Abstract
The peptide hormone ghrelin is the only known protein modified with an O-linked octanoyl side group, which occurs on its third serine residue. This modification is crucial for ghrelin's physiological effects including regulation of feeding, adiposity, and insulin secretion. Despite the crucial role for octanoylation in the physiology of ghrelin, the lipid transferase that mediates this novel modification has remained unknown. Here we report the identification and characterization of human GOAT, the ghrelin O-acyl transferase. GOAT is a conserved orphan membrane-bound O-acyl transferase (MBOAT) that specifically octanoylates serine-3 of the ghrelin peptide. Transcripts for both GOAT and ghrelin occur predominantly in stomach and pancreas. GOAT is conserved across vertebrates, and genetic disruption of the GOAT gene in mice leads to complete absence of acylated ghrelin in circulation. The occurrence of ghrelin and GOAT in stomach and pancreas tissues demonstrates the relevance of GOAT in the acylation of ghrelin and further implicates acylated ghrelin in pancreatic function.
Keywords: acylation, membrane-bound O-acyl transferase
Ghrelin is a 28-aa peptide hormone produced principally by stomach tissue with an unusual acyl modification on its critical serine-3 residue. Ghrelin is the endogenous ligand for the growth hormone secretagogue receptor 1a (GHSR1a), and its acyl modification is critical for the activation of the GHSR1a (1). In addition to stimulating growth hormone release from pituitary, ghrelin also promotes food intake, carbohydrate utilization, and adiposity (2–5). Accordingly, ghrelin levels are modulated by changes in nutritional status such as feeding and fasting or exposure to high-fat diets (5, 6). Importantly, ghrelin is the only peptide hormone of peripheral tissue origin that increases food intake (2).
More recent studies have identified roles for acylated ghrelin in regulating insulin secretion and blood glucose. Acylated ghrelin occurs in pancreas tissues, and GHSR1a receptor blockade with specific antagonists or treatments with antiserum against acylated ghrelin enhance glucose-induced increases in insulin release whereas acylated ghrelin decreases insulin release (5, 7–10). These observations have further implicated acylated ghrelin in the regulation of metabolism.
In stomach tissue and in circulation, acylated forms of ghrelin are modified via an ester linkage with n-octanoic acid and to a lesser extent with decanoyl and decenoyl fatty acids (1, 3). Importantly, the acyl modification in ghrelin is essential for function, with octanoyl and decanoyl fatty acids being optimal (11). Ghrelin is highly conserved in vertebrates, and the third serine residue, which is uniquely modified by the ester-linked acyl group, occurs in all mammal, avian, and fish species (3).
The enzyme(s) responsible for acylation of ghrelin has remained unknown. Work by Takada et al. (12) described that porcupine, an enzyme with structural similarities to membrane-bound O-acyl transferases (MBOAT), is required for serine-209 acylation with palmitoleic acid and for transport of Wnt3a from the endoplasmic reticulum for secretion. Ghrelin and Wnt3a are the only proteins known to possess acylated serine residues. Intriguingly, the enzyme porcupine has been localized at the endoplasmic reticulum, the same cellular compartment through which ghrelin is expected to pass during its processing (13). These observations raised the possibility that perhaps an acyl transferase belonging to the MBOAT family of enzymes may also mediate the acyl modification in ghrelin.
In 2001, Kanamoto et al. (14) exploited immunohistochemistry and radioimmunoassays with reagents capable of detecting des-octanoyl ghrelin (des-acyl ghrelin) or acyl ghrelin to show that the human medullary thyroid carcinoma cell line (TT cell line) produces ghrelin peptides, with des-acyl ghrelin being the most prominent form. Importantly, however, low levels of acyl ghrelin immunoreactivity were also reported, suggesting that these cells possess the necessary enzymatic machinery for the acylation of ghrelin.
To further understand the mechanism by which ghrelin is acylated, we used the TT cell line along with siRNA gene-silencing strategies with MS-based assays to search for genes capable of modulating the octanoylation of ghrelin. Here we report the identification and characterization of a member of the MBOAT family of acyl transferases capable of specifically octanoylating ghrelin on its critical serine-3 residue.
Results
Stimulation of Ghrelin Octanoylation in TT Cells.
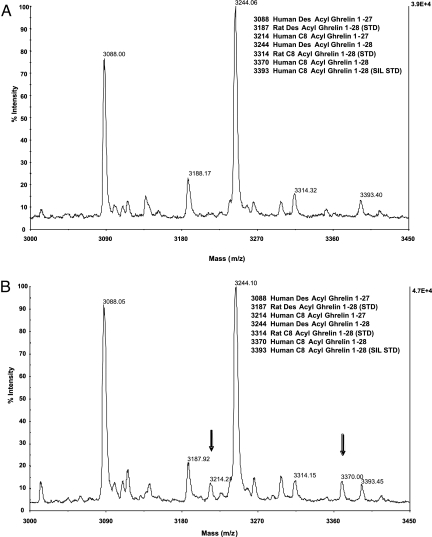
We used an immunoprecipitation MALDI-TOF assay to detect both acyl and des-acyl forms of ghrelin peptides from cell culture media of human medullary thyroid carcinoma (TT) cells (14). We detected only des-acyl ghrelin peptides 1–28 (m/z 3,244) and 1–27 (m/z 3,088) (Fig. 1A), suggesting that the cells generate low levels of acylated ghrelin. We reasoned that limiting fatty acid levels in our culture system may preclude ghrelin acylation. Accordingly, we supplemented cell culture media with various levels of octanoic acid and then readily demonstrated the octanoylation of ghrelin 1–28 and 1–27 (Fig. 1B). Additional peptide fragmentation and tandem MS (MS/MS) analyses confirmed that the octanoylation occurs exclusively at serine-3 (data not shown). These TT cell culture conditions provided a useful system for identifying the ghrelin acyl transferase.
