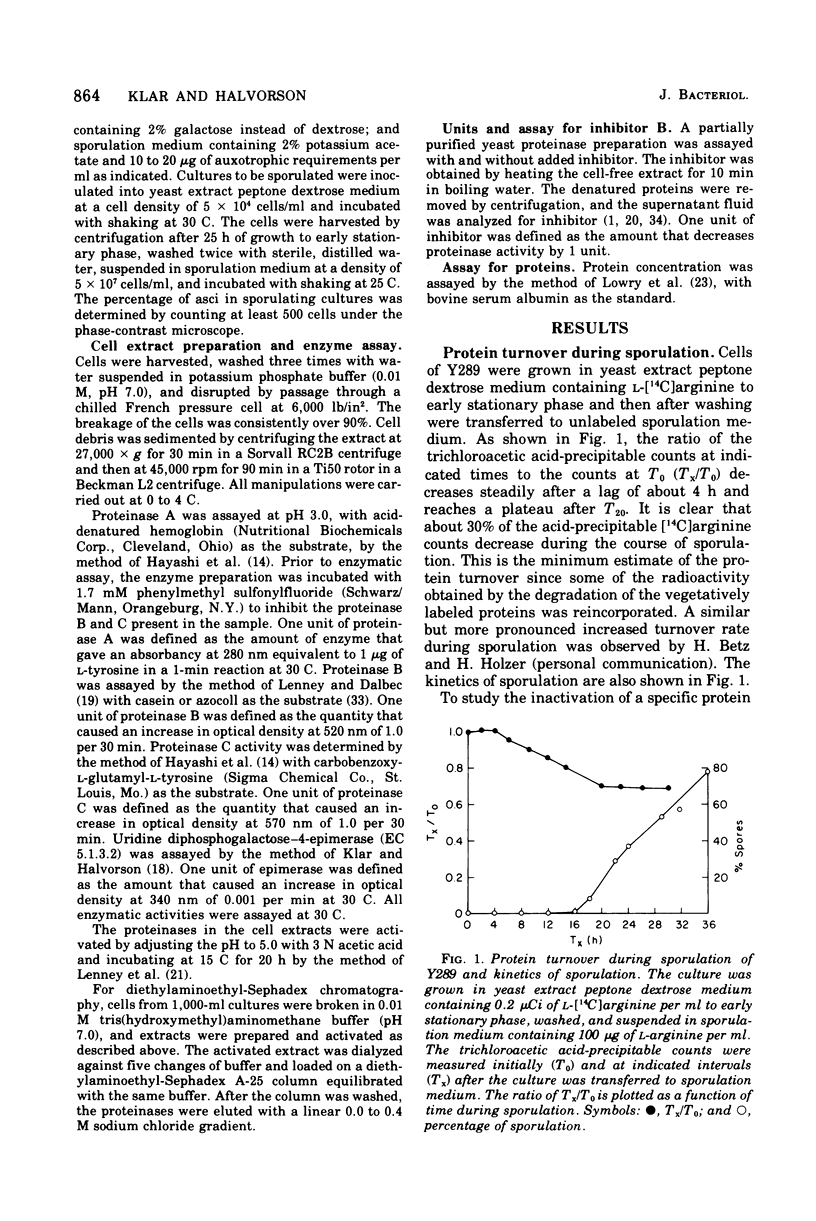
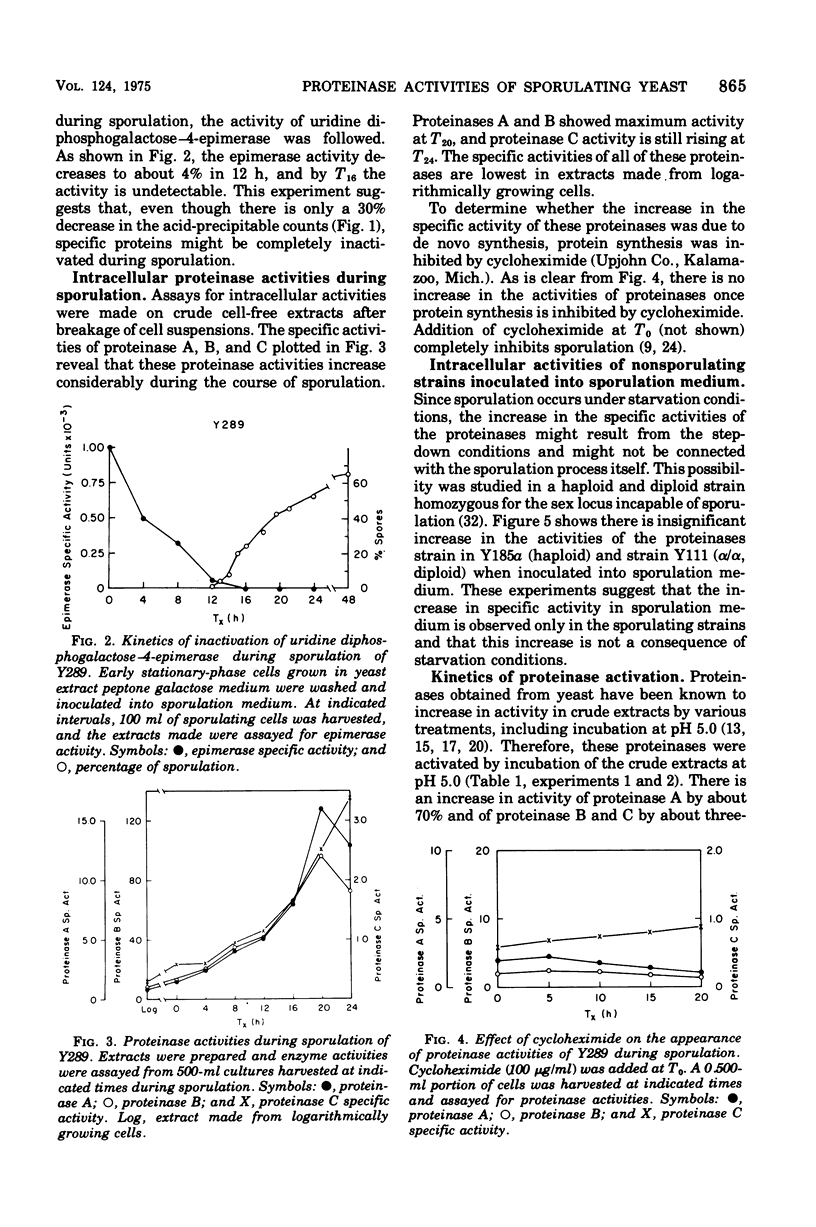
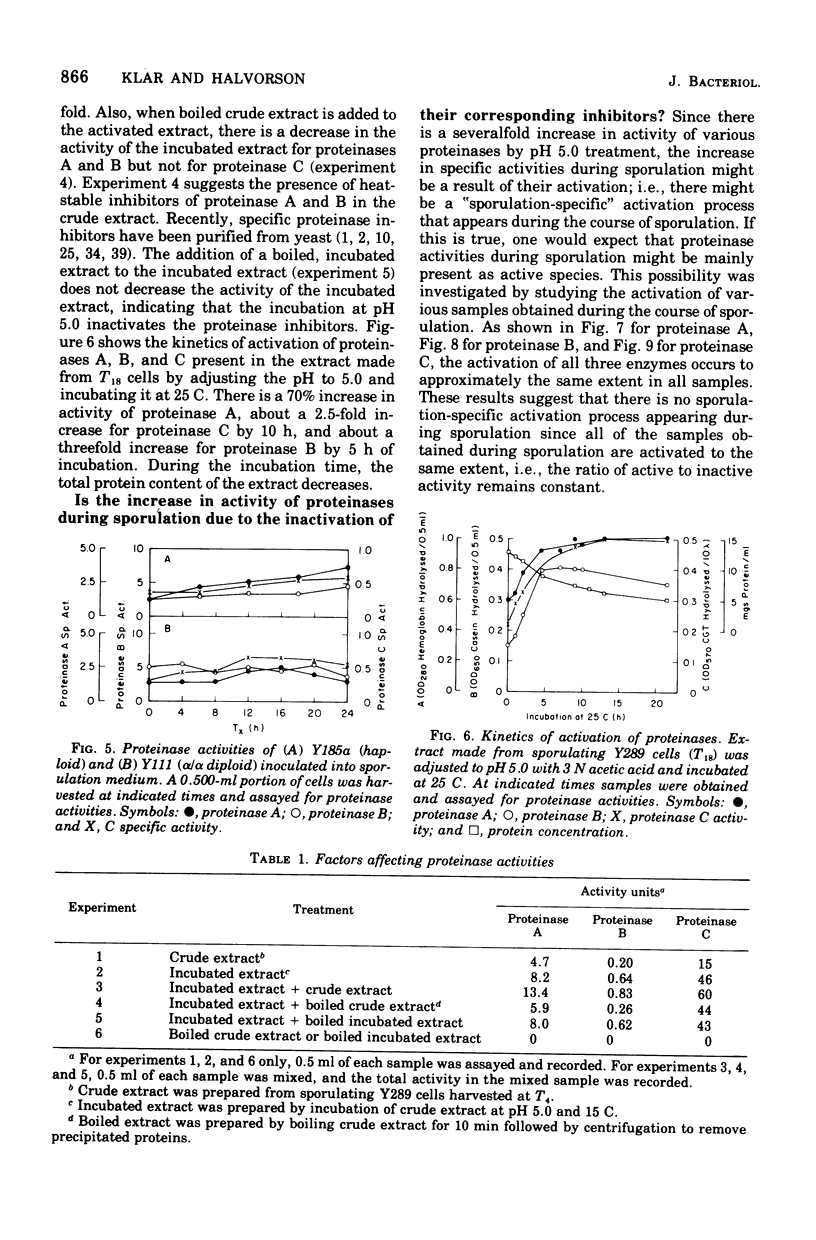
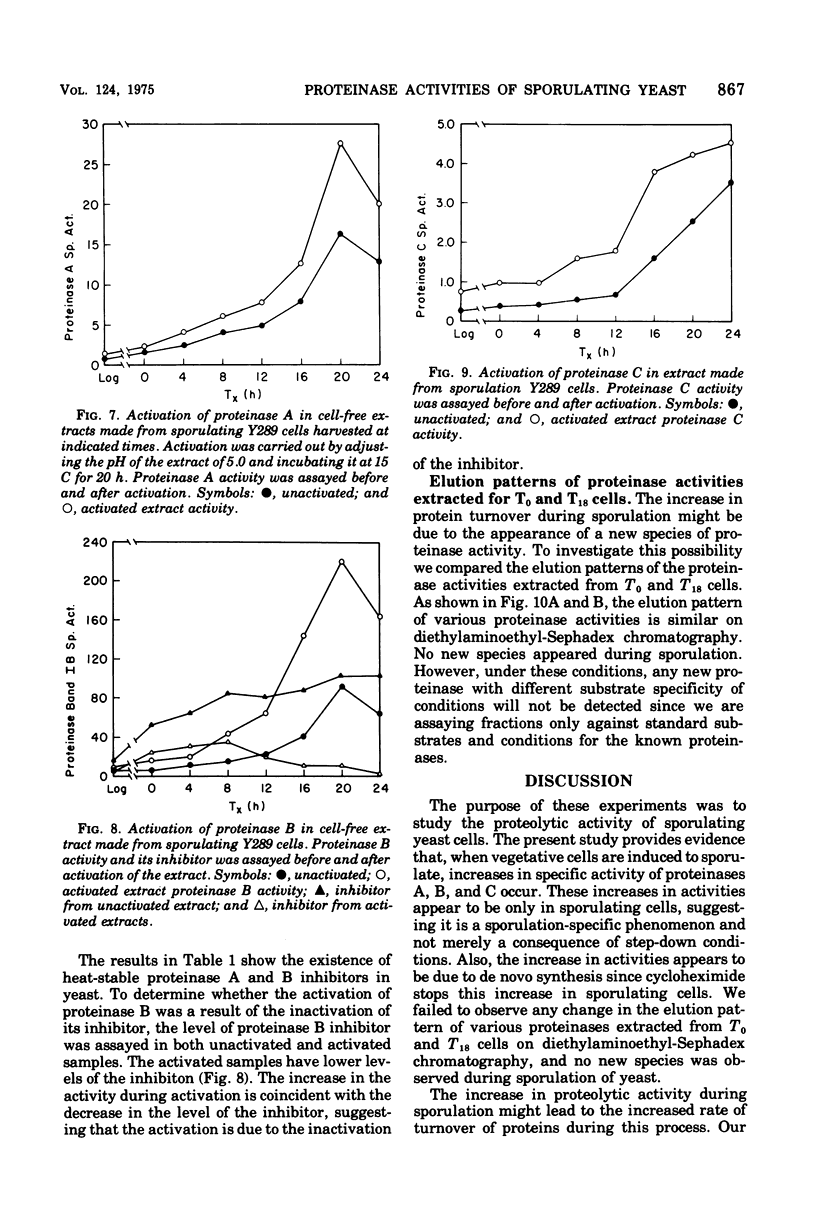
Sporulation in Saccharomyces cerevisiae occurs in the absence of a exogenous nitrogen source. Thus, the internal amino acid pool and the supply of nitrogen compounds from protein and nucleic acid turnover must be sufficient for new protein synthesis. Since sporulation involves an increased rate of protein turnover, an investigation was conducted of the changes in the specific activity of various proteinases. A minimum of 30% of the vegetative proteins was turned over during the course of sporulation. There was a 10- to 25-fold increase in specific activity of various proteinases, with a maximum activity around 20 h after transfer into the sporulation medium. The increase in activities was due to de novo synthesis since inhibition of protein synthesis by cycloheximide blocks both an increase in proteinase activities and sporulation. There was no increase observed in proteinase activities of nonsporogenic cultures (a and alpha/alpha strains) inoculated into the sporulation medium, suggesting that the increase in proteinase activities is "sporulation specific" and not a consequence of step-down conditions. The elution patterns through diethylaminoethyl-Sephadex chromatography of various proteinases extracted from T0 and T18 cells were similar, and no new species was observed.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Betz H., Hinze H., Holzer H. Isolation and properties of two inhibitors of proteinase B from yeast. J Biol Chem. 1974 Jul 25;249(14):4515–4521. [PubMed] [Google Scholar]
