Abstract
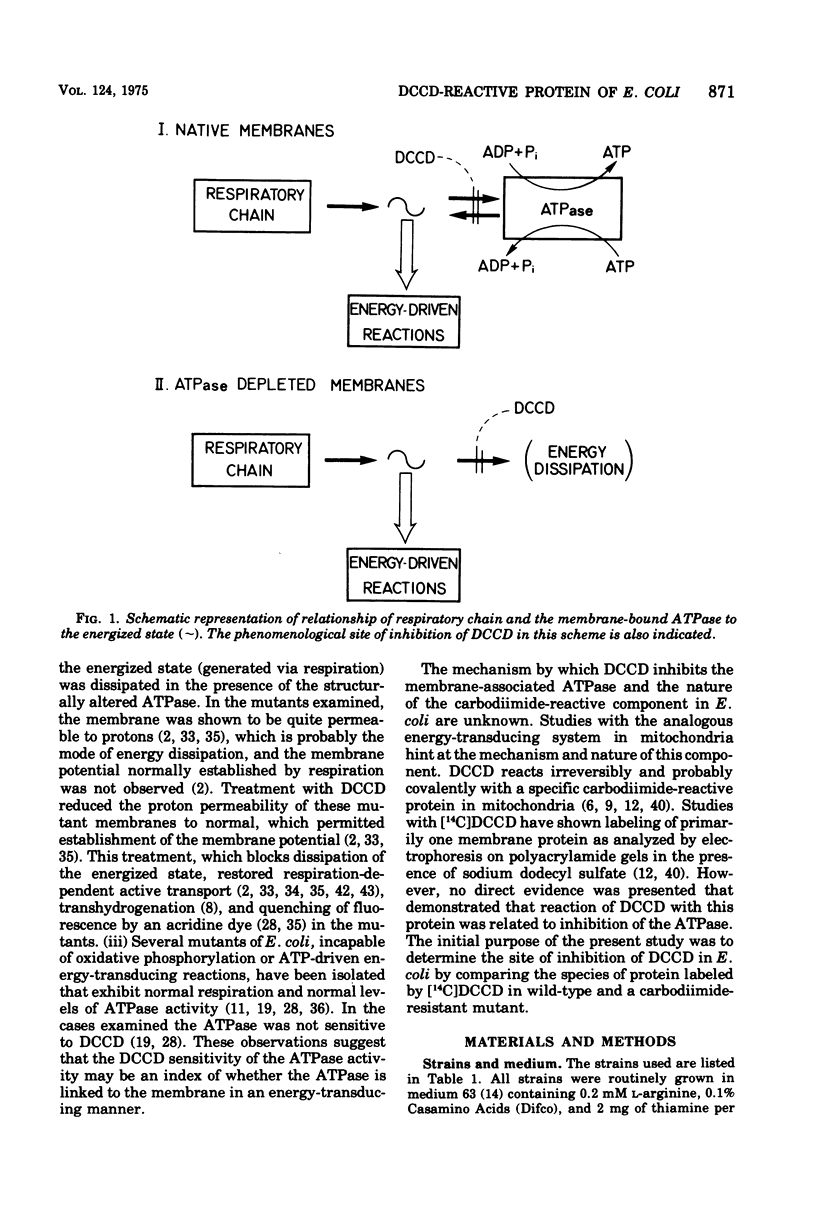
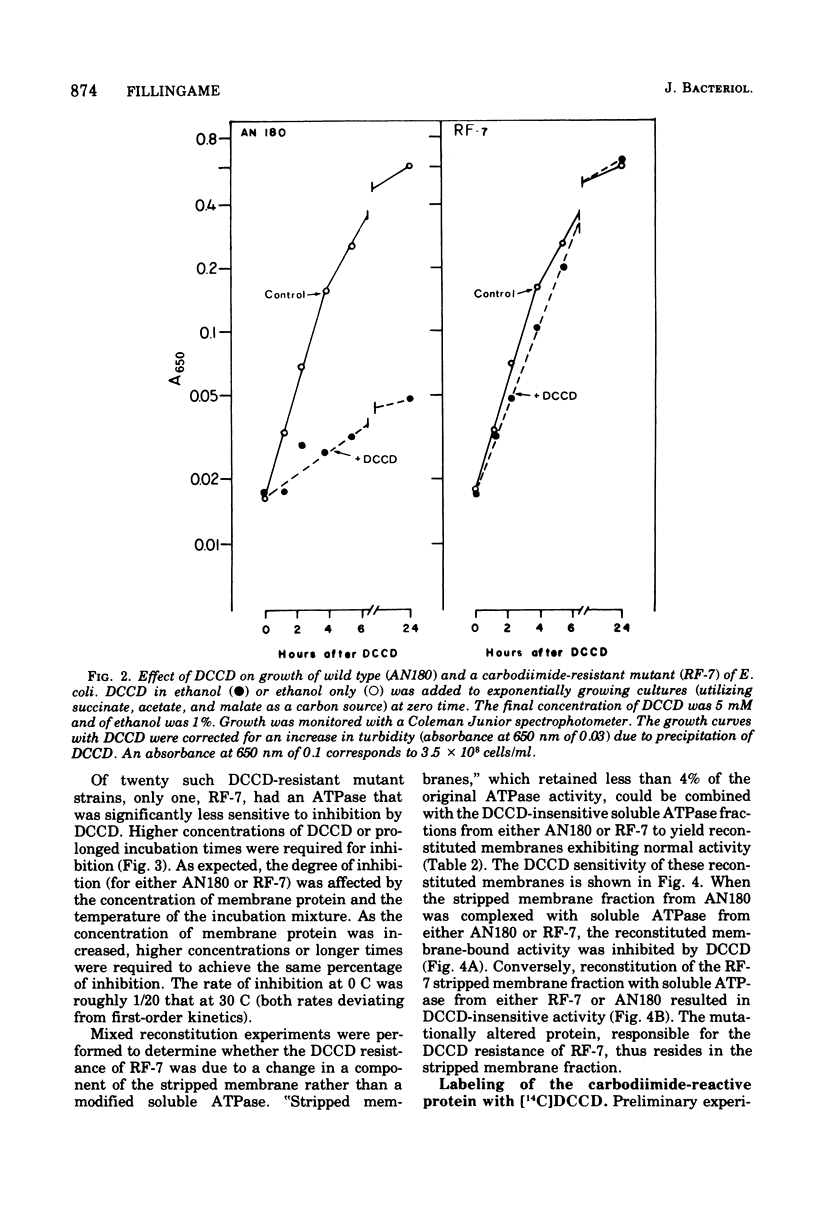
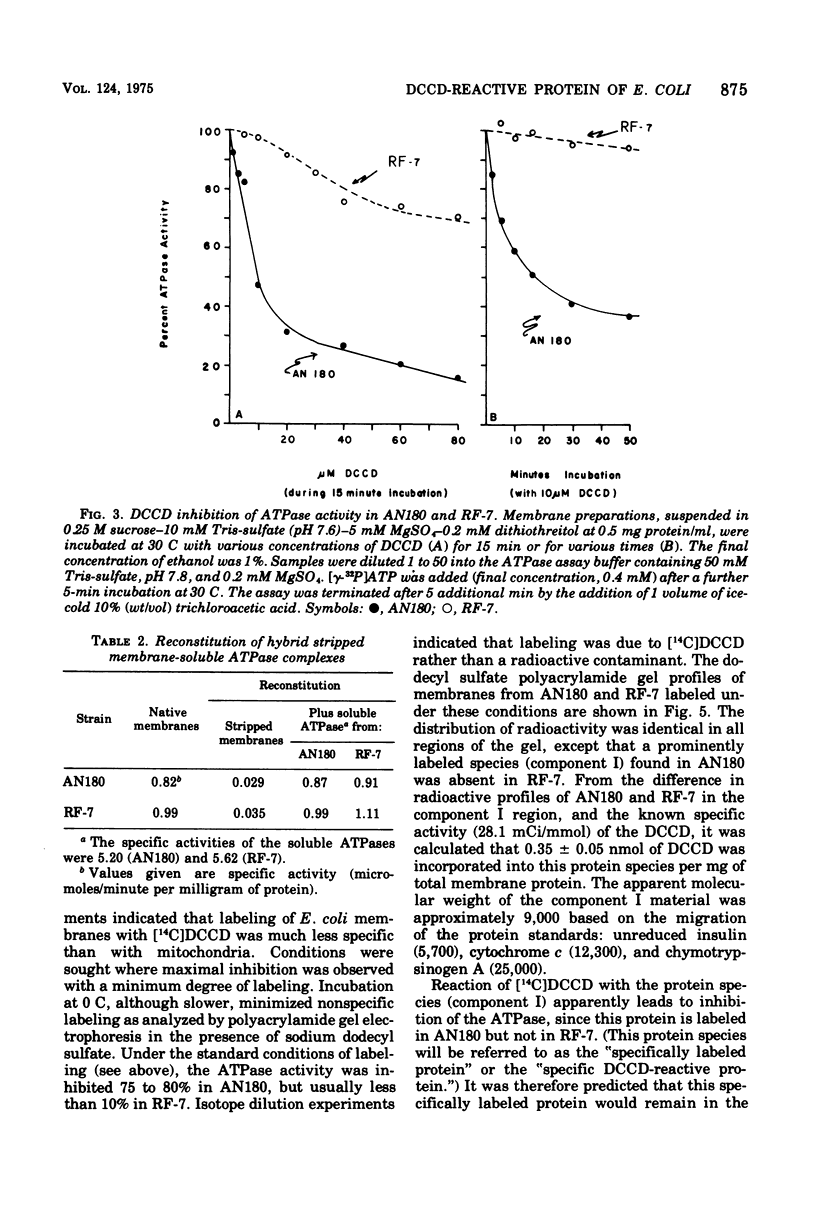
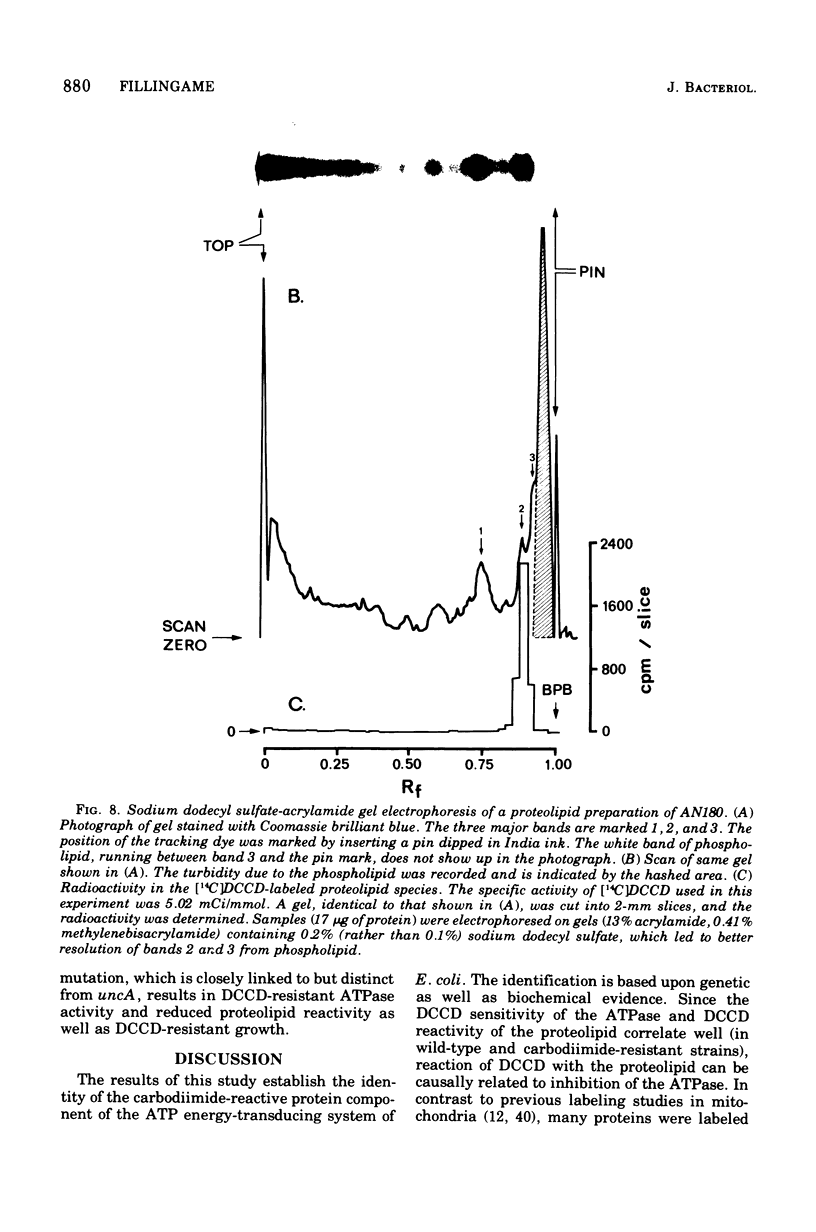
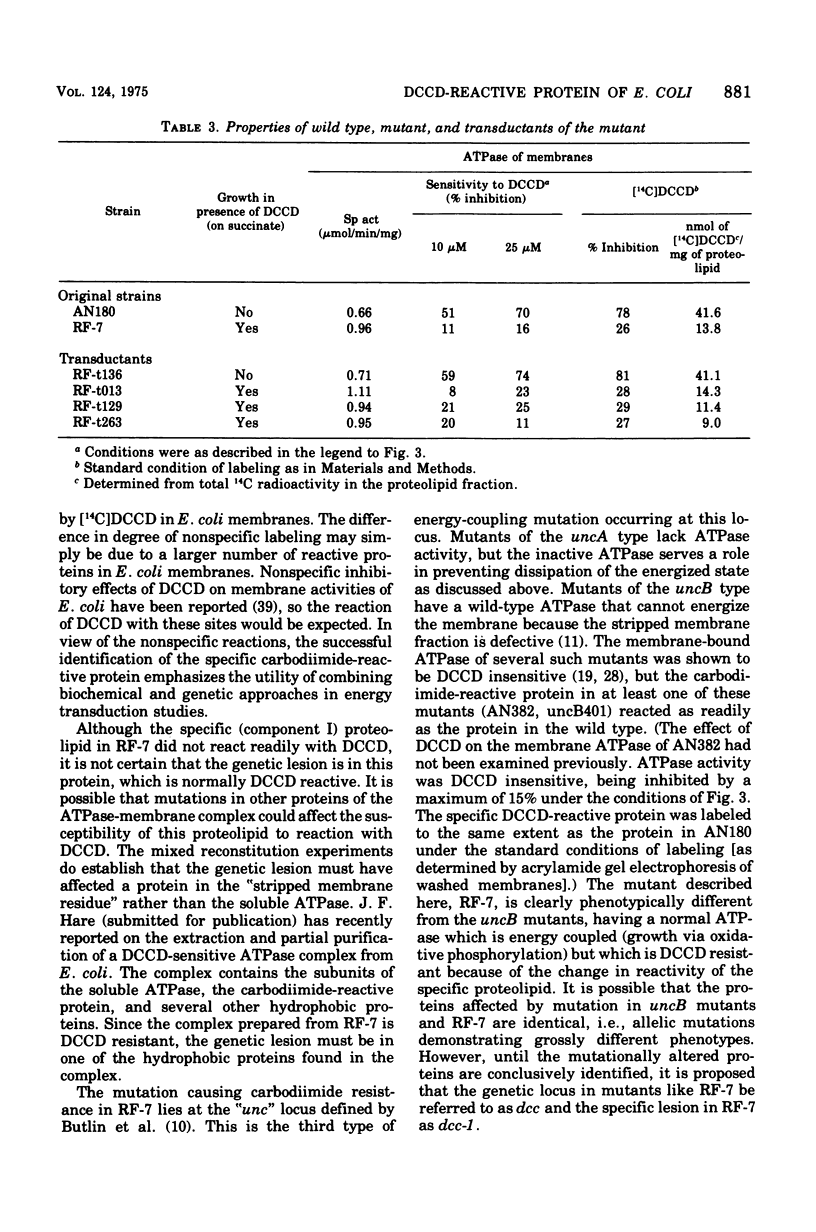
Membranes of Escherichia coli contain an adenosine 5'-triphosphate (ATP) energy-transducing system that is inhibited by treatment with dicyclohexylcarbodiimide (DCCD). The carbodiimide-reactive protein component of this system has been identified after treatment with [14C]DCCD. This protein has an apparent molecular weight of 9,000 as judged from acrylamide gel electrophoresis in the presence of sodium dodecyl sulfate and is extracted from the membrane with chloroform-methanol (2:1). These properties are similar to the analogous protein previously identified in mitochondria (Cattell et al., 1971). A mutant strain, RF-7, has been isolated which derives energy from oxidative phosphorylation in the presence of 5 mM DCCD. The ATP hydrolase activity of the membraned system in the mutant was considerably less sensitive to inhibition by DCCD than that in the wild type. The carbodiimide-reactive protein, which was easily labeled by [14C]DCCD in the wild type, was labeled much less rapidly in the carbodiimide-resistant mutant. It is thus concluded that the reaction of DCCD with this specific protein leads to inhibition of the ATP energy-transducing reactions. The mutation causing carbodiimide resistance in strain RF-7 was mapped. It is cotransduced with the uncA gene at a frequency exceeding 90%. The mutationally altered protein causing the carbodiimide resistance was not conclusively identified. However, reconstitution