Fig. 1.
Generation of octanoylated ghrelin peptides by octanoic acid treatment in TT cells. (A) Ghrelin immunoprecipitation MALDI-TOF MS (IPMS) analyses of TT cell culture media under control conditions. Des-acyl ghrelin 1–28 (m/z 3,244) and 1–27 (m/z 3,088) were observed within 6 days. (B) Exposure of TT cells to octanoic acid (125 μg/ml) induced production of octanoylated ghrelin 1–28 (m/z 3,370) and 1–27 (m/z 3,214) peptides by the cells. Treatment-dependent generation of octanoylated ghrelin peptides is denoted by downward arrows. Ghrelin peptide standards were added at the start of the culture period (m/z 3,187, 3,314, and 3,393 for rat des-acyl ghrelin, rat octanoylated ghrelin, and human SIL octanoylated ghrelin peptides).
Searching for the Acyl Transferase.
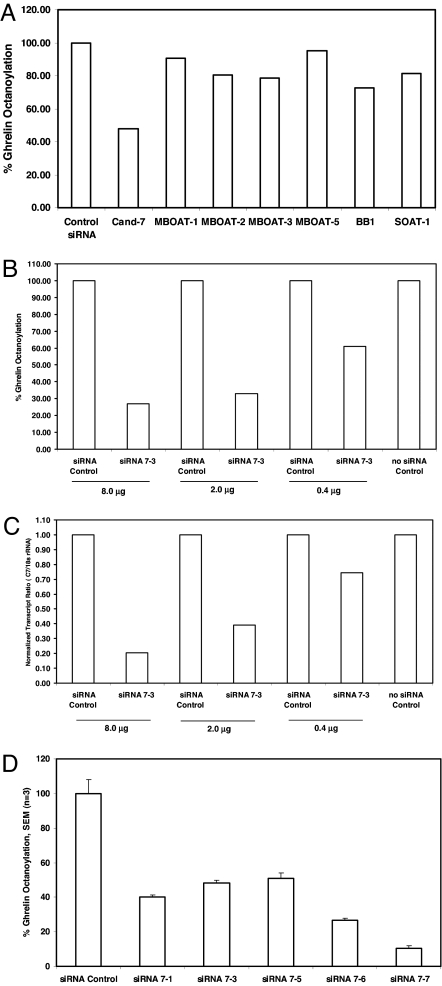
Candidate sequences were selected for gene-silencing experiments according to the following criteria: (i) similarity to known acyltransferase sequences; (ii) presence of a human homologue; and (iii) the function of the gene was unknown. Twelve candidate genes were identified, most of which were orphan MBOATs. Silencing RNAs were generated and used for determination of ghrelin acylation in TT cells. Fig. 2 shows the effects of candidate gene silencing and the accumulation of octanoylated ghrelin (m/z 3,370). Remarkably, TT cell treatment with reagents designed to knock down one of the candidate genes (candidate 7; also described as FKSG89, MBOAT4, or OACT4), but none of the other sequences, including several MBOAT candidates, greatly diminished octanoyl ghrelin synthesis (Fig. 2A). Dose–response studies with siRNA7–3, targeting candidate 7, showed that diminished ghrelin octanoylation levels correlated with knockdown of targeted transcripts (Fig. 2 B and C). Furthermore, exposure of TT cells to five distinct siRNAs targeting the FKSG89 transcript decreased ghrelin octanoylation by ≈50–90% (Fig. 2D). These data defined an essential role for the transcripts targeted by these siRNA reagents in ghrelin acylation.
Fig. 2.
GOAT is essential for ghrelin octanoylation in TT cells. (A) TT cells were exposed to targeting siRNAs (2 μg) specific for candidate 7 (Cand-7), MBOAT-1, MBOAT-2, MBOAT-3, MBOAT-5, human BB1, SOAT-1, or nontargeting control siRNAs and assayed for ghrelin octanoylation by using the ghrelin IPMS assay. Ghrelin octanoylation levels were normalized to cells treated with nontargeting siRNA control. (B) Dose-dependent decrease in octanoylated ghrelin levels in TT cells treated with candidate 7 gene siRNA 7-3. (C) Dose-dependent effects of candidate 7 gene siRNA 7-3 on normalized GOAT transcripts (GOAT/18s rRNA). (D) Exposure of TT cells to targeting siRNAs (2 μg) to five distinct regions of the candidate 7 transcript decrease the levels of octanoylated ghrelin (1–28) relative to siRNA control treatment.
Candidate 7 Is a Member of the MBOAT Family of Proteins.