- Bukhari A. I., Zipser D. Mutants of Escherichia coli with a defect in the degradation of nonsense fragments. Nat New Biol. 1973 Jun 20;243(129):238–241. doi: 10.1038/newbio243238a0. [DOI] [PubMed] [Google Scholar]
- Cabib E., Ulane R., Bowers B. Yeast chitin synthetase. Separation of the zymogen from its activating factor and recovery of the latter in the vacuole fraction. J Biol Chem. 1973 Feb 25;248(4):1451–1458. [PubMed] [Google Scholar]
- Chen A. W., Miller J. J. Proteolytic activity of intact yeast cells during sporulation. Can J Microbiol. 1968 Sep;14(9):957–963. doi: 10.1139/m68-159. [DOI] [PubMed] [Google Scholar]
- Esposito M. S., Esposito R. E., Arnaud M., Halvorson H. O. Acetate utilization and macromolecular synthesis during sporulation of yeast. J Bacteriol. 1969 Oct;100(1):180–186. doi: 10.1128/jb.100.1.180-186.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson A. R., Katsunuma T., Betz H., Holzer H. Purification and properties of an inhibitor of the tryptophan-synthase-inactivating enzymes in yeast. Eur J Biochem. 1973 Feb 1;32(3):444–450. doi: 10.1111/j.1432-1033.1973.tb02626.x. [DOI] [PubMed] [Google Scholar]
- Goldberg A. L. Effects of protease inhibitors on protein breakdown and enzyme induction in starving Escherichia coli. Nat New Biol. 1971 Nov 10;234(45):51–52. doi: 10.1038/newbio234051a0. [DOI] [PubMed] [Google Scholar]
- HALVORSON H. Intracellular protein and nucleic acid turnover in resting yeast cells. Biochim Biophys Acta. 1958 Feb;27(2):255–266. doi: 10.1016/0006-3002(58)90332-9. [DOI] [PubMed] [Google Scholar]
