Abstract
The Saccharomyces cerevisiae protein Bro1p is required for sorting endocytic cargo to the lumen of multivesicular bodies (MVBs). The mammalian ortholog of Bro1p is not known; although Alix, a structurally related protein, supports the topologically similar process of virus budding, functional studies have so far failed to identify a role for Alix in MVB formation. To establish whether Alix or similar protein(s) participate in endosomal sorting, we attached a retroviral peptide that binds Alix to a reporter receptor. This chimera was sorted efficiently away from the early endosome to the lumen of late endocytic compartments. Surprisingly, sorting was not prevented by depleting Alix but instead required the Alix-related protein His domain phosphotyrosine phosphatase (HD-PTP)/His-Domain/Type N23 protein tyrosine phosphatase (PTPN23). Depletion of HD-PTP also reduced transfer of fluid-phase markers and EGF receptor to lysosomes, caused the accumulation of ubiquitinated proteins on endosomal compartments and disrupted the morphogenesis of MVBs. Rescue experiments using an RNAi-resistant version of HD-PTP and HD-PTP mutants demonstrated an essential role for the HD-PTP Bro1 domain, with ESCRT-III binding correlating with full biological activity.
Keywords: Alix, endocytosis, ESCRT
Mitogenic receptor down-regulation involves their internalization and ubiquitin-dependent sorting to the multivesicular body (MVB). The molecular basis for MVB sorting is partly defined, with several evolutionarily conserved, cytosolic endosomal sorting complex required for transport (ESCRT) complexes (ESCRT-0/h, I, II, III) being recruited to the endosome as the MVB forms and then recycled back to the cytosol by the AAA ATPase, VPS4 (1). Factors other than ESCRT complexes have been implicated in MVB formation, including the Saccharomyces cerevisiae protein Bro1p (2), which binds ESCRT-III via its N-terminal Bro1 domain (3) and recruits the deubiquitinating enzyme, Doa4p (4). In contrast to the ESCRT complexes, the conservation of Bro1p function in mammalian cells is unclear.
Cellular components involved in MVB biogenesis support late events during retroviral budding, where they are recruited via conserved peptides within “Late Domains” of viral Gag proteins (5). The p6 Late Domain of HIV binds the ESCRT-I subunit TSG101 via a PTAP peptide and binds the Bro1p-related protein Alix via a YPD-LXXLF motif (6–9). The latter discovery provides indirect evidence that Alix might also contribute to MVB sorting. However, although Alix is structurally related to Bro1p (10–12), localizes to endosomes (13), and binds ESCRT proteins in vitro (6, 7), functional studies to date have failed to reveal such a role (10, 12).
Results and Discussion
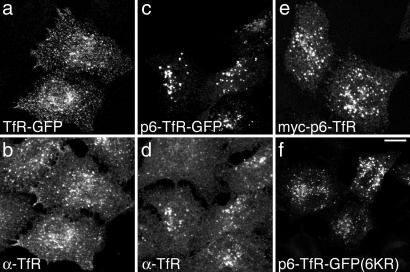
To investigate whether Alix recruitment could support MVB sorting, we first attached HIV p6 to the cytoplasmic domain of transferrin receptor (TfR), which normally cycles between the endosome and the cell surface [see supporting information (SI) Fig. S1 for details of reporters]. Although TfR-GFP distribution was similar to endogenous TfR (Fig. 1 a and b), most p6-TfR-GFP localized to intracellular foci, consistent with relocation to later endocytic compartments (Fig. 1 c and d). Myc-tagged p6-TfR localized to similar structures (Fig. 1e). Levels of TfR chimeras were not high relative to endogenous TfR, judged by the TfR fluorescence signal in transfected cells compared with neighboring cells (Fig. 1) and by Western blot analysis (Fig. S2A). Hence, their trafficking is unlikely to have been influenced by high expression levels. A trivial explanation for the localization of p6-TfR to later endocytic compartments might be that it is recognized as unfolded and subject to ubiquitin-dependent post-Golgi quality control (14). However, ubiquitination of the chimera could not be detected (Fig. S2B). Additionally, mutation of all lysines within p6 and the TfR cytoplasmic tail to arginine, to prevent ubiquitination, did not affect p6-TfR-GFP distribution (Fig. 1f).
Fig. 1.
The HIV p6 Late Domain diverts TfR-GFP from the endocytic recycling pathway. Cells were transfected with TfR-GFP (a and b), p6-TfR-GFP (c and d), myc-p6-TfR (e), or p6-TfR-GFP(6KR) (f) were visualized for GFP fluorescence (a, c, and f) and stained for TfR (b and d) or myc (e). (Scale bar, 10 μm.)