experiments indicate that the altered protein is not one of the subunits of the soluble ATP hydrolase activity, which can be removed from the membrane by washing with 1 mM tris(hydroxymethyl)aminomethane buffer lacking Mg2+. The carbodiimide-reactive protein remains with the membrane residue after removal of the soluble ATP hydrolase and is thus distinct from these subunits as well.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abrams A., Smith J. B., Baron C. Carbodiimide-resistant membrane adenosine triphosphatase in mutants of Streptococcus faecalis. I. Studies of the mechanism of resistance. J Biol Chem. 1972 Mar 10;247(5):1484–1488. [PubMed] [Google Scholar]
- Altendorf K., Harold F. M., Simoni R. D. Impairment and restoration of the energized state in membrane vesicles of a mutant of Escherichia coli lacking adenosine triphosphatase. J Biol Chem. 1974 Jul 25;249(14):4587–4593. [PubMed] [Google Scholar]
- Beechey R. B., Holloway C. T., Knight I. G., Roberton A. M. Dicyclohexylcarbodiimide--an inhibitor of oxidative phosphorylation. Biochem Biophys Res Commun. 1966 Apr 6;23(1):75–80. doi: 10.1016/0006-291x(66)90271-3. [DOI] [PubMed] [Google Scholar]
- Beechey R. B., Roberton A. M., Holloway C. T., Knight I. G. The properties of dicyclohexylcarbodiimide as an inhibitor of oxidative phosphorylation. Biochemistry. 1967 Dec;6(12):3867–3879. doi: 10.1021/bi00864a033. [DOI] [PubMed] [Google Scholar]
- Bragg P. D., Hou C. Purification of a factor for both aerobic-driven and ATP-driven energy-dependent transhydrogenases of Escherichia coli. FEBS Lett. 1972 Dec 15;28(3):309–312. doi: 10.1016/0014-5793(72)80738-5. [DOI] [PubMed] [Google Scholar]