Candidate 7 encodes an uncharacterized protein with structural motifs present in the MBOAT family of acyltransferases [supporting information (SI) Fig. S1]. The predicted proteins described in the public databases with homology to FKSG89 exhibit large diversity specific for their respective N termini. To ensure authenticity of the human gene for candidate 7, RT-PCR and 5′ RACE reactions were performed with human TT cell mRNA using primers specific for this gene. The 5′ RACE results from TT cells or human stomach mRNA showed only the presence of larger amplification products and not those expected for the FKSG89 gene. Nucleotide analyses of human chromosome 8 (region 8p12) supported the presence of two additional exons upstream of the single-exon FKSG89 gene, which confirmed the full-length transcript that we observed experimentally. We named the predicted protein encoded by the longer transcript of candidate 7 the ghrelin O-acyl transferase, GOAT (Fig. S2).
GOAT Acylates Ghrelin at the Critical Serine-3.
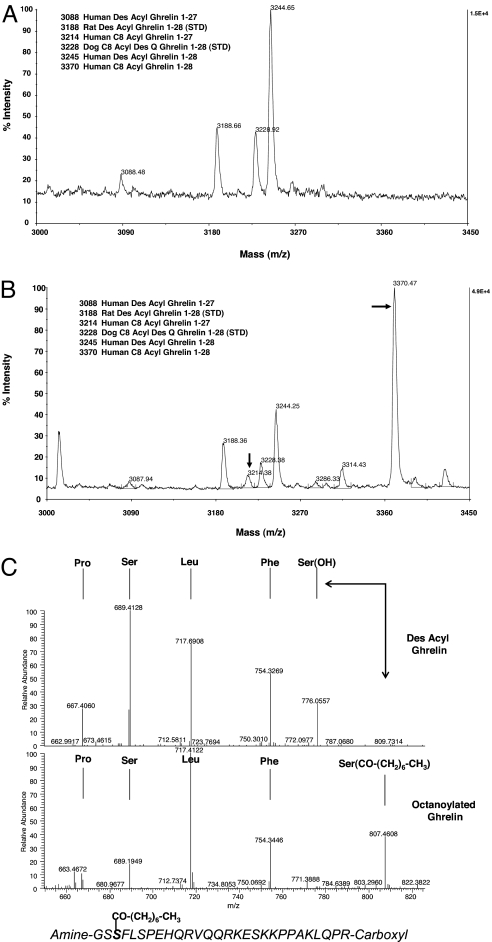
To ascertain whether GOAT can octanoylate ghrelin protein, we performed transient transfections in human embryonic kidney (HEK-293) cells. Media from HEK-293 cells 72 h after transfection with ghrelin alone contained only the des-acyl forms of ghrelin 1–28 and 1–27 (Fig. 3A). Strikingly, cotransfection with GOAT yielded an intense peak at m/z 3,370 and a minor peak at m/z 3,214, corresponding to octanoylated ghrelin 1–28 and 1–27 (Fig. 3B). Further MS fragmentation confirmed that the octanoylation occurs only at serine-3 (Fig. 3C). Interestingly, we also detected minor levels of serine-3 acetylated and serine-3 butyrylated (Fig. 3B, m/z 3,286 and 3,314, respectively) ghrelin peptides, suggesting that GOAT can also use additional fatty acid substrates.
Fig. 3.
GOAT octanoylates ghrelin peptide in HEK-293 cells. (A) HEK-293 cells transiently transfected with human preproghrelin cDNA secreted des-acyl ghrelin peptides 1–28 (m/z 3,244) and 1–27 (m/z 3,088). (B) Transient cotransfection of HEK-293 cells with human preproghrelin and GOAT cDNAs produced principally octanoylated ghrelin peptides 1–28 (m/z 3,370) and 1–27 (m/z 3,214). Ghrelin peptides standards were rat des-acyl ghrelin (m/z 3,188) and dog octanoyl des Q ghrelin (m/z 3,228). (C) MS fragmentation analyses showing the GOAT-mediated octanoyl modification of serine-3 in ghrelin. Immunoprecipitated ghrelin peptides from cotransfected cells were subjected to MS/MS analyses. (Upper) Fragmentation pattern for +5 ions (m/z 649.56) for des-acyl ghrelin denoting the presence of daughter y″ ions up to the unmodified serine-3 residue. (Lower) Fragmentation pattern for +5 ions (m/z 674.78) for octanoylated ghrelin. Daughter y″ ion fragmentation pattern for octanoylated ghrelin shows a shift for the modified serine-3 residue corresponding to the covalent octanoylation of this residue. Arrows denote mass shift at serine-3 for des-acyl and octanoylated ghrelin. The amino acid sequence for human octanoylated ghrelin is shown within the figure.
To determine whether other fatty acids, besides octanoic acid, can be used as substrates by HEK-293 cells in the ghrelin acylation system, we supplemented the media with fatty acids ranging from acetate (C2) to hexadecanoic acid (C16). These studies showed that GOAT can acyl modify the serine-3 residue of ghrelin with fatty acids up to tetradecanoic acid, suggesting that GOAT has greater fatty acid selectivity beyond octanoic and decanoic acid (Fig. S3).
The specificity of GOAT as the acyl transferase for ghrelin and a member of the MBOAT family of proteins was further tested by using the HEK-293 cell system. Of the MBOATs tested only GOAT is capable of acyl modifying ghrelin (Fig. S4). We also wanted to determine whether the MBOAT-conserved histidine residue (GOAT H338) is critical for ghrelin acylation. Alanine replacement of histidine-338 in GOAT completely abolished its ability to octanoylate ghrelin, further supporting the observation that GOAT is a member of the MBOAT family of proteins (Fig. S5).