To characterize the compartment containing p6-TfR-GFP, cells were stained for endosomal markers. Although puncti of TfR-GFP colocalized extensively with EEA1 (Fig. S3), far fewer p6-TfR-GFP structures did so. Very little p6-TfR-GFP localized with LAMP1 (Fig. S3). Two well characterized endosomal markers are the lipids LBPA, which localizes mainly to lysosomes and late endosomes (15, 16), and cholesterol, which localizes to MVB [but is also found in other endosomal (15) and biosynthetic compartments]. There was little colocalization between p6-TfR-GFP and LBPA (Fig. S3). In contrast, p6-TfR-GFP consistently colocalized with the cholesterol marker filipin (Fig. S3). These data suggest that at steady state most p6-TfR-GFP is found in a compartment between early endosomes and lysosomes.
Electron microscopy was used to ascertain whether HIV p6 supports inward vesiculation of cargo within these compartments. Most p6-TfR-GFP localized to the lumen of vacuoles (Fig. 2Aa, arrowheads and Table S1). Myc-p6-TfR, epitope tagged on the cytoplasmic domain, also labeled the lumen (Fig. 2Ab, arrowheads and Table S1), consistent with the chimera sorting to this location. The morphology of these vacuoles, with abundant internal vesicles and some additional content, was similar to that of mature MVBs/late endosomes in untransfected cells (data not shown). Their mean diameter (590 ± 77 nm; n = 55) was similar to that of MVBs/late endosomes in control cells (602 ± 92 nm; n = 46). TfR-GFP (Fig. 2Ac) or myc-TfR (data not shown) labeled the plasma membrane, and intracellular labeling predominated on tubules or the limiting membranes of vacuoles containing few or no internal vesicles, consistent with early endosomes (Table S1).
Fig. 2.
p6-TfR is trafficked to the lumen of late endocytic compartments. (A) Cells transfected with p6-TfR-GFP (a), myc-p6-TfR (b), or TfR-GFP (c) were immunolabeled for EM, using antibodies to GFP or myc. Examples of nanogold-enhanced particles labeling endosomes are indicated by arrowheads. (Scale bars, 0.4 μm.) (B) (Left) Cells transfected with myc-p6-TfR were pulse-chased with Alexa594Tf, then fixed and stained with anti-myc. Arrows show examples of colocalization. Asterisks indicate nontransfected cells. (Scale bar, 10 μm.) (Right) Transfected cells treated as above were imaged by wide-field microscopy. Average Tf fluorescent intensities were calculated for each cell [nontransfected (cont) 124 cells; myc-TfR 40 cells; myc-p6-TfR 45 cells]. Values are means ± SEM.
To establish whether p6-TfR is routed to late endocytic compartments from the biosynthetic pathway or is first exposed on the cell surface, its ability to internalize and retain Tf was examined. Cells were pulsed briefly with Alexa594Tf and chased with leupeptin present, to limit degradation of retained Tf. When efficiently recycled from untransfected cells or those containing myc-TfR, Alexa594Tf was retained in cells transfected with myc-p6-TfR (P < 0.0001, Mann–Whitney two-tailed test), much of it colocalizing with myc-p6-TfR (Fig. 2B).
HIV either buds directly from the plasma membrane or is released via a secretory “exosome” pathway after budding into endosomes or endosome-like domains of the plasma membrane (17). These fates do not occur for p6-TfR. First, no p6-TfR-GFP was released from cells (Fig. S4A). Second, incubation of cells with lysosomal protease inhibitors increased levels of p6-TfR-GFP but had little effect on TfR-GFP as judged by Western blotting or by quantifying fluorescence levels (Fig. S4 B and C). Under these conditions, most of p6-TfR-GFP, but not TfR-GFP, localized with LAMP1 (Fig. S4D). Consistent with this, pulse–chase analysis showed that, whereas some TfR-GFP and p6-TfR-GFP were degraded shortly after their biosynthesis (most likely by ER-associated degradation), thereafter p6-TfR-GFP was degraded faster than TfR-GFP (Fig. S4E). In conclusion, p6-TfR-GFP follows a pathway leading to lysosomal degradation. Expression of p6-TfR-GFP did not disrupt trafficking of endogenous MVB cargo, because EGF degradation was unaffected in cells expressing p6-TfR-GFP (Fig. S5). In addition, most compartments containing EGF also contained p6-TfR-GFP, suggesting that p6-TfR-GFP enters newly forming EGF-containing MVBs.