- Holzer H., Katsunuma T., Schött E. G., Ferguson A. R., Hasilki A., Betz H. Studies on a tryptophan synthase inactivating system from yeast. Adv Enzyme Regul. 1973;11:53–60. doi: 10.1016/0065-2571(73)90008-3. [DOI] [PubMed] [Google Scholar]
- Hopper A. K., Magee P. T., Welch S. K., Friedman M., Hall B. D. Macromolecule synthesis and breakdown in relation to sporulation and meiosis in yeast. J Bacteriol. 1974 Aug;119(2):619–628. doi: 10.1128/jb.119.2.619-628.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juni E., Heym G. A. Properties of yeast pyruvate decarboxylase and their modification by proteolytic enzymes. II. Selective alteration by yeast proteases. Arch Biochem Biophys. 1968 Sep 20;127(1):89–100. doi: 10.1016/0003-9861(68)90205-1. [DOI] [PubMed] [Google Scholar]
- Lenney J. F., Dalbec J. M. Purification and properties of two proteinases from Saccharomyces cerevisiae. Arch Biochem Biophys. 1967 Apr;120(1):42–48. doi: 10.1016/0003-9861(67)90595-4. [DOI] [PubMed] [Google Scholar]
- Lenney J. F., Dalbec J. M. Yeast proteinase B: identification of the inactive form as an enzyme-inhibitor complex. Arch Biochem Biophys. 1969 Jan;129(1):407–409. doi: 10.1016/0003-9861(69)90194-5. [DOI] [PubMed] [Google Scholar]
- Lenney J. F., Matile P., Wiemken A., Schellenberg M., Meyer J. Activities and cellular localization of yeast proteases and their inhibitors. Biochem Biophys Res Commun. 1974 Oct 23;60(4):1378–1383. doi: 10.1016/0006-291x(74)90350-7. [DOI] [PubMed] [Google Scholar]