GOAT Is Conserved Across Vertebrate Animals.
GOAT is predicted to be conserved across vertebrates. To determine whether this conservation was indeed functional, we obtained the GOAT cDNAs for a diverse group of vertebrates, including rat, mouse, and zebrafish, and coexpressed them with ghrelin in HEK-293 cells. These studies showed that the mouse, rat, and zebrafish forms of GOAT can faithfully octanoylate human ghrelin (Fig. S6). Amino acid sequence comparisons for human, mouse, rat, and zebrafish GOAT showed percentage amino acid similarities of ≈90% for human, mouse, and rat GOAT proteins and ≈60% for mammalian and zebrafish GOAT proteins (data not shown).
GOAT Is the Lipid Acyl Transferase for Ghrelin.
GOAT gene knockout mice were generated and used to characterize circulating acyl and des-acyl ghrelin peptides with the ghrelin IPMS assay. Results from these initial studies show the complete absence of octanoylated ghrelin in the blood of GOAT-null mice in contrast to wild-type littermate animals (Fig. 4). These results show that GOAT is the acyl transferase required for the acylation of ghrelin.
Fig. 4.
GOAT gene-null mice lack octanoylated ghrelin in circulation. Blood profiles for acylated and des-acyl ghrelin in either wild-type (Upper) or GOAT gene-disrupted (Lower) mice were determined by using the ghrelin IPMS assay. Arrow denotes location of octanoylated ghrelin. Ghrelin peptide standards were mouse SIL acyl (m/z 3,338) and des-acyl ghrelin (m/z 3,211) peptides, respectively.
Stomach and Pancreas Tissues Express GOAT Transcripts.
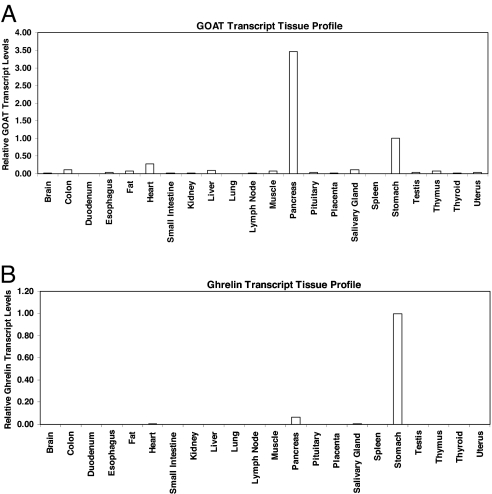
We evaluated the distribution of GOAT by transcript profiling in 48 human tissues. We found that GOAT is a message of relatively low abundance because its measured cycle threshold value was 32.7 for stomach tissue. This is in contrast to ghrelin's transcript cycle threshold measurements of 20.6 in the same tissue (data not shown). However, there are elevated levels of GOAT transcripts in stomach and pancreas and very low levels in most other tissues (Fig. 5A). By comparison, ghrelin showed abundant transcript expression in stomach tissue, modest expression in pancreas, and relatively low levels in most other tissues (Fig. 5B). The concordant expression of both ghrelin and GOAT in stomach agrees with observations that octanoylated ghrelin is principally produced in gastric tissue. In addition, the elevated levels for both GOAT and ghrelin in pancreas are consistent with recent work showing that octanoylated ghrelin regulates insulin secretion (8).
Fig. 5.
GOAT and ghrelin transcript profiles in human tissues. Origene's TissueScan Real-Time 48 human tissue panel was used for these studies. Ghrelin and GOAT relative transcript levels were normalized to β-actin transcript amounts and then calibrated to stomach expression in each profile, which was given an arbitrary value of 1. Relative transcript levels for 22 major tissues are shown. (A) GOAT is expressed mainly in stomach and pancreas human tissues. (B) Ghrelin transcripts were abundantly detected in stomach and modestly in pancreas human tissues.
Discussion
The structure of ghrelin is unprecedented, with a unique octanoyl modification on its serine-3 residue. This modification is absolutely essential for its growth hormone, orexigenic, metabolic, and insulin secretion effects. It is always present on the third hydroxyl-containing residue. In vivo, ghrelin is mainly modified with octanoyl and to lesser degree decanoyl fatty acids. The mechanism responsible for this modification has remained elusive (3,4–5, 15). Because of the essential nature of ghrelin acylation, the enzyme or enzyme complex responsible for this modification has been sought as a key regulatory step. Our discovery of human GOAT describes the metabolic activation of the peptide hormone ghrelin.
Establishment of a ghrelin octanoylation cell culture system was key to our identification of GOAT. Kanamoto et al. (14) reported that TT cells secrete ghrelin into cell culture media. Our supplementation studies with octanoic acid and our stabilization of ghrelin with an antibody specific to the acylated form were crucial. Nevertheless, no more than 10% of ghrelin secreted by our TT cells was acylated, suggesting that acylating machinery is of relatively low abundance in these cells (Fig. 1B).