- Bragg P. D., Hou C. Reconstitution of energy-dependent transhydrogenase in ATPase-negative mutants of Escherichia coli. Biochem Biophys Res Commun. 1973 Feb 5;50(3):729–736. doi: 10.1016/0006-291x(73)91305-3. [DOI] [PubMed] [Google Scholar]
- Bulos B., Racker E. Partial resolution of the enzymes catalyzing oxidative phosphorylation. XVII. Further resolution of the rutamycin-sensitive adenosine triphosphatase. J Biol Chem. 1968 Jul 25;243(14):3891–3900. [PubMed] [Google Scholar]
- Butlin J. D., Cox G. B., Gibson F. Oxidative phosphorylation in Escherichia coli K-12: the genetic and biochemical characterisations of a strain carrying a mutation in the uncB gene. Biochim Biophys Acta. 1973 Feb 22;292(2):366–375. doi: 10.1016/0005-2728(73)90043-1. [DOI] [PubMed] [Google Scholar]
- Butlin J. D., Cox G. B., Gibson F. Oxidative phosphorylation in Escherichia coli K12. Mutations affecting magnesium ion- or calcium ion-stimulated adenosine triphosphatase. Biochem J. 1971 Aug;124(1):75–81. doi: 10.1042/bj1240075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COHEN G. N., RICKENBERG H. V. Concentration spécifique réversible des amino acides chez Escherichia coli. Ann Inst Pasteur (Paris) 1956 Nov;91(5):693–720. [PubMed] [Google Scholar]
- Cattell K. J., Lindop C. R., Knight I. G., Beechey R. B. The identification of the site of action of NN'-dicyclohexylcarbodi-imide as a proteolipid in mitochondrial membranes. Biochem J. 1971 Nov;125(1):169–177. doi: 10.1042/bj1250169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cockrell R. S. Energy-linked ion translocation in submitochondrial particles. 3. Transport of monovalent cations by submitochondrial particles. J Biol Chem. 1973 Oct 10;248(19):6828–6833. [PubMed] [Google Scholar]