- Lieberman M. M., Markovitz A. Depression of guanosine diphosphate-mannose pyrophosphorylase by mutations in two different regulator genes involved in capsular polysaccharide synthesis in Escherichia coli K-12. J Bacteriol. 1970 Mar;101(3):965–972. doi: 10.1128/jb.101.3.965-972.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MANDELSTAM J. The intracellular turnover of protein and nucleic acids and its role in biochemical differentiation. Bacteriol Rev. 1960 Sep;24(3):289–308. doi: 10.1128/br.24.3.289-308.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MUNDKUR B. Electron microscopical studies of frozendried yeast. III. Formation of the tetrad in Saccharomyces. Exp Cell Res. 1961 Oct;25:24–40. doi: 10.1016/0014-4827(61)90304-4. [DOI] [PubMed] [Google Scholar]
- Magee P. T., Hopper A. K. Protein synthesis in relation to sporulation and meiosis in yeast. J Bacteriol. 1974 Sep;119(3):952–960. doi: 10.1128/jb.119.3.952-960.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matern H., Hoffmann M., Holzer H. Isolation and characterization of the carboxypeptidase Y inhibitor from yeast. Proc Natl Acad Sci U S A. 1974 Dec;71(12):4874–4878. doi: 10.1073/pnas.71.12.4874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matile P., Wiemken A. The vacuole as the lysosome of the yeast cell. Arch Mikrobiol. 1967 Feb 20;56(2):148–155. doi: 10.1007/BF00408765. [DOI] [PubMed] [Google Scholar]
- Roman H, Phillips M M, Sands S M. Studies of Polyploid Saccharomyces. I. Tetraploid Segregation. Genetics. 1955 Jul;40(4):546–561. doi: 10.1093/genetics/40.4.546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SVIHLA G., DAINKO J. L., SCHLENK F. ULTRAVIOLET MICROSCOPY OF THE VACUOLE OF SACCHAROMYCES CEREVISIAE DURING SPORULATION. J Bacteriol. 