Abstract
Separation of chemical and physical carcinogenesis into the stages of initiation (mutation) and promotion (selection) established that incipient neoplastic cells could persist in the organism indefinitely without expression. Spontaneous mutations associated with cancer also lie dormant in untreated normal tissue. Without selection, there is no tumor development. Experiments in cell culture showed that confluent normal fibroblasts suppress growth of contacting transformed fibroblasts, and that normal keratinocytes similarly suppress tumor formation by adjacent papilloma cells. With cells that are generally more susceptible to transformation, however, prolonged contact inhibition progressively selects mutants that favor neoplastic growth. Selection of individual mutant cells allows them to become a significant fraction of the population and creates an enlarged target for additional genetic hits. Crucially, this enrichment step, not the initial mutation step, is the numerically limiting factor in tumor development. Unexpectedly, variants that are resistant to spontaneous transformation are selected in vitro by growing cells for many low density passages at maximal exponential rate. Confluent cultures of resistant variants suppress the growth and normalize the morphology of contacting transformed cells. Varying the conditions for selection shows that tumorigenic transformation is preceded by intermediate steps of progressively higher saturation density that are increasingly permissive for the expression of the more neoplastic cells in the population. There is also evidence of increasing permissiveness with age of normal tissues in vivo for solitary cancer cells transplanted in their midst. Spontaneous transformation in culture can be used to identify dietary components that are required for promotion and may therefore be applicable in prevention of human cancer.
Keywords: tumor initiation, promotion, progression, microenvironment
Most cancer researchers would agree that “most if not all cancer cells contain genetic damage that appears to lie at the heart of tumorigenesis” and “there is little doubt that the genetic paradigm dominates research on cancer” (1). However, there is good reason to believe that there is another fundamental aspect of cancer that is as important as genetic change in determining the actual growth of a tumor, but has received much less attention in recent years. That aspect concerns the selective conditions that underlie the development of tumors from cells that carry the requisite oncogenic mutations, but otherwise remain dormant.
Cancer-Related Mutations Without Neoplastic Expression
These conditions first came to light in classical studies on the mechanism of carcinogenesis in the skin of mice treated with polycyclic aromatic hydrocarbons (PAHs). Repeated painting of the skin with a carcinogenic PAH for more than 8 weeks is required for papillomas to appear subsequently, and an even longer period is required for carcinomas to ultimately develop (2, 3). However, a single application of the carcinogen followed by repeated application of an agent that is itself not carcinogenic in most mouse strains will produce tumors (4). The single application of carcinogen is called initiation, and the repeated application of the noncarcinogenic agent is known as promotion. The promoting agent can be started shortly after initiation with a high yield of tumors, or 1 year later with a general decrease in tumor yield, but that leaves unmistakable evidence of the persistence of the initiated effect (5). The early onset of initiation and its long persistence are characteristic of mutations and are generally interpreted as such. In contrast, interrupting the promotional treatment for an extended period before it is completed returns the skin almost to its original postinitiation state (6), although more quickly responsive to reestablishment of promotion (7). Without promotion there is no tumor formation. The physiological changes of promotion include terminal differentiation of most of the epidermal keratinocytes, releasing the initiated cells, which escape differentiation, to multiply and form papillomas (8, 9).
The differential effect of promotion on normal and initiated epidermal cells implies that, in the absence of promotion, the normal basal keratinocytes suppress the neoplastic development of the initiated cells. That implication was borne out in cell culture by the partial inhibition of colony formation by initiated cells when seeded in an excess of keratinocytes in sufficient calcium (10), and of papilloma formation in skin grafts with a similar excess of keratinocytes (11). There was no inhibition of epidermal carcinoma cells in two-dimensional cultures, but early stage carcinoma cells were inhibited in three-dimensional organotypic cultures of skin (12). A role of selection is also seen in experimental carcinogenesis of the liver in rats in which the promoting agent is toxic to normal hepatocytes, thereby allowing the formation of nodules by those hepatocytes that had been rendered resistant to the promoter by prior initiation with carcinogen (13, 14). That conclusion is reinforced by the normalization of transplanted aneuploid rat hepatocarcinoma cells distributed as solitary cells among the liver cords (15). The stages of initiation and promotion have been identified in the development of mammary adenocarcinoma and many other neoplasms of animals and humans (6). The process may therefore be considered as a general characteristic of carcinogenesis.
There is evidence that spontaneously initiated cells occur in the normal epidermis of the SENCAR line of mice, which has been selected for sensitivity to skin carcinogenesis (16). Long-term repetitive treatment of uninitiated skin of this line with the strong promoter 12-O-tetradecanoylphorbol 13-acetate (TPA) produced papillomas and even carcinomas in some mice; most of the tumors harbored a mutation in a specific codon of the Ha-ras oncogene (17). It appears that the promoters drove the expansion of clones bearing a spontaneous mutation of the Ha-ras gene to papilloma formation, which provided the opportunity for further mutations and progression to carcinomas.
Similarly, the repetitive painting of the Oslo strain of uninitiated hairless mice with TPA led to tumors in about one-fourth of them in 4 months (18). Because TPA is nonmutagenic in mammalian cells (19), and does not require metabolic activation for its promoting effect in mouse skin (20), it was surmised that the tumors arose by promotion of spontaneous mutations in the epidermis (3). These results take on great significance in view of the evidence that most human cancers arise from selection of cells with spontaneous tumor-related mutations (21). The extended periods of time required for the appearance of tumors by repeated promoter treatment of uninitiated skin suggests that there are intermediate stages that precede tumor formation. That would be consistent with genetic reconstruction of a hereditary form of human colorectal cancer associated with mutation of mismatch repair genes. The reconstruction shows that 90% of the mutations that ultimately contribute to tumor development involve clone sizes below a threshold of clinical detection (22). Therefore, this occult prologue to visible neoplasia is much longer than generally appreciated.
The initiation phase of two-stage chemical carcinogenesis in mouse skin can be replaced by infecting the epidermis with the activated v-ras gene of the mouse sarcoma virus (23). The mere establishment of the v-ras gene was insufficient to produce any tumors, but when establishment was followed by repeated painting with TPA, papillomas appeared in almost all of the mice. The latent period for papilloma appearance in these mice was considerably shorter than for those initiated with a carcinogenic PAH. This shortening of time to papilloma appearance was thought to reflect the higher levels of expression of the mutated ras genes driven by viral gene promotion than are observed in chemically induced tumors associated with activated ras genes. The TPA treatment was equally effective begun immediately after the virus was introduced or 4 months later, showing that the activated ras alone could persist for extended periods without any sign of neoplastic expression.