- Evans D. J. Membrane Mg-(Ca)-Activated Adenosine Triphosphatase of Escherichia coli: Characterization in the Membrane-Bound and Solubilized States. J Bacteriol. 1970 Dec;104(3):1203–1212. doi: 10.1128/jb.104.3.1203-1212.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FOLCH J., LEES M. Proteolipides, a new type of tissue lipoproteins; their isolation from brain. J Biol Chem. 1951 Aug;191(2):807–817. [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- Futai M., Sternweis P. C., Heppel L. A. Purification and properties of reconstitutively active and inactive adenosinetriphosphatase from Escherichia coli. Proc Natl Acad Sci U S A. 1974 Jul;71(7):2725–2729. doi: 10.1073/pnas.71.7.2725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutnick D. L., Kanner B. I., Postma P. W. Oxidative phosphorylation in mutants of Escherichia coli defective in energy transduction. Biochim Biophys Acta. 1972 Nov 17;283(2):217–222. doi: 10.1016/0005-2728(72)90237-x. [DOI] [PubMed] [Google Scholar]
- Hare J. F., Olden K., Kennedy E. P. Heterogeneity of membrane vesicles from Escherichia coli and their subfractionation with antibody to ATPase. Proc Natl Acad Sci U S A. 1974 Dec;71(12):4843–4846. doi: 10.1073/pnas.71.12.4843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harold F. M., Baarda J. R., Baron C., Abrams A. Inhibition of membrane-bound adenosine triphosphatase and of cation transport in Streptococcus faecalis by N,N'-dicyclohexylcarbodiimide. J Biol Chem. 1969 May 10;244(9):2261–2268. [PubMed] [Google Scholar]