1964 Aug;88:449–456. doi: 10.1128/jb.88.2.449-456.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saheki T., Holzer H. Comparisons of the tryptophan synthase inactivating enzymes with proteinases from yeast. Eur J Biochem. 1974 Mar 1;42(2):621–626. doi: 10.1111/j.1432-1033.1974.tb03377.x. [DOI] [PubMed] [Google Scholar]
- Saheki T., Matsuda Y., Holzer H. Urification and characterization of macromolecular inhibitors of proteinase A from yeast. Eur J Biochem. 1974 Sep 1;47(2):325–332. doi: 10.1111/j.1432-1033.1974.tb03697.x. [DOI] [PubMed] [Google Scholar]
- Setlow P. Identification of several unique low molecular weight basic proteins in dormant spores of Bacillus megaterium and their degradation during spore germination. Biochem Biophys Res Commun. 1974 Dec 23;61(4):1110–1117. doi: 10.1016/s0006-291x(74)80398-0. [DOI] [PubMed] [Google Scholar]
- Shineberg B., Zipser D. The ion gene and degradation of beta-galactosidase nonsense fragments. J Bacteriol. 1973 Dec;116(3):1469–1471. doi: 10.1128/jb.116.3.1469-1471.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takano I., Oshima Y. Mutational nature of an allele-specific conversion of the mating type by the homothallic gene HO alpha in Saccharomyces. Genetics. 1970 Jul;65(3):421–427. doi: 10.1093/genetics/65.3.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulane R. E., Cabib E. The activating system of chitin synthetase from Saccharomyces cerevisiae. Purification and properties of an inhibitor of the activating factor. J Biol Chem. 1974 Jun 10;249(11):3418–3422. [PubMed] [Google Scholar]