Gene silencing of GOAT from these cells decreased acyl ghrelin production, indicating that GOAT is essential. This inhibition of ghrelin acylation was achieved with several siRNAs to distinct regions of GOAT, confirming its role in ghrelin acylation. The GOAT gene-silencing effect was dose-dependent and correlated with ghrelin octanoylation levels in TT cells (Fig. 2 B and C). Silencing of other MBOAT members in TT cells had no effect on the octanoylation of ghrelin, implicating GOAT's function specifically in the acylation of ghrelin. Transient coexpression of GOAT and ghrelin in HEK-293 cells recapitulated the secretion of octanoylated ghrelin. HEK-293 cells do not express either GOAT or ghrelin (data not shown). MS/MS fragmentation analyses of GOAT acylated ghrelin shows that the acylation is on serine-3 identical to stomach-produced acyl ghrelin.
GOAT shares structural similarities with members of the MBOAT family of acyl transferases (16–18). When we coexpressed ghrelin with other members of the MBOAT family, including MBOAT1, MBOAT2, MBOAT3, MBOAT5, human-BB1, porcupine, and FKSG89, we were able to achieve ghrelin octanoylation only with GOAT. Moreover, mutating the conserved catalytic histidine residue in GOAT to alanine (H338A) abolished its activity, demonstrating that GOAT is an MBOAT.
GOAT can also acylate ghrelin with other fatty acids, besides octanoate, ranging from acetate to tetradecanoic acid. Peak intensities for acyl-modified forms of ghrelin corresponding to C7 to C12 appear most intense; however, it is important to point out that these data are affected by at least three factors: (i) the uptake of fatty acids by the HEK-293 cells; (ii) the selectivity of the protecting antibody in our assay (to prevent ghrelin desacylation); and (iii) the fatty acid selectivity by GOAT. Nevertheless, these data are relevant because tissue-derived and circulating ghrelin forms are mainly modified with octanoate and decanoate fatty acid residues. Our observations suggest that there is some specific and perhaps unique fatty acid metabolism occurring in the ghrelin-producing gastric X/A cells ensuring that primarily octanoate and decanoate fatty acid substrates are available for GOAT to acylate ghrelin.
GOAT is conserved across vertebrates. Our GOAT and ghrelin coexpression studies show that functional GOAT is also present in rats, mice, and zebrafish. These observations are consistent with the identification of octanoylated forms of ghrelin peptides across vertebrates, including lower vertebrates such as zebrafish (3, 5). Zebrafish GOAT, which shares ≈60% amino acid similarity to human GOAT, is capable of acyl modifying human ghrelin, suggesting that structural domains important for acylation are conserved across the distinct GOAT species. Structure to activity relationship studies for GOAT's function will be needed to define these domains. The ability of zebrafish GOAT to octanoylate human ghrelin also highlights the structural conservation of ghrelin as a substrate for octanoylation by GOAT. Human and zebrafish forms of ghrelin differ in amino acid length and composition. However, they share a 7-aa conserved region in their N termini composed of GSSFLSP and GTSFLSP for human and zebrafish ghrelin, respectively. GOAT may recognize structural aspects of this motif to specifically acyl modify ghrelin.
GOAT is essential for the acylation of ghrelin in mice. In vivo, loss of function of GOAT in mice yields the absence of octanoylated ghrelin, conclusively demonstrating the critical role of GOAT in its acylation (Fig. 5). Future studies with these GOAT-null mice are needed to determine the physiological consequences of the specific deficiency of acylated ghrelin.
Stomach and pancreas tissues express transcripts for ghrelin and GOAT. This concordant transcript expression for GOAT and ghrelin in human stomach and pancreas tissues is consistent with GOAT being the acyltransferase for ghrelin. It is well established that stomach is the principal tissue for acylated ghrelin production and that changes in ghrelin production in this tissue greatly impact fluctuations caused by metabolic adaptation in organisms (19, 20). Acylated ghrelin also regulates insulin secretion; however, the source of acylated ghrelin in pancreas remains controversial. That GOAT is coexpressed with ghrelin in pancreas may indicate that locally produced acylated ghrelin mediates the observed effects on insulin secretion.
Disrupting ghrelin signaling by targeting the ghrelin or ghrelin receptor genes blunts weight gain from a high-fat diet. Specifically, GHSR-null mice eat less food, metabolize more fat, become less adipose, and remain more insulin-sensitive (21). Similarly, ghrelin-null mice resist weight induced by early exposure to diets containing high fat. These mice display decreased adiposity and an increase in energy expenditure (20). Of added interest is the phenotype of the ghrelin and ghrelin receptor double knockout mice, which show decreased body weight, increased energy expenditure, and increased motor activity on a standard chow diet (22). Because octanoylated ghrelin promotes food intake and adiposity and also suppresses insulin secretion and impairs glucose tolerance, GOAT may provide a critical molecular target in developing novel therapeutics for obesity and type 2 diabetes.
Materials and Methods
Reagents.
Rat and dog ghrelin peptides (1–28) were obtained from Phoenix Pharmaceuticals. Stable isotope-labeled (SIL) human ghrelin peptides (1–28) were from Midwest Biotech. Two monoclonal antibodies against ghrelin were used for these studies and prepared at Lilly Research Laboratories. Their specificities were toward the octanoylated N terminus (antibody C2-5A1) and the carboxyl terminus (antibody D4-7.1). Fatty acids and N-octyl glucopyranoside detergent (catalog no. O3757) were from Sigma. TT Cells were from American Type Culture Collection (catalog no. CRL-1803). Cell culture reagents, magnetic beads (catalog no. 142.04), and GripTite 293 MSR Cells (catalog no. R795-07) were from Invitrogen.