- Kanner B. I., Gutnick D. L. Energy linked nicotinamide adenine dinucleotide transhydrogenase in a mutant of Escherichia coli K12 lacking membrane Mg(2+)&z.sbnd;Ca(2+)-activated adenosine triphosphatase. FEBS Lett. 1972 May 1;22(2):197–199. doi: 10.1016/0014-5793(72)80043-7. [DOI] [PubMed] [Google Scholar]
- Klein W. L., Boyer P. D. Energization of active transport by Escherichia coli. J Biol Chem. 1972 Nov 25;247(22):7257–7265. [PubMed] [Google Scholar]
- Knowles A. F., Guillory R. J., Racker E. Partial resolution of the enzymes catalyzing oxidative phosphorylation. XXIV. A factor required for the binding of mitochondrial adenosine triphosphatase to the inner mitochondrial membrane. J Biol Chem. 1971 Apr 25;246(8):2672–2679. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuis F. J., Kanner B. I., Gutnick D. L., Postma P. W., van Dam K. Energy conservation in membranes of mutants of Escherichia coli defective in oxidative phosphorylation. Biochim Biophys Acta. 1973 Oct 19;325(1):62–71. doi: 10.1016/0005-2728(73)90151-5. [DOI] [PubMed] [Google Scholar]
- Or A., Kanner B. I., Gutnick D. L. Active transport in mutants of Escherichia coli with alterations in the membrane ATPase complex. FEBS Lett. 1973 Sep 15;35(2):217–219. doi: 10.1016/0014-5793(73)80288-1. [DOI] [PubMed] [Google Scholar]
- PATTERSON M. S., GREENE R. C. MEASUREMENT OF LOW ENERGY BETA-EMITTERS IN AQUEOUS SOLUTION BY LIQUID SCINTILLATION COUNTING OF EMULSIONS. Anal Chem. 1965 Jun;37:854–857. doi: 10.1021/ac60226a017. [DOI] [PubMed] [Google Scholar]