Mutations in chromosomal regions associated with human mammary cancer were found in histologically normal breasts of one-half of the women examined (24). The presence of such lesions does not necessarily lead to cancer even many years later (25). The finding of about 100 mutations and large genomic rearrangements in every cell of the small intestine and other organs of young normal mice, with more than a 3-fold increase of their number in old mice (26, 27), is a guarantee that cancer-related mutations are common, but neoplastically dormant in normal tissues. Skin fibroblasts from people genetically predisposed to colorectal cancer became tumorigenic when grown in the presence of TPA (28), consistent with the initiated state of the cells. Numerous examples have been given of specific mutations of the ras oncogene in chemically induced tumors in which the mutation had arisen spontaneously and was selected rather than induced by the treatment (29).
Studies on Neoplastic Suppression and Selection in Cell Culture
The foregoing results established that cancer-related mutations exist without neoplastic expression in normal tissues unless physiological changes are brought about that elicit tumors. However, detailed analysis of the dynamics of neoplastic suppression and selection were limited in an organism. Development of an assay for transformation of cells in culture by Rous sarcoma virus (RSV) (30) opened up the quantitative study of transformation, which led to the genetic era of cancer investigation (1). An early finding in the study of RSV-induced transformation of chicken fibroblasts was that the addition of newly transformed cells to a confluent, contact-inhibited culture of chicken fibroblasts would result in the morphologic normalization of the RSV-transformed cells and inhibition of their proliferation (31). Transformed focus formation of RSV-infected cells was suppressed by substituting fetal bovine serum (FBS) for calf serum (CS) in the medium, or raising the concentration of the latter, but suppression was effective only when the infected cells were surrounded by normal cells (32). Mammalian fibroblasts transformed by polyoma virus were also inhibited by contact with a quiescent layer of mouse fibroblasts (33). That was also true for mammalian fibroblasts irradiated with UV, but continuous treatment with TPA brought on focus formation (34, 35). These and other studies with fibroblasts showed that transformed fibroblasts could be inhibited by contact with quiescent normal fibroblasts, but the single-step expression of transformation did not reveal intermediary aspects of the process. These studies did not explore whether the suppressive capacity of cells could be altered. These deficiencies were eventually overcome in studies of spontaneous transformation in culture.
Spontaneous transformation was first encountered in untreated controls during experiments on chemical carcinogenesis of mouse fibroblasts in culture (36). Spontaneously transformed cells capable of producing sarcomas in mice began to appear after 4 months in culture and continued occurring over a 4-year period of investigation (37). It was also observed in epithelial cells from liver, kidney, epidermis, and parotid gland (38). A key observation was that mouse fibroblasts repeatedly seeded and maintained at high density to maximize contacts between cells became tumorigenic in syngeneic mice, whereas those subcultured at low density to minimize contact did not (39). The onset of tumorigenicity in the cultured cells was accompanied by an increase in their saturation density. It was implied that the high-density cultures selected for cells with the capacity to overcome contact inhibition and thereby drive tumorigenesis. In retrospect, this finding seems to present a paradox because cell–cell contact interactions in different situations either suppress or select the neoplastic phenotype.
A related experiment was done with a diploid line of rat liver epithelium, which was either maintained at confluence for extended periods of time at each passage to maximize selection or subcultured more frequently to limit selection (40). Some cultures in both groups were also treated with a mutagenic carcinogen. The cells underwent spontaneous transformation much earlier under selective than nonselective conditions. Transformation was accelerated somewhat under nonselective conditions by treatment with the mutagenic carcinogen, but it did not approach the early onset of spontaneous transformation under selective conditions. It is apparent that selection was the dominant driving force in spontaneous transformation.
Neither of the above studies (39, 40) examined conditions for spontaneous transformation other than cell–cell contact, nor did they inquire about intermediate steps in that transformation or shed light on the dual consequences of cell–cell interactions noted above. An opportunity to approach these problems became available with the NIH 3T3 established line of cells that had originated from mouse embryo fibroblasts (41). Spontaneous transformation was commonly encountered in this line in the form of foci of morphologically altered cells in confluent cultures (42–44). The NIH 3T3 cells were of special interest because they were uniquely sensitive to transformation by transfection with DNA from some human cancers (45). The sensitivity of the NIH 3T3 cells to transformation by the mutated ras gene from a human bladder cancer was widely interpreted to mean that the cancer resulted from mutation in that single gene and was the spark that set off intense search for causal mutations in human cancer. However, the common occurrence of spontaneous transformation in the target NIH 3T3 cells indicates they had already progressed to a preneoplastic state that required only the selective promotion of confluence for neoplastic expression. That view was reinforced by the appearance of large numbers of transformed foci when the cells were grown in a medium specially formulated to maximize clonal growth of mouse 3T3 cells (46). The incidence of spontaneous transformation was then so high in the conventional 10% concentration of CS that it had to be reduced to 2% CS to eliminate such foci in a single round of confluence (1° assay). By thus raising the bar to transformation, it was possible to study various conditions that bring on transformation.
Alternative Conditions That Reduce or Promote Spontaneous Transformation
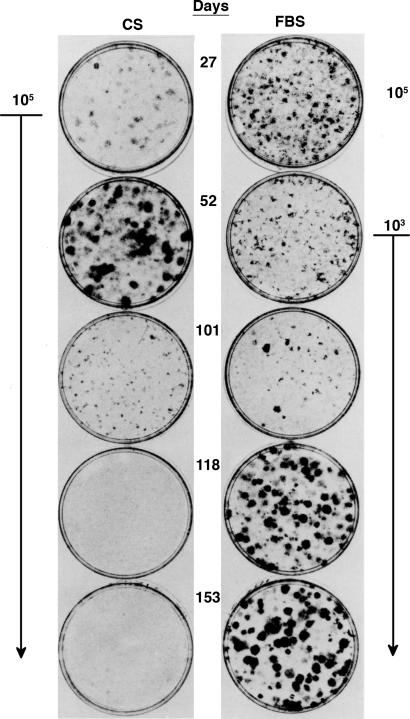
A 5-fold reduction of CS concentration slowed the exponential growth rate of the NIH 3T3 cells at low density only slightly, but it reduced their saturation density in direct proportion to the reduced CS concentration (47). A few low-density passages (LDPs) in the low CS (2%) resulted in the appearance of transformed foci when the cells were tested at confluence (48). In contrast, LDP in conventional high CS (10%) produced no increase in focus-forming capacity; indeed, the cultures exhibited a decrease in focus formation (Fig. 1). Parallel LDPs in high FBS, which made only a minimal decrease in exponential growth rate (47), eventually resulted in a large increase in transformed focus formation (Fig. 1). Continued LDP beyond the formation of small light foci produced an increase in the size and density of the individual foci to a degree concordant with tumor-producing capacity (49). Because no foci approaching such size and density had appeared in previous assays with fewer LDPs carried in high FBS, mutations must have been occurring continually to traverse the multiple steps required for such development. These mutations required selection under the slightly reduced growth rates in FBS to increase the number of target cells for the additional mutations necessary for progression to full tumorigenic capacity. Mutations must have been occurring at an even higher frequency per unit time under the maximal growth rate in high CS, but the lack of selection did not allow the increase in the collective target size required to accumulate the further mutations needed for progression to tumorigenic capacity. In fact, there was a gradual selection for cells resistant to spontaneous transformation. Clearly, a high rate of mutation without selection is insufficient to drive neoplastic transformation.