Transcript Silencing in TT Cells.
TT cells were cultured in Ham's F12K media (ATCC 30-2004) as described by the supplier. Introduction of specific siRNA complexes into cells was done with the Amaxa Nucleofector II Device using 5 × 106 cells following the manufacturer's recommended protocol. The following five double-stranded RNA-silencing sequences were used to target GOAT transcript: siRNA7-1, 5′-UGU UGC AGA CAU UUG CCU UCU-3′ (siRNA 7-1); siRNA7-3, 5′-AAU GCC UAA ACG UGG CAG UGA-3′ (siRNA 7-3); stealth-1, 5′-CAG AUU CUU GGA CUA GAA UGC CUA A-3′ (siRNA 7-5); stealth-2, 5′-CGG GAC UGA CUG AUU GCC AGC AAU U-3′ (siRNA 7-6); and stealth-3, 5′-AGC UGA CUA CCU GAU UCA CUC CUU U-3′ (siRNA 7-7). As a control, cells were treated with the nontargeting control (NTC-2) siRNA (catalog no. D-001210-02-05 from Dharmacon). Double-stranded silencing RNAs stealth 1–3 were from Invitrogen, and siRNA7-1 and siRNA7-3 (catalog no. 1027020) were custom siRNAs from Qiagen. Cells in TT cell media were allowed to adhere overnight to T-25 tissue culture flasks. Cell media were replaced with TT cell culture media supplemented with 125 μg/ml octanoic acid, 0.4 ng/ml SIL human octanoylated ghrelin, and 10 μg/ml C2-5A1 antibody to prevent ghrelin desacylation. Cells were allowed to incubate for 6 days. After this period, cell media were acidified to 50 mN HCl and stored at −80°C until ready for ghrelin IPMS analyses. Cell pellets were scraped and stored at −80°C until ready for total RNA isolation.
Determination of GOAT Transcript Levels in TT Cells.
GOAT mRNA levels were determined with total RNA by using the RNeasy kit from Qiagen. One microgram of total RNA was converted to cDNA by using a High Capacity cDNA Reverse Transcription kit (Applied Biosystems). Quantitative RT-PCR was performed by employing a standard curve method using a 7900HT instrument (Applied Biosystems). Twenty-microliter PCRs were prepared containing 1× Universal master mix (catalog number 4305719, Applied Biosystems), 0.8 μM custom forward primer (5′-GGCTCTCTGTGCTCCTTCCA-3′), 0.8 μM custom reverse primer (5′-AGAGTGTCTGGGATGCAAAGC-3′), 0.2 μM probe containing a 5′ 6-FAM label with 3′ black hole quencher 1 (BHQ1) (5′ FAM-CTGGACCCTTGAACACGAGCCTGAAA-BHQ1–3′), and 4 μl of template cDNA diluted 1:50 in 10 mM Tris (pH 7.5). Primers and probes were from Biosource International. Ribosomal RNA (rRNA) levels were with the assay from Applied Biosystems (catalog no. 4310893E). PCR conditions for GOAT and 18s rRNA were as follows: 50°C for 2 min, 95°C for 10 min, followed by 40 cycles of 95°C for 15 s and 60°C for 1 min. The data from GOAT were normalized to the 18s rRNA and calibrated relative to nontargeting control.
Ghrelin and GOAT Gene Transient Transfection in Griptite 293 MSR Cells.
The full-length ghrelin cDNA was obtained from Origene (catalog no. TC123546). The 5′ GOAT sequence was obtained with the SMART RACE kit (5′ RACE; catalog no. 634914, Clontech) with TT cell RNA. GOAT transcript specific primers (5′-CCTCCTCTCCAGGGCTCTGACCAAGCTC-3′ and 5′-GGCAGTGCCTTACACACATGCTCAGAC-3′), SMART II A oligo, and the universal primer mix provided with the Smart RACE kit were used as described by the manufacturer. Nucleotide primers 5′-CACCATGGAGTGGCTTTGGCTG-3′ and 5′-TCAGTTACATTTGTGCTTTCTCTTCGCC-3′, designed to encompass the entire coding region for the GOAT cDNA, were used with TT cell cDNA generated with the SMART RACE kit. The amplified GOAT gene was cloned into pcDNA3.2/V5/GW/D-TOPO and sequenced on both strands (Agencourt). Cell transfection studies were performed in Griptite 293 MSR cells by using Mirus transfection reagents. Cells were allowed to adhere overnight to T-25 cell culture flasks in DMEM containing 10% FBS, 0.1 mM MEM nonessential amino acids, and 600 μg/ml geneticin (Griptite 293 MSR growth medium). After overnight incubation, the medium was replaced with the Griptite 293 MSR growth medium supplemented with 125 μg/ml octanoic acid, 0.4 ng/ml SIL human octanoylated ghrelin, and 10 μg/ml C2-5A1 antibody. Cells were allowed to incubate for 3 days. Cell media were acidified to 50 mM HCl and stored at −80°C until ready for ghrelin IPMS analyses.
Ghrelin Immunoprecipitation Reactions.