Inhibition of the AAA ATPase VPS4 disrupts ESCRT function and prevents MVB sorting and forward transport of cargo (18). In cells expressing dominant-negative (dn) VPS4, p6-TfR-GFP was found in large vacuoles and clusters (Fig. 3A). Hence, p6-TfR-GFP behaves as a bona fide MVB cargo. HIV p6 contains two peptides (PTAP and LYP-LRSLF), which mediate viral release by recruiting the ESCRT-I subunit, TSG101, and Alix, respectively (6, 8, 9, 19) (Fig. 3B). Mutation of the PTAP motif, which abolished binding of GST-p6 to TSG101 (data not shown) did not affect localization of p6-TfR-GFP (Fig. 3B). In contrast, p6-TfR-GFP lacking a functional LYP-LRSLF motif localized similarly to TfR-GFP (Fig. 3B). Hence, sorting of p6-TfR-GFP correlates with an ability to bind Alix but not TSG101. Effective recruitment of TSG101 by HIV p6 may depend on factor(s) besides the PTAP peptide. One of these factors is most likely ubiquitin, given its ability to increase the avidity of TSG101 for the PTAP peptide (19) and the role of ubiquitination in viral budding (20).
Fig. 3.
Molecular characterization of p6-TfR-GFP sorting. (A) Cells transfected with p6-TfR-GFP and either WT (Upper) or dn (Lower) VPS4B-myc, were immunostained with anti-myc. (B) (Upper) diagram of p6-TfR-GFP indicating the PTAP and LYP-LRSLF motifs. (Lower) Cells were transfected with p6-TfR-GFP (Left), or with p6-TfR-GFP containing mutations in the LYP-LRSLF (Center) or PTAP (Right) motifs as indicated. (C) Cells depleted for Alix were transfected with p6-TfR-GFP, incubated with lysosomal inhibitors, then labeled for EEA1 (Upper) or LAMP1 (Lower). Arrows indicate example positions of p6-TfR-GFP structures that lack EEA1 and those that contain LAMP1. (Scale bar, 10 μm.)
Surprisingly, in the light of this correlation, conditions that depleted Alix to essentially undetectable levels (Fig. S6A) did not substantially alter the distribution of p6-TfR-GFP (data not shown) or prevent p6-TfR-GFP being sorted away from early endosomes and accumulating in lysosomes when its degradation was prevented (Fig. 3C). Likewise, Alix depletion only modestly affected EGF trafficking (Fig. S6 B and C; P = 0.007, Student's paired test, two-tailed). In contrast to the effect of ESCRT-I loss (21, 22), EGF did not accumulate within early endosomes, and there was no increase in the endosomal pool of ubiquitinated proteins (data not shown). Previous studies failed to detect an effect of Alix depletion on EGF degradation (10, 12). These findings, together with the continued trafficking of p6-TfR, suggest that other factors may account for the Bro1p-related activity at the endosome or at least compensate for the loss of Alix.
Mammalian cells contain another Bro1p-like protein, His domain phosphotyrosine phosphatase (HD-PTP)/His-Domain/Type N23 protein tyrosine phosphatase (PTPN23) (23). Like Alix, HD-PTP possesses a Bro1 domain and binds ESCRTs in vitro (24) but otherwise remains largely uncharacterized. The predicted secondary structure of HD-PTP suggests that the V domain of Alix, which binds the LYP-LRSLF motif within HIV p6 (8, 9), is also conserved (Fig. S7). When HA-tagged HD-PTP was coexpressed with dn VPS4, it was found to be clustered (Fig. S8) and to overlap partially with VPS4, EEA1, and LAMP1 (Fig. 4A). In contrast, HD-PTP was largely cytosolic when expressed alone (data not shown) or with WT VPS4 (Fig. S8). This behavior is consistent with that of a Class E vps protein and suggests that HD-PTP may be involved in the ESCRT pathway. Indeed, upon silencing HD-PTP expression (Fig. S9 A and B), p6-TfR-GFP localized in clusters closely associated with EEA1 (Fig. 4B), suggesting that its forward trafficking was impaired. No additional phenotype was observed upon codepleting Alix with HD-PTP (data not shown).
Fig. 4.