Fig. 1.
Effects of frequent LDP in CS or FBS on the capacity of cells for focus formation. NIH 3T3 cells were thawed and subjected to LDP three times a week either in 10% CS at a maximal growth rate or in 10% FBS with a 10–15% reduction of maximal growth rate. After the indicated total number of days in LDP, aliquots of cells were assayed for focus formation in a 2° assay at confluence in 2% CS. Note that all of the assays of the cells that had been in LDP with 10% CS were seeded with 105 cells, as shown on the left. In contrast, only the first assay at 27 days of the cells that had been in LDP with 10% FBS used 105 cells, whereas all of the subsequent assays were diluted to 103 cells, as shown on the right, and cocultured with 105 non-focus-forming cells (50).
Selection for Resistance to Spontaneous Transformation and for Capacity to Suppress Growth of Contiguous Cells
Continued LDP at maximal growth rate in high CS over an extended period resulted in more than a 2-fold decrease in saturation density of the NIH 3T3 cells (50). Those cells became refractory to spontaneous transformation even in three and four serial rounds of confluence (3° and 4° assays) (51, 52). In addition, they suppressed focus formation when they were cocultured in large numbers with small numbers of fully transformed cells, for which they formed a confluent background. The suppression began only after the suppressing cells reached confluence and small foci derived from the added transformed cells began to appear, but those beginning foci disappeared in the following few days (Fig. 2A). In contrast, early foci formed in cocultures of the fully transformed cells on a confluent background of transformable cells continued to expand to form enormous foci. It would appear that the transformation-resistant cells perform the same type of cell–cell suppression of fully transformed cells as did quiescent confluent cultures of normal freshly explanted chick embryo fibroblasts in contact with RSV-transformed fibroblasts (31). In contrast, those NIH 3T3 cells that were themselves capable of undergoing spontaneous transformation at confluence allowed continuous expansion of colonies from fully transformed cells distributed in their midst.
Fig. 2.
Suppression vs. expression of transformed foci depending on the resistance or susceptibility to spontaneous transformation of the confluent, contact-inhibited, background cells. (A) Suppression vs. expression of focus formation by transformed cells cocultured with an excess of resistant (173c) or susceptible (27M/28M) cells. One thousand transformed cells of the 28H subline were cocultured with 105 cells of the 173c subline that had become resistant to spontaneous transformation by virtue of 294 LDP in 10% CS; or with 105 cells of the 27M or 28M subline that were still sensitive to transformation. The cultures were fixed and stained at 8, 10, and 14 days (51). (B) Partial suppression of focus formation by coculture with an excess of partly resistant cells. A clone of NIH 3T3 cells that repeatedly produced 1–2 small, dense foci in 1° assay of 105 cells was put through a 2° assay using 105 cells and a 3° assay using 500 cells cocultured with 105 cells that had become partially resistant by virtue of 108 LDP in 10% CS (53).
To study an intermediate level of suppression, a clone of NIH 3T3 cells was chosen that produced large dense foci in two rounds of confluence (53). Diluting these cells from the 2° assay with an excess of cells that were moderately refractory to spontaneous transformation resulted in a decrease in the size and density of foci from the former in a 3° assay (Fig. 2B). The number of foci, however, remained as expected from the cell dilution. The results indicated there are many degrees of susceptibility to transformation that are negatively correlated with the capacity to suppress focus formation by fully transformed cells.
Dynamics of Spontaneous Neoplastic Transformation
It is evident from the increasing size and density of foci generated by successive LDPs (Fig. 1) and from serial rounds of confluence (54) that there are intermediate degrees of focus formation. But such foci might be only the tip of the iceberg in view of the evidence that most of the mutations in human cancer occur in an occult stage before a tumor becomes clinically evident (22). Increase in saturation density would serve as an accurate quantitative indicator of increased growth capacity of a cell population before focus formation, and the increase resembles the hyperplasia that is a preneoplastic change in experimental and human cancer (55–58). Evidence for progressive preneoplastic states came from a multilineage experiment with NIH 3T3 cells in which a primary round of confluence (1° assay) was done in low or high concentrations of CS to vary saturation densities, and therefore vary the total number of cell divisions in which mutations occur (59). The cells were relatively refractory to spontaneous transformation in low CS, which would allow expression of preneoplastic stages of progression. The period of incubation in the 1° assay was also varied to account for the kinetics of change. After the 1° assay each of the lineages underwent serial 2°, 3°, and 4° assays, all in the same low CS to maintain constant conditions in which saturation densities were measured in each of the four groups, as shown in Table 1. There were graduated increases of saturation density in the serial 2°, 3°, and 4° assays in each category, but the increases were much greater in those started from 1° assays in high CS than in low CS, in keeping with the much larger number of cell divisions and progressive mutations occurring in the 1° assay of the former under the constraint of confluence. In addition, the saturation densities of the serial assays increased significantly with length of incubation from 2 to 3 weeks in the 1° assays, although the total number of cells in the 1° assays showed only a small fractional increase in the extra week. That relationship indicates that the mutations accumulated in that fraction of cells that could continue multiplying after the majority population had become quiescent.
Table 1.