Monoclonal antibodies (D4-7.1) were covalently coupled to Invitrogen/Dynal magnetic beads following the manufacturer's procedure. Acidified media were extracted on tC18 Sep Pak cartridges (catalog no. WAT036805, Millipore). Peptides were eluted with 60% acetonitrile in 0.1% trifluoroacetic acid and lyophilized. Pellets were suspended in 275 μl of buffer (140 mM Tris·HCl/50 mM Hepes/150 mM NaCl/0.1% N-octyl glucopyranoside, pH 7.5), exposed to ≈1 μg of anti-ghrelin antibody (D4-7.1) bound to magnetic beads and incubated either overnight at 4°C or for 2 h at room temperature. Antibody–antigen complexes were washed (500 μl) twice in 50 mM Tris·HCl, 50 mM Hepes, and 150 mM NaCl (pH 7.5) and twice in distilled water. Immunocomplexes were acidified with 10 μl of a solution of 0.1% trifluoroacetic acid, removed from magnetic beads, and processed by using C18 ZipTips (catalog no. ZTC18S, Millipore). Ghrelin peptides were eluted by using 3.0 μl of 50% acetonitrile/0.1% TFA saturated with α-cyano-4-hydroxy-cinammic acid matrix. A 1-μl volume from each eluate was spotted on target plates as described previously and analyzed by using MALDI-TOF MS (23). We refer to the ghrelin immunoprecipitation reactions combined with the MALDI-TOF MS analysis as the ghrelin IPMS assay.
MALDI-TOF MS.
An Applied Biosciences 4700 MALDI-TOF mass spectrometer (Applied Biosystems) was used for MS analysis under optimized conditions for ghrelin peptide detection.
Transcript Profiling in Human Tissues.
Transcript profiling was performed by using TissueScan Real-Time human cDNA panels (Origene Technologies) according to the manufacturer's protocol. The cDNA-specific gene expression assays were obtained from Applied Biosystems: ghrelin, HS00175082_m1; β-actin, 4310881E. GOAT custom primers and a probe specific for the exon 1 and exon 2 splice junction were designed with Primer Express (Applied Biosystems) and supplied by Applied Biosystems [forward primer, 5′-CCCTTTGCACTTCTCTTCAATTATC; reverse primer, 5′-CGAGCACGGCGTAGGAA; probe, 5′-FAM-TCGTGCCAGGTACCT-MGBNFQ-3′ containing 5′-6-FAM label and 3′-minor groove binding nonfluorescent quencher (MGBNFQ)].
Blood Ghrelin Profiling in GOAT-Null Mice.
GOAT-null mice were generated by Taconic/Artemis using C57BL/6N cells from C57/TacN mice. Blood from homozygous GOAT-null or wild-type littermate animals was collected and immediately mixed 1:1 in a solution of 25 mM EDTA, 1 mg/ml protease inhibitor mixture (catalog no. 11 873 580 001, Roche), 1 M NaCl, and 200 mM HCl to prevent ghrelin desacylation. Ghrelin peptide standards for acyl and des-acyl ghrelin were added at 500 and 250 pg/ml, respectively. The acidified whole blood was ethanol-precipitated (3:1 ethanol:blood ratio), and the resulting supernatants were further ether-precipitated. Pellets were briefly dried, suspended in Tris-Hepes buffer, and processed as described above for ghrelin immunoprecipitation and MS analyses.
Supplementary Material
Acknowledgments.
We thank Dr. David S. Bredt for his constructive comments in the preparation of this manuscript and Drs. Tamer Coskun, Lawrence Gelbert, Niles Fox, and Mark Heiman for discussions and support during the implementation of these studies. We also acknowledge Jeff Arnold, Yue-Wei Qian, Cara Ruble, Brent Sexton, and He Wang for their bioinformatic and technical assistance in this effort.
Note Added in Proof.
While this manuscript was in revision, Yang et al. described a similar observation for GOAT as the acyl transferase for ghrelin (24).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The GOAT sequence reported in this paper has been deposited in the GenBank database [EU518498 (human), EU518496 (rat), EU518495 (mouse), and EU518497 (zebrafish)].
See Commentary on page 6213.
This article contains supporting information online at www.pnas.org/cgi/content/full/0800708105/DCSupplemental.