- Roberton A. M., Holloway C. T., Knight I. G., Beechey R. B. A comparison of the effects of NN'-dicyclohexylcarbodi-imide, oligomycin A and aurovertin on enrgy-linked reactions in mitochondria and submitochondrial particles. Biochem J. 1968 Jul;108(3):445–456. doi: 10.1042/bj1080445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roisin M. P., Kepes A. The membrane ATPase of Escherichia coli. I. Release into solution, allotopic properties and reconstitution of membrane-bound ATPase. Biochim Biophys Acta. 1973 May 30;305(2):249–259. doi: 10.1016/0005-2728(73)90173-4. [DOI] [PubMed] [Google Scholar]
- Rosen B. P., Adler L. W. The maintenance of the energized membrane state and its relation to active transport in Escherichia coli. Biochim Biophys Acta. 1975 Apr 14;387(1):23–36. doi: 10.1016/0005-2728(75)90049-3. [DOI] [PubMed] [Google Scholar]
- Rosen B. P. Beta-galactoside transport and proton movements in an adenosine triphosphatase-deficient mutant of Escherichia coli. Biochem Biophys Res Commun. 1973 Aug 21;53(4):1289–1296. doi: 10.1016/0006-291x(73)90605-0. [DOI] [PubMed] [Google Scholar]
- Rosen B. P. Restoration of active transport in an Mg2+-adenosine triphosphatase-deficient mutant of Escherichia coli. J Bacteriol. 1973 Dec;116(3):1124–1129. doi: 10.1128/jb.116.3.1124-1129.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schairer H. U., Gruber D. Mutants of Escherichia coli K12 defective in oxidative phosphorylation. Eur J Biochem. 1973 Aug 17;37(2):282–286. doi: 10.1111/j.1432-1033.1973.tb02986.x. [DOI] [PubMed] [Google Scholar]
- Schairer H. U., Haddock B. A. -Galactoside accumulation in a Mg 2+ -,Ca 2+ -activated ATPase deficient mutant of E.coli. Biochem Biophys Res Commun. 1972 Aug 7;48(3):544–551. doi: 10.1016/0006-291x(72)90382-8. [DOI] [PubMed] [Google Scholar]