Progressive increase in saturation densities in successive serial assays of NIH 3T3 cells, beginning with a 1° assay in 2% and 10% CS, followed by 2°, 3°, and 4° assays, all in 2% CS
| Saturation density of cells × 10−5 cells |
||
|---|---|---|
| 2-wk 1° assay | 3-wk 1° assay | |
| Low CS (2%), 1° | ||
| 1° | 4.3 ± 0.1 | 4.7 ± 0.1 |
| 2° | 4.6 ± 0.2 | 5.3 ± 0.1 |
| 3° | 7.3 ± 0.3 | 8.8 ± 0.3 |
| 4° | 8.7 ± 1.3 | 13.0 ± 0.8 |
| High CS (10%) 1° | ||
| 1° | 21.4 ± 0.5 | 21.9 ± 1.3 |
| 2° | 7.0 ± 0.3 | 15.3 ± 9.9 |
| 3° | 11.2 ± 5.4 | 64.8 ± 8.5 |
| 4° | 40.5 ± 17.9 | ND |
Multiple 1° assays were prepared in 2% and 10% CS. At either 2 or 3 wk, groups of cultures were trypsinized and counted for saturation densities, and all were subcultured as separate lineages in 2% CS only and for 2 wk only in serial 2°, 3°, and 4° assays. They show that progressive increases in saturation density vary with the serum concentration, saturation density, and incubation period of the 1° assay. (The complete data on saturation densities of each lineage are in ref. 94.) Density refers to number of cells per 20-cm2 culture dish. Results are mean ± SD. ND, not determined.
The major point of the experiment, however, was that the lineages started in low CS showed no transformed foci in the 2° and 3° serial assays, and only very small foci in the 4° assays (59). Where there were progressive increases in saturation density, there were, in effect, extended periods of hyperplasia at high density with no or very little sign of clonal neoplasia in the form of transformed foci. A similar result appeared in the 2° assay of cells started in high CS for 2 weeks, but careful microscopic examination of the 2° assay cultures revealed uniform numbers from lineage to lineage of very small, light foci, indicative of selection of many variants capable of limited overgrowth. All these cultures with limited increases in saturation density but no clearly neoplastic foci resemble the preclinical hyperplasia recorded in experimental and human carcinogenesis (55–58).
The unique category started in high CS for 3 weeks had lineages that already showed transformed foci in the 2° assay (59). These would represent a telescoping of progressive transformation in the 2° assays which masks the intermediary steps of hyperplasia, just as they might not be readily apparent in histological examination of clinical tumors. Finally, the variance in saturation densities was very low where there were no large foci (Table 1). Where the variances were high, they were associated with the appearance of cultures with many dense foci, which drove large increases in saturation density. The high variances are indicative of the rarity of cells that had accumulated enough mutations to progress to the type of dense foci identified with tumorigenic capacity (49).
Correlating the in Vivo and in Vitro Evidence for Suppression and Selection of Neoplastic Development
The process of initiation and promotion in chemical carcinogenesis established unequivocally that cells with neoplastic potential could persist indefinitely within the tissue of origin without forming recognizably neoplastic lesions unless promotional treatment is brought to bear (4, 5). With the advent of the molecular era of cancer investigation, many other types of evidence have reinforced this conclusion. It was of particular significance that Peter Brookes, coauthor of the first paper that reported covalent bonding of PAHs to DNA in the order of their carcinogenicity (60), editorialized that many of the ras mutations found in experimental carcinogenesis are not induced by the carcinogenic treatment but result from selection of spontaneous mutations (29). Selection has been inferred as the driving force in human cancer of the colon (61), skin (62, 63), lung (64, 65), and other tissues (21).
Although it was long known that the neoplastic phenotype of transformed fibroblasts could be suppressed in cell culture by contact with an excess of normal fibroblasts (31, 33), it was not until parallel observations were made with papilloma cells and normal epidermal cells (10, 11) that the role of normal cells in suppressing tumorigenic proliferation of initiated cells became apparent. A particularly significant observation was that a line of epidermal cells exposed to carcinogen that behaved like initiated cells in vitro but formed normal epidermis in skin grafts had lost their capacity to suppress tumor formation by papilloma cells in mixed grafts (11). This observation implies that the cells surrounding an incipient papilloma cell in initiated skin may have become more permissive for tumor development during promotion. Similarly, a line of immortalized but nontumorigenic human keratinocytes lost the capacity to suppress the growth of early stage carcinoma cells in three-dimensional organotypic cultures of skin (66). The effect is simulated in the NIH 3T3 cultures during serial rounds of confluence, which raise the saturation density of the population and its permissiveness for transformed focus formation (Table 1). It supports the idea of regional neoplasia in which large areas of a tissue exposed to carcinogen are involved in the neoplastic process although the tumors arise locally (67). The process is strikingly exemplified after repeated paintings of rabbit ears with carcinogen, whereupon papillomas appear and disappear, with new ones continuing to appear for years after the paintings have ceased (68).
Another source of decreased suppressive capacity of phenotypically normal cells is aging of the organism, as seen in the increased permissiveness of the rat liver with age for multiplication of transplanted rat hepatocarcinoma cells (15). This permissiveness may be related to the marked increase with human age in the incidence of solid epithelial cancer. The number of mutations per proliferating cell increases more than 3-fold with age (27), as does heterogeneity of cell cycle time (69) and of gene expression (70), all of which are consistent with a reduction in the regulatory capacity of the entire population of the cells.
The contact interactions among cells that underlie neoplastic suppression and selection are mediated by the activity of the plasma membrane (71, 72). They depend on the adhesion among cells that is responsible for contact inhibition of growth (73) but can be modulated by the growth-promoting activity of serum. A sharp lowering of the concentration of CS in confluent NIH 3T3 cells markedly diminishes transformed focus formation, whereas doing it to rapidly proliferating cells in LDP provides the selective conditions for transformation (59). In other words, too steep a combined shutdown in cellular metabolism and growth is inhibitory to progressive selection, whereas the slight decrease in growth rate in LDP encourages selection. The choice between suppression and selection is conditionally dependent. However, epithelial cells in the organism are in continuous contact with one another, and no cells in the adult organism are in continuous exponential proliferation as they are in LDP cell culture. Therefore, cell–cell contact interaction is a crucial aspect of both suppression and selection in tumor development.
It was proposed by the pioneer molecular geneticist Rollin Hotchkiss that a fundamental feature of the living state is ordered heterogeneity (74). The similar basic concept of macrodeterminism over microdeterminism grew out of studies of embryological development (75). It was exemplified as “order invariant at the cellular level relative to irregularity at the molecular level,” and it generalized for all levels of biological organization as the first principle of a theory of organisms (76). In keeping with the principle of ordered heterogeneity, there appears to be a breakdown in the ordering capacity of tissues with age, and with exposure to carcinogenic conditions (73).