References
- 1.Kojima M, et al. Ghrelin is a growth-hormone releasing acylated peptide from stomach. Nature. 1999;402:656–660. doi: 10.1038/45230. [DOI] [PubMed] [Google Scholar]
- 2.Cummings DE, Overduin J. Gastrointestinal regulation of food intake. J Clin Invest. 2007;117:13–23. doi: 10.1172/JCI30227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kojima M, Kangawa K. Ghrelin: Structure and function. Physiol Rev. 2005;85:495–522. doi: 10.1152/physrev.00012.2004. [DOI] [PubMed] [Google Scholar]
- 4.Tschop M, Smiley DL, Heiman ML. Ghrelin induces adiposity in rodents. Nature. 2000;407:908–913. doi: 10.1038/35038090. [DOI] [PubMed] [Google Scholar]
- 5.Van Der Lely AJ, Tschop M, Heiman ML, Ghigo E. Biological, physiological, pathophysiological, and pharmacological aspects of ghrelin. Endocr Rev. 2004;25:426–457. doi: 10.1210/er.2002-0029. [DOI] [PubMed] [Google Scholar]
- 6.Monteleone P, Bencivenga R, Longobardi N, Serritella C, Maj M. Differential responses of circulating ghrelin to high-fat or high-carbohydrate meal in healthy women. J Clin Endocrin Metab. 2003;88:5510–5514. doi: 10.1210/jc.2003-030797. [DOI] [PubMed] [Google Scholar]
- 7.Date Y, et al. Ghrelin is present in pancreatic α-cells of humans and rats and stimulates insulin secretion. Diabetes. 2002;51:124–129. doi: 10.2337/diabetes.51.1.124. [DOI] [PubMed] [Google Scholar]
- 8.Dezaki K, et al. Endogenous ghrelin in pancreatic islets restricts insulin release by attenuating Ca2+ signaling in β-cells: Implication in the glycemic control in rodents. Diabetes. 2004;53:3142–3151. doi: 10.2337/diabetes.53.12.3142. [DOI] [PubMed] [Google Scholar]
- 9.Sun Y, Asnicar M, Saha PK, Chan L, Smith RG. Ablation of ghrelin improves the diabetic but not the obese phenotype in ob/ob mice. Cell Metab. 2006;3:379–386. doi: 10.1016/j.cmet.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 10.Wierup N, Yang S, McEvilly RJ, Mulder H, Sundler F. Ghrelin is expressed in a novel endocrine cell type in developing rat islets and inhibits insulin secretion from INS-1 (832/13) cells. J Histochem Cytochem. 2004;52:301–310. doi: 10.1177/002215540405200301. [DOI] [PubMed] [Google Scholar]
- 11.Bednarek MA, et al. Structure-function studies on the new growth hormone-releasing peptide, ghrelin: Minimal sequence of ghrelin necessary for activation of growth hormone secretagogue receptor 1a. J Med Chem. 2000;43:4370–4376. doi: 10.1021/jm0001727. [DOI] [PubMed] [Google Scholar]
- 12.Takada R, et al. Monounsaturated fatty acid modification of Wnt protein: Its role in Wnt secretion. Dev Cell. 2006;11:791–801. doi: 10.1016/j.devcel.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 13.Tanaka K, Kitagawa Y, Kadowaki T. Drosophila segment polarity gene product porcupine stimulates the posttranslational N-glycosylation of wingless in the endoplasmic reticulum. J Biol Chem. 2002;277:12816–12823. doi: 10.1074/jbc.M200187200. [DOI] [PubMed] [Google Scholar]
- 14.Kanamoto N, et al. Substantial production of ghrelin by a human medullary thyroid carcinoma cell line. J Clin Endocrin Metab. 2001;86:4984–4990. doi: 10.1210/jcem.86.10.7891. [DOI] [PubMed] [Google Scholar]
- 15.Kojima M, Ida T, Sato T. Structure of mammalian and nonmammalian ghrelins. Vitam Horm. 2008;77:31–46. doi: 10.1016/S0083-6729(06)77003-0. [DOI] [PubMed] [Google Scholar]
- 16.Hofmann K. A superfamily of membrane-bound O-acyltransferases with implications for wnt signaling. Trends Biochem Sci. 2000;25:111–112. doi: 10.1016/s0968-0004(99)01539-x. [DOI] [PubMed] [Google Scholar]
- 17.Chamoun Z, et al. Skinny hedgehog, an acyltransferase required for palmitoylation and activity of the hedgehog signal. Science. 2001;293:2080–2084. doi: 10.1126/science.1064437. [DOI] [PubMed] [Google Scholar]
- 18.Liang JJ, et al. Overexpression of human diacylglycerol acyltransferase 1, acyl-coa:cholesterol acyltransferase1, or acyl-coa:cholesterol acyltransferase 2 stimulates secretion of apolipoprotein B-containing lipoproteins in McA-RH7777 cells. J Biol Chem. 2004;279:44938–44944. doi: 10.1074/jbc.M408507200. [DOI] [PubMed] [Google Scholar]
- 19.Martos-Moreno GA, Barrios V, Soriano-Guillen L, Argente J. Relationship between adiponectin levels, acylated ghrelin levels, and short term body mass index changes in children with diabetes mellitus type 1 at diagnosis and after insulin therapy. Eur J Endocrinol. 2006;155:757–761. doi: 10.1530/eje.1.02273. [DOI] [PubMed] [Google Scholar]
- 20.Wortley KE, et al. Absence of ghrelin protects against early-onset obesity. J Clin Invest. 2005;115:3573–3578. doi: 10.1172/JCI26003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zigman JM, et al. Mice lacking ghrelin receptors resist the development of diet-induced obesity. J Clin Invest. 2005;115:3564–3572. doi: 10.1172/JCI26002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pfluger PT, et al. Simultaneous deletion of ghrelin and its receptor increases motor activity and energy expenditure. Am J Physiol. 2008;294:G610–G618. doi: 10.1152/ajpgi.00321.2007. [DOI] [PubMed] [Google Scholar]
- 23.Gutierrez JA, et al. Quantitative determination of peptides using matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. BioTechniques. 2005;(June Suppl):13–17. doi: 10.2144/05386su02. [DOI] [PubMed] [Google Scholar]
- 24.Yang J, Brown MS, Liang G, Grishin NV, Goldstein JL. Identification of the acyltransferase that octanoylates ghrelin, an appetite-stimulating peptide hormone. Cell. 2008;132:387–396. doi: 10.1016/j.cell.2008.01.017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.