- Senior A. E. The structure of mitochondrial ATPase. Biochim Biophys Acta. 1973 Dec 31;301(3):249–277. doi: 10.1016/0304-4173(73)90006-2. [DOI] [PubMed] [Google Scholar]
- Singh A. P., Bragg P. D. Effect of dicyclohexylcarbodiimide on growth and membrane-mediated processes in wild type and heptose-deficient mutants of Escherichia coli K-12. J Bacteriol. 1974 Jul;119(1):129–137. doi: 10.1128/jb.119.1.129-137.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stekhovan F. S., Waitkus R. F., Van Moerkerk H. T. Identification of the dicyclohexylcarbodiimide-binding protein in the oligomycin-sensitive adenosine triphosphatase from bovine heart mitochondria. Biochemistry. 1972 Mar 28;11(7):1144–1150. doi: 10.1021/bi00757a005. [DOI] [PubMed] [Google Scholar]
- Swank R. T., Munkres K. D. Molecular weight analysis of oligopeptides by electrophoresis in polyacrylamide gel with sodium dodecyl sulfate. Anal Biochem. 1971 Feb;39(2):462–477. doi: 10.1016/0003-2697(71)90436-2. [DOI] [PubMed] [Google Scholar]
- Tsuchiya T., Rosen B. P. Restoration of active calcium transport in vesicles of an Mg2+-ATPase mutant of Escherichia coli by wild-type Mg2+-ATPase. Biochem Biophys Res Commun. 1975 Apr 21;63(4):832–838. doi: 10.1016/0006-291x(75)90642-7. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- van Thienen G., Postma P. W. Coupling between energy conservation and active transport of serine in Escherichia coli. Biochim Biophys Acta. 1973 Oct 25;323(3):429–440. doi: 10.1016/0005-2736(73)90188-0. [DOI] [PubMed] [Google Scholar]