Prospectus
Most of the insights about the role of cell–cell contact in suppression of neoplastic development have come from operational experiments with fibroblasts (31, 33), epidermal cells (10, 11), and liver cells (15, 77). However, much remains to be done in this approach. Whereas neoplastic epithelial cells were suppressed by contact with homotypic epithelial cells but not with fibroblasts (10), it is not known whether heterotypic epithelial cells are suppressive. While epidermal cells in early stages of initiation have lost their capacity to suppress the growth of papilloma cells, it is not known whether direct treatment of normal epidermal cells by PAHs reduces their suppressive capacity. The suppressive capacity of normal cells for homotypic neoplastic cells needs to be determined in a larger variety of tissues. However, the well established capacity of fibroblasts to modulate neoplastic expression of transformed fibroblasts (Fig. 2) can be applied to determine whether it is correlated with the intrinsic susceptibility of mouse strains and of humans to cancer. It is noteworthy that fibroblasts of humans with inherited susceptibility to cancer exhibit increased sensitivity to transformation by various means in cell culture (78–82, †). Skin fibroblasts from patients with breast cancer and other neoplasms often display properties associated with neoplastic cells (83, 84), suggesting those patients may be a susceptible minority of the population (85). It would be of great interest to determine whether fibroblasts from cancer-resistant families have an increased capacity to suppress the neoplastic phenotype of spontaneously transformed NIH 3T3 fibroblasts, or have a decreased capacity for spontaneous transformation themselves.
Given that both suppression and selection are mediated by contact interactions at the plasma membrane, the molecular basis of those interactions needs further study. Promising results have recently been obtained in Drosophila on mutations in plasma membrane proteins that lead to malignancy of imaginal discs during larval development (86). Those mutations disrupt polarity of epithelial tissue. Chronic activation of cellular protooncogene c-Myc during development of three-dimensional organotypic mammary acinar structure in culture results in cellular hyperproliferation and transformed acinar morphology (87). However, the same oncogene activation in preformed, mature, quiescent acini fails to initiate the cell cycle or transform the structures. The capacity of c-Myc to reinitiate the cell cycle in those acinar structures is restored by the loss of a cell polarity protein. The suppressive effects of stable acinar structures resemble those of the NIH 3T3 cells that become effective only after strong contact inhibition has been established (Fig. 2A). It would be of great interest to determine which plasma membrane proteins are functional in the suppression and selection of neoplastic development of the NIH 3T3 cells that have proved so amenable to analysis in culture. It should also help to account for the well established decrease in adhesiveness of neoplastic cells (88, 89).
Spontaneous transformation of NIH 3T3 cells is highly dependent on the concentration of glutamine in the medium (90). Glutamine not only is important in the synthesis of protein, nucleotides, and other molecules involved in nitrogen metabolism, but also is a major oxidative energy source for cells in culture (91, 92). Caloric restriction is the most consistently observed tumor suppressive dietary modification and operates only on the promotion not the initiating stage of tumorigenesis (93). Since spontaneous transformation is equivalent to promotion, it suggests that the former methodology may be useful in formulating a diet that is effective in the prevention of cancer.
Acknowledgments.
I am grateful for manuscript preparation and editing by Dorothy M. Rubin. The manuscript benefited from comments by Drs. Richmond Prehn, Peter Vogt, and Stuart Yuspa. The project was partly supported by the Elsasser Family Fund.
Footnotes
The author declares no conflict of interest.
This article is a PNAS Direct Submission.
Tainsky MA, et al. Normal fibroblasts derived from patients with Li-Fraumeni cancer syndrome spontaneously transform in vitro. Proceedings of the 80th Annual Meeting of the American Association for Cancer Research, January 22–26, 1989, San Diego, CA, Vol 30, p 315.
References
- 1.Bishop JM. Molecular themes in oncogenesis. Cell. 1991;64:235–248. doi: 10.1016/0092-8674(91)90636-d. [DOI] [PubMed] [Google Scholar]
- 2.Hieger I. On the mechanism of carcinogenesis by chemical compounds. Am J Cancer. 1936;28:522–529. [Google Scholar]
- 3.Rubin H. Synergistic mechanisms in carcinogenesis by polycyclic aromatic hydrocarbons and tobacco smoke: A biohistorical perspective with updates. Carcinogenesis. 2001;22:1903–1930. doi: 10.1093/carcin/22.12.1903. [DOI] [PubMed] [Google Scholar]
- 4.Berenblum I, Shubik P. The role of croton oil applications associated with a single painting of a carcinogen in tumor induction of the mouse's skin. Brit J Cancer. 1947;1:379–382. doi: 10.1038/bjc.1947.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Van Duuren BL, et al. The effect of aging and interval between primary and secondary treatment in two-stage carcinogenesis on mouse skin. Cancer Res. 1975;35:502–505. [PubMed] [Google Scholar]
- 6.Pitot HC. Fundamentals of Oncology. New York: Dekker; 2002. [Google Scholar]
- 7.Burns FJ, Vanderlaan M, Sivak A, Albert RE. Regression kinetics of mouse skin papillomas. Cancer Res. 1976;36:1422–1427. [PubMed] [Google Scholar]
- 8.Parkinson EK. Defective responses of transformed keratinocytes to terminal differentiation stimuli: Their role in epidermal tumour promotion by phorbol esters and by deep wounding. Brit J Cancer. 1985;52:479–493. doi: 10.1038/bjc.1985.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hennings H, Michael D, Lichti U, Yuspa SH. Response of carcinogen-altered mouse epidermal cells to phorbol ester tumor promoters and calcium. J Invest Dermatol. 1987:60–65. doi: 10.1111/1523-1747.ep12465014. [DOI] [PubMed] [Google Scholar]