HD-PTP is essential for endocytic trafficking. (A) Cells were transfected with HA-tagged HD-PTP and dn VPS4B-myc, then immunostained as indicated. (B) Cells treated with HD-PTP RNAi were transfected with p6-TfR-GFP, incubated with lysosomal inhibitors, and imaged for GFP or EEA1 staining. (C) Cells depleted of lamin A or HD-PTP were pulse-labeled with 125I EGF and chased, then assayed for EGF degradation (squares, solid lines), recycling (triangles, dotted lines), or cellular retention (circles, dashed lines). Values are means ± SEM from three experiments, each performed in duplicate. (D) Cells depleted of lamin A or HD-PTP and transfected with HA-Ubiquitin (Center Right) were pulse-chased with Alexa555EGF and stained as indicated. Arrowheads highlight colocalization of EGF with markers, an arrow highlights an example of HA-Ub positive structure lacking EGF. (E) Cells depleted as indicated were incubated with EGF for 10 min, washed to remove surface label, and fixed. (Scale bars, 10 μm.).
To ascertain whether HD-PTP silencing affected other MVB cargo, we measured 125I-EGF degradation. In these experiments, 125I-EGF was internalized briefly, and the cell surface label was removed by a low pH wash before a subsequent chase to focus on the fate of the endosomal pool of 125I-EGF (Fig. 4C). There was substantially reduced 125I-EGF degradation (P = 0.0015, Student's paired test, two-tailed) and increased intracellular retention of 125I-EGF (P = 0.05) compared with control cells. Separate experiments indicated that depletion of HD-PTP caused a modest inhibition of 125I-EGF uptake and/or slightly enhanced fast recycling (data not shown).
Internalized EGF localized to EEA1-positive clusters and remained in these for at least 3 h (Fig. 4D Left). In contrast to control cells (Fig. 4D Center), HD-PTP depleted cells expressing HA-Ub (Fig. 4D Right) showed intense labeling for HA over EGF clusters, which also labeled for endogenous ubiquitinated proteins (data not shown). Ubiquitinated proteins also accumulated on late endocytic compartments (data not shown). Shorter uptake times confirmed that EGF was internalized in HD-PTP depleted cells, although its localization was more peripheral than in control cells (Fig. 4E). Hence, depletion of HD-PTP, like that of ESCRT-I (25), causes ubiquitinated proteins to accumulate on aberrant endosomes. Overexpression of HA-Ub did not overcome the arrest in EGF trafficking, indicating that it was not merely a consequence of depleted levels of free ubiquitin.
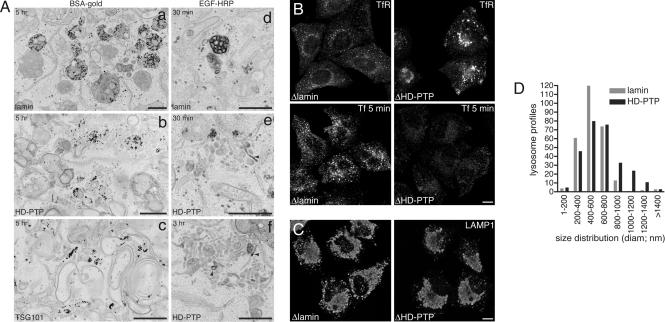
A major role for ESCRTs is generating intralumenal vesicles within the MVBs. However, some ESCRTs may also have a broader function in organizing the early endosome within mammalian cells (21, 22). Loss of HD-PTP also dramatically affected the structural organisation of endosomes, giving rise to defects in the passage of both cargo receptors and fluid phase markers. Although internalized BSA-gold localized almost exclusively to lysosomes after a chase in control cells, it failed to do so in cells depleted of HD-PTP, and instead accumulated in striking membrane clusters (Fig. 5A a and b and Table S2). These were reminiscent of the aberrant endosomes formed upon depletion of the ESCRT-1 subunit TSG101 (Fig. 5Ac), although the stacked cisternae (22) and elongated linear tubules (21) characteristic of TSG101 depletion were absent. This general similarity cannot be explained by a secondary loss of ESCRT-I upon silencing of HD-PTP, because TSG101 levels were unaffected (data not shown).
Fig. 5.
HD-PTP is essential for maintaining early endosome morphology and function. (A) Cells depleted of lamin A (a and d), HD-PTP (b, e, and f), or TSG101 (c) were pulse-chased with BSA-15 nm gold (a–c) or HRP-EGF (d–f) as indicated, then processed for EM. Arrowheads indicate regions of HRP-EGF concentration. (Scale bar, 600 nm.) (B) (Upper) RNAi-treated cells were immunostained with anti-TfR. (Lower) Cells were pulsed with Alexa594Tf and fixed. Identical acquisition settings were used for each sample. (Scale bar, 10 μm.) (C) RNAi-treated cells were fixed and stained for LAMP1. (Scale bar, 10 μm.) (D) Diameters of 280 lysosomes from RNAi-treated cells were measured and grouped into the size ranges indicated.