- 10.Hennings H, et al. Development of an in vitro analogue of initiated mouse epidermis to study tumor promoters and antipromoters. Cancer Res. 1990;50:4794–4800. [PubMed] [Google Scholar]
- 11.Strickland JE, Ueda M, Hennings H, Yuspa SH. A model for initiated mouse skin: Suppression of cells in grafts on athymic nude mice. Cancer Res. 1992;52:1439–1444. [PubMed] [Google Scholar]
- 12.Javaherian A, Vaccariello M, Fusenig NE, Garlick JA. Normal keratinocytes suppress early stages of neoplastic progression in stratified epithelium. Cancer Res. 1998;58:2200–2208. [PubMed] [Google Scholar]
- 13.Solt D, Farber E. New principle for the analysis of chemical carcinogenesis. Nature. 1976;263:701–703. [Google Scholar]
- 14.Farber E. The multistep nature of cancer development. Cancer Res. 1984;44:4217–4223. [PubMed] [Google Scholar]
- 15.McCullough KD, et al. Plasticity of the neoplastic phenotype in vivo is regulated by epigenetic factors. Proc Natl Acad Sci USA. 1998;95:15333–15338. doi: 10.1073/pnas.95.26.15333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rubin H. The role of selection in progressive neoplastic transformation. Adv Cancer Res. 2001;83:159–207. doi: 10.1016/s0065-230x(01)83006-2. [DOI] [PubMed] [Google Scholar]
- 17.Pelling JC, Neades R, Strawhecker J. Epidermal papillomas and carcinomas induced in uninitiated mouse skin by tumor promoters alone contain a point mutation in the 61st codon of the Ha-ras oncogene. Carcinogenesis. 1988;9:665–667. doi: 10.1093/carcin/9.4.665. [DOI] [PubMed] [Google Scholar]
- 18.Iversen OH. TPA (12-O-tetradecanoyl-phorbol-13-acetate) as a carcinogen for mouse skin. Virchows Arch B Cell Pathol Incl Mol Pathol. 1985;49:129–135. doi: 10.1007/BF02912091. [DOI] [PubMed] [Google Scholar]
- 19.Lankas GR, Baxter CS, Christian RT. Effect of tumor promoting agents on mutation frequencies in cultured V79 Chinese hamster cells. Mutat Res. 1977;45:153–156. doi: 10.1016/0027-5107(77)90053-7. [DOI] [PubMed] [Google Scholar]
- 20.Berry DL, et al. Metabolic conversion of 12-O-tetradecanoylphorbol-13-acetate in adult and newborn mouse skin and mouse liver microsomes. Cancer Res. 1978;38:2301–2306. [PubMed] [Google Scholar]
- 21.Krawczak M, et al. Somatic spectrum of cancer-associated single basepair substitutions in the TP53 gene is determined mainly by endogenous mechanisms of mutation and by selection. Human Mutat. 1995;5:48–57. doi: 10.1002/humu.1380050107. [DOI] [PubMed] [Google Scholar]
- 22.Tsao J-L, et al. Genetic reconstruction of individual colorectal tumor histories. Proc Natl Acad Sci USA. 2000;97:1236–1241. doi: 10.1073/pnas.97.3.1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brown K, et al. V-ras genes from Harvey and Balb murine sarcoma viruses can act as initiators of two-stage mouse skin carcinogenesis. Cell. 1986;46:447–456. doi: 10.1016/0092-8674(86)90665-3. [DOI] [PubMed] [Google Scholar]
- 24.Larson PS, et al. Genetically abnormal clones in histologically normal breast tissue. Am J Pathol. 1998;152:1591–1598. [PMC free article] [PubMed] [Google Scholar]
- 25.Kasami M, et al. Loss of heterozygosity and microsatellite instability in breast neoplasia: No obligate correlation of these genetic alterations with subsequent malignancy. Am J Pathol. 1997;150:1925–1932. [PMC free article] [PubMed] [Google Scholar]
- 26.Dollé MET, et al. Distinct spectra of somatic mutations accumulated with age in mouse heart and small intestine. Proc Natl Acad Sci USA. 2000;97:8403–8408. doi: 10.1073/pnas.97.15.8403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rubin H. What keeps cells in tissues behaving normally in the face of myriad mutations? BioEssays. 2006;28:515–524. doi: 10.1002/bies.20403. [DOI] [PubMed] [Google Scholar]
- 28.Kopelovich L, Bias NE, Helson L. Tumour promoter alone induces neoplastic transformation of fibroblasts from humans genetically predisposed to cancer. Nature. 1979;282:619–621. doi: 10.1038/282619a0. [DOI] [PubMed] [Google Scholar]
- 29.Brookes P. Chemical carcinogens and ras gene activation. Mol Carcinog. 1989;2:305–307. doi: 10.1002/mc.2940020604. [DOI] [PubMed] [Google Scholar]
- 30.Temin H, Rubin H. Characteristics of an assay for Rous sarcoma virus and Rous sarcoma cells in tissue culture. Virology. 1958;6:669–688. doi: 10.1016/0042-6822(58)90114-4. [DOI] [PubMed] [Google Scholar]
- 31.Rubin H. An analysis of the assay of Rous sarcoma cells in vitro by the infective center technique. Virology. 1960;10:29–49. doi: 10.1016/0042-6822(60)90004-0. [DOI] [PubMed] [Google Scholar]
- 32.Rubin H. The suppression of morphological alterations in cells infected with Rous sarcoma virus. Virology. 1960;12:14–31. doi: 10.1016/0042-6822(60)90146-x. [DOI] [PubMed] [Google Scholar]
- 33.Stoker M, Shearer M, O'Neill C. Growth inhibition of polyoma-transformed cells by contact with static normal fibroblasts. J Cell Sci. 1966;1:297–310. doi: 10.1242/jcs.1.3.297. [DOI] [PubMed] [Google Scholar]
- 34.Herschman HR, Brankow DW. Ultraviolet irradiation transforms C3H 10T1/2 cells to a unique, suppressible phenotype. Science. 1986;234:1385–1388. doi: 10.1126/science.3787250. [DOI] [PubMed] [Google Scholar]
- 35.Herschman HR, Brankow DW. Colony size, cell density and nature of the tumor promoter are critical variables in expression of a transformed phenotype (focus formation) in cocultures of UV-TDTx and C3H10T1/2 cells. Carcinogenesis. 1987;8:993–998. doi: 10.1093/carcin/8.7.993. [DOI] [PubMed] [Google Scholar]
- 36.Earle WR. Production of malignancy in vitro. IV. The mouse fibroblast cultures and changes in the living cells. J Natl Cancer Inst. 1943;4:165–212. [Google Scholar]
- 37.Sanford KK, et al. Production of malignancy in vitro. XII. Further transformation of mouse fibroblasts to sarcomatous cells. J Natl Cancer Inst. 1950;11:351–375. [PubMed] [Google Scholar]
- 38.Sanford KK, Evans VJ. A quest for the mechanism of ‘spontaneous’ malignant transformation in culture with associated advances in culture technology. J Natl Cancer Inst. 1982;68:895–913. [PubMed] [Google Scholar]
- 39.Aaronson SA, Todaro GJ. Basis for the acquisition of malignant potential by mouse cells cultivated in vitro. Science. 1968;162:1024–1026. doi: 10.1126/science.162.3857.1024. [DOI] [PubMed] [Google Scholar]
- 40.Lee LW, Tsao M-S, Grisham JW, Smith GJ. Emergence of neoplastic transformants spontaneously or after exposure to N-methyl-N′-nitro-N-nitrosoguanidine in populations of rat liver cells cultured under selective and nonselective conditions. Am J Pathol. 1989;135:63–71. [PMC free article] [PubMed] [Google Scholar]
- 41.Jainchill JL, Aaronson SA, Todaro GJ. Murine sarcoma and leukemia viruses: Assay using clonal lines of contact-inhibited mouse cells. J Virol. 1969;4:549–553. doi: 10.1128/jvi.4.5.549-553.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Copeland NG, Zelenetz AD, Cooper GM. Transformation of NIH/3T3 mouse cells by DNA of Rous sarcoma virus. Cell. 1979;17:993–1002. doi: 10.1016/0092-8674(79)90338-6. [DOI] [PubMed] [Google Scholar]
- 43.Shih C, et al. Passage of phenotypes of chemically transformed cells via transfection of DNA and chromatin. Proc Natl Acad Sci USA. 1979;76:5714–5718. doi: 10.1073/pnas.76.11.5714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Perucho M, et al. Human-tumor-derived cell lines contain common and different transforming genes. Cell. 1981;27:467–476. doi: 10.1016/0092-8674(81)90388-3. [DOI] [PubMed] [Google Scholar]
- 45.Varmus HE. The molecular genetics of cellular oncogenes. Annu Rev Genet. 1984;18:553–612. doi: 10.1146/annurev.ge.18.120184.003005. [DOI] [PubMed] [Google Scholar]
- 46.Rubin H, Xu K. Evidence for the progressive and adaptive nature of spontaneous transformation in the NIH 3T3 cell line. Proc Natl Acad Sci USA. 1989;86:1860–1864. doi: 10.1073/pnas.86.6.1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yao A, Rubin H. Sensitivity of transformation to small differences in population density during serial passage of NIH 3T3 cells. Proc Natl Acad Sci USA. 1992;89:7486–7490. doi: 10.1073/pnas.89.16.7486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yao A, Rubin AL, Rubin H. Progressive state selection of cells in low serum promotes high density growth and neoplastic transformation in NIH 3T3 cells. Cancer Res. 1990;50:5171–5176. [PubMed] [Google Scholar]
- 49.Rubin AL, Arnstein P, Rubin H. Physiological induction and reversal of focus formation and tumorigenicity in NIH-3T3 cells. Proc Natl Acad Sci USA. 1990;87:10005–10009. doi: 10.1073/pnas.87.24.10005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rubin H. Adaptive evolution of degrees and kinds of neoplastic transformation in cell culture. Proc Natl Acad Sci USA. 1992;89:977–981. doi: 10.1073/pnas.89.3.977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rubin H. Cellular epigenetics: Control of the size, shape, and spatial distribution of transformed foci by interactions between the transformed and nontransformed cells. Proc Natl Acad Sci USA. 1994;91:1039–1043. doi: 10.1073/pnas.91.3.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rubin H. Microenvironmental regulation of the initiated cell. Adv Cancer Res. 2003;90:1–62. doi: 10.1016/s0065-230x(03)90001-7. [DOI] [PubMed] [Google Scholar]
- 53.Chow M, Rubin H. The cellular ecology of progressive neoplastic transformation: A clonal analysis. Proc Natl Acad Sci USA. 1999;96:2093–2098. doi: 10.1073/pnas.96.5.2093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rubin H. Experimental control of neoplastic progression in cell populations: Foulds' rules revisited. Proc Natl Acad Sci USA. 1994;91:6619–6623. doi: 10.1073/pnas.91.14.6619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Setala K, et al. Mechanism of experimental tumorigenesis. I. Epidermal hyperplasia in mouse caused by locally applied tumor initiator and dipole-type tumor promoter. J Natl Cancer Inst. 1959;23:925–951. [PubMed] [Google Scholar]
- 56.Auerbach O, Stout AP, Hammond EC, Garfinkel L. Changes in bronchial epithelium in relation to cigarette smoking and in relation to lung cancer. N Eng J Med. 1961;265:255–267. doi: 10.1056/NEJM196108102650601. [DOI] [PubMed] [Google Scholar]
- 57.Chang W. Histogenesis of symmetrical 1,2-dimethylhydrazine-induced neoplasms of the colon in the mouse. J Natl Cancer Inst. 1978;60:1405–1418. doi: 10.1093/jnci/60.6.1405. [DOI] [PubMed] [Google Scholar]
- 58.Koss LG. Diagnostic Cytology and Its Histopathologic Bases. Philadelphia: Lippincott; 1979. [Google Scholar]
- 59.Rubin H. Degrees and kinds of selection in spontaneous neoplastic transformation: An operational analysis. Proc Natl Acad Sci USA. 2005;102:9276–9281. doi: 10.1073/pnas.0503688102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Brookes P, Lawley PD. Evidence for the binding of polynuclear aromatic hydrocarbons to the nucleic acids of mouse skin: Relation between carcinogenic power of the hydrocarbons and their binding to deoxyribonucleic acid. Nature. 1964;202:781–784. doi: 10.1038/202781a0. [DOI] [PubMed] [Google Scholar]
- 61.Tomlinson I, Bodmer W. Selection, the mutation rate and cancer: Ensuring that the tail does not wag the dog. Nat Med. 1999;5:11–12. doi: 10.1038/4687. [DOI] [PubMed] [Google Scholar]
- 62.Zhang W, et al. Escaping the stem cell compartment: Sustained UVB exposure allows mutant keratinocytes to colonize adjacent epidermal proliferating units without incurring additional mutations. Proc Natl Acad Sci USA. 2001;98:13948–13953. doi: 10.1073/pnas.241353198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Brash DE, Zhang W, Grossman D, Takeuchi S. Colonization of adjacent stem cell compartments by mutant keratinocytes. Sem Cancer Biol. 2005;15:97–102. doi: 10.1016/j.semcancer.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 64.Rodin SN, Rodin AS. Human lung cancer and p53: The interplay between mutagenesis and selection. Proc Natl Acad Sci USA. 2000;97:12244–12249. doi: 10.1073/pnas.180320897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rodin SN, Rodin AS. Origins and selection of p53 mutations in lung carcinogenesis. Sem Cancer Biol. 2005;15:103–112. doi: 10.1016/j.semcancer.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 66.Vaccariello M, et al. Cell interactions control the fate of malignant keratinocytes in an organotypic model of early neoplasia. J Invest Dermatol. 1999;113:384–391. doi: 10.1046/j.1523-1747.1999.00701.x. [DOI] [PubMed] [Google Scholar]
- 67.Foulds L. Neoplastic Development. Vol. 1. New York: Academic; 1969. [Google Scholar]
- 68.Friedewald WF, Rous P. The pathogenesis of deferred cancers. A study of the after effects of methylcholanthrene upon rabbit skin. J Exp Med. 1950;91:459–484. doi: 10.1084/jem.91.5.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fry RJM, Tyler SA, Lesher S. In: Radiation and Ageing. Lindop PJ, Sacher GA, editors. London: Taylor & Francis; 1966. pp. 43–55. [Google Scholar]
- 70.Bahar R, et al. Increased cell-to-cell variation in gene expression in ageing mouse heart. Nature. 2006;441:1011–1014. doi: 10.1038/nature04844. [DOI] [PubMed] [Google Scholar]
- 71.Hennings H, et al. Activity of diverse tumor promoters in a keratinocyte co-culture model of initiated epidermis. Carcinogenesis. 1992;13:2145–2151. doi: 10.1093/carcin/13.11.2145. [DOI] [PubMed] [Google Scholar]
- 72.Alexander DB, et al. Normal cells control the growth of neighboring transformed cells independent of gap junctional communication and Src activity. Cancer Res. 2004;64:1347–1358. doi: 10.1158/0008-5472.can-03-2558. [DOI] [PubMed] [Google Scholar]
- 73.Rubin H. Ordered heterogeneity and its decline in cancer and aging. Adv Cancer Res. 2007;98:117–147. doi: 10.1016/S0065-230X(06)98004-X. [DOI] [PubMed] [Google Scholar]
- 74.Gerard RW. Concepts of biology. Behav Sci. 1958;3:92–215. [Google Scholar]
- 75.Weiss P. The Science of Life. Mt. Kisco, NY: Futura; 1973. [Google Scholar]
- 76.Elsasser WM. Reflections on a Theory of Organisms. Baltimore: Johns Hopkins Univ Press; 1998. [Google Scholar]
- 77.Coleman WB, Wennerberg AE, Smith GJ, Grisham JW. Regulation of the differentiation of diploid and some aneuploid rat liver epithelial (stemlike) cells by the hepatic microenvironment. Am J Pathol. 1993;142:1373–1382. [PMC free article] [PubMed] [Google Scholar]
- 78.Todaro GJ, Green H. Cell growth and the initiation of transformation by SV 40. Proc Natl Acad Sci USA. 1966;55:302–308. doi: 10.1073/pnas.55.2.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Miller RW, Todaro CJ. Viral transformation of cells from persons at high risk of cancer. Lancet. 1969:81–82. doi: 10.1016/s0140-6736(69)91094-0. [DOI] [PubMed] [Google Scholar]
- 80.Chang KSS. Susceptibility of xeroderma pigmentosum cells to transformation by murine and feline sarcoma. Cancer Res. 1976;36:3294–3299. [PubMed] [Google Scholar]
- 81.Danes SB. Heritable colonic cancer syndrome: Induction of anchorage-independence in dermal cultures derived from patients with adenomatosis of the colon and rectum. Oncology. 1980;37:386–389. doi: 10.1159/000225478. [DOI] [PubMed] [Google Scholar]
- 82.Kopelovich L. The transformed (initiated) human cell phenotype: Study of cultured skin fibroblasts from individuals predisposed to cancer. Mutat Res. 1988;199:369–385. doi: 10.1016/0027-5107(88)90215-1. [DOI] [PubMed] [Google Scholar]
- 83.Azzarone B, et al. Abnormal properties of skin fibroblasts from patients with breast cancer. Int J Cancer. 1984;33:759–764. doi: 10.1002/ijc.2910330608. [DOI] [PubMed] [Google Scholar]
- 84.Azzarone B, Macieira-Coelho A. Further characterization of skin fibroblasts from cancer patients. J Cell Sci. 1987;87:155–162. doi: 10.1242/jcs.87.1.155. [DOI] [PubMed] [Google Scholar]
- 85.Peto J, Mack TM. High constant incidence in twins and other relatives of women with breast cancer. Nat Genet. 2000;26:411–414. doi: 10.1038/82533. [DOI] [PubMed] [Google Scholar]
- 86.Bilder D. Epithelial polarity and proliferation control: Links from Drosophila neoplastic tumor suppressors. Genes Dev. 2004;18:1909–1925. doi: 10.1101/gad.1211604. [DOI] [PubMed] [Google Scholar]
- 87.Partanen JI, Nieminen AI, Mäkelä TP, Klefstrom J. Suppression of oncogenic properties of c-Myc by LKB1-controlled epithelial organization. Proc Natl Acad Sci USA. 2007;104:14694–14699. doi: 10.1073/pnas.0704677104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Coman DR. Decreased mutual adhesiveness, a property of cells from squamous cell carcinomas. Cancer Res. 1944;4:625–629. [Google Scholar]
- 89.McCutcheon M, Coman DR, Moore FB. Studies on invasiveness of cancer. Adhesiveness of malignant cells in various human adenocarcinomas. Cancer. 1948;1:460–467. doi: 10.1002/1097-0142(194809)1:3<460::aid-cncr2820010313>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 90.Rubin AL. Suppression of transformation by and growth adaptation to low concentrations of glutamine in NIH-3T3 cells. Cancer Res. 1990;50:2832–2839. [PubMed] [Google Scholar]
- 91.Reitzer LJ, Wice BM, Kennell D. Evidence that glutamine, not sugar, is the main energy source for cultured HeLa cells. J Biol Chem. 1979;254:2669–2676. [PubMed] [Google Scholar]
- 92.Zielke HR, Zielke CL, Ozand PT. Glutamine: A major energy source for cultured mammalian cells. Fed Proc. 1984;43:121–125. [PubMed] [Google Scholar]
- 93.Tannenbaum A. The dependence of the genesis of induced skin tumors on the caloric intake during different stages of carcinogenesis. Cancer Res. 1944;4:673–677. [Google Scholar]
- 94.Rubin H. Incipient and overt stages of neoplastic transformation. Proc Natl Acad Sci USA. 1994;91:12076–12080. doi: 10.1073/pnas.91.25.12076. [DOI] [PMC free article] [PubMed] [Google Scholar]