Clusters contained an array of tubules and small vacuolar elements. Vacuoles often possessed some intralumenal content, although were not as electron dense as late endosomes in control cells. Hence, inward invagination of membrane appeared reduced but not completely prevented. A further characteristic of HD-PTP depletion was a reduction in the volume density of MVBs from 0.45% to 0.13% (Table S2). To examine cargo sorting, cells were pulse-labeled with HRP-EGF (Fig. 5A d–f). In control cells, HRP-EGF passed through endosomes containing abundant internal vesicles en route to lysosomes. In HD-PTP depleted cells, HRP-EGF localized to clusters and the amount of MVBs containing HRP-EGF was reduced by 94%. HRP-EGF remained in clusters during a 3-h chase; indeed, the volume density of clusters containing HRP-EGF increased (data not shown). HRP-EGF was often concentrated within selected, tubular regions of clusters (Fig. 5A e and f, arrowheads). The impairment of EGF segregation into the vacuoles and the failure of these vacuoles to develop fully and to separate away from tubular domains point to a role for HD-PTP in endosomal sorting and MVB biogenesis.
Loss of endosomal organisation was accompanied by redistribution of TfR into endosomal clusters and reduced uptake of Alexa594Tf, most likely because of a diminution of the surface pool of TfR (Fig. 5B). Such a failure to recycle cargo would explain why p6-TfR-GFP was found associated with clustered EEA1 upon depleting HD-PTP. Later endocytic compartments were also affected by HD-PTP depletion. LAMP1-labeled structures appeared larger by immunofluorescence (Fig. 5C). By EM analysis, although the volume density of late endosomes/lysosomes increased only slightly (Table S2), their size distribution altered significantly (Fig. 5D; P < 0.0001, Mann–Whitney 2-tailed test).
When cells treated with HD-PTP RNAi were transfected with an HA-tagged RNAi-resistant version of HD-PTP (Fig. S9C), but not a control vector (data not shown), they fully recovered their ability to sort endocytosed cargo, as judged by the rescue of the EGF clustering phenotype and reversion to WT ubiquitin staining patterns (Fig. S10, Table 1, and Table S3). Rescued cells could also sort p6-TfR-GFP to lysosomes (Fig. S11). Such rescue experiments allowed dissection of HD-PTP function.
Table 1.
Molecular dissection of HD-PTP function
| Construct | n | WT EGF |
Clustered EGF |
||
|---|---|---|---|---|---|
| Trans | Untrans | Trans | Untrans | ||
| FL HD-PTP | 6 | 99 ± 2 | 18 ± 5 | 1 ± 2 | 82 ± 5 |
| L202 | 4 | 88 ± 8 | 25 ± 12 | 12 ± 8 | 75 ± 12 |
| L202/I206 | 4 | 91 ± 7 | 28 ± 13 | 9 ± 7 | 72 ± 13 |
| Bro1 | 4 | 17 ± 5 | 9 ± 3 | 83 ± 5 | 91 ± 3 |
| Bro1-V | 7 | 85 ± 11 | 14 ± 5 | 15 ± 11 | 86 ± 5 |
| Bro1-V L202 | 3 | 77 ± 15 | 11 ± 3 | 23 ± 15 | 89 ± 3 |
| Bro1-V L202/I206 | 3 | 43 ± 6 | 7 ± 7 | 57 ± 6 | 93 ± 7 |
| ΔBro1 | 2 | 22 | 17 | 78 | 83 |
Cells depleted of HD-PTP were transfected with RNAi-resistant full-length (FL) HA-tagged HD-PTP or the indicated mutant. Bro1 refers to the Bro1 domain alone, and Bro1-V to the Bro1 and V domains alone. Cells were scored for the accumulation of fluorescent EGF in clusters or WT patterns of residual EGF staining. Neighboring untransfected cells (Untrans) from each sample were also scored. The number of experimental repeats is indicated. Values are means ± SD where appropriate.
HD-PTP binds CHMP4B via its Bro1 domain, and TSG101 via a central domain that contains two PS/TAP motifs and other proline-rich sequences (24). The CHMP4B binding site within Alix, but not its TSG101 binding site, is essential for its ability to drive HIV budding (8). To ascertain whether ESCRT binding correlates with HD-PTP function, we first examined the effects of deleting domains in RNAi rescue experiments. HD-PTP lacking the Bro1 domain could not restore function (Table 1, Table S3, and Fig. S11), and neither could the Bro1 domain alone (Table 1, Table S3, Fig. S10, and Fig. S11). Surprisingly, HD-PTP lacking the entire proline-rich region and the C-terminal PTPase domain was able to restore EGF trafficking close to levels observed with full-length HD-PTP (Table 1) and largely restore p6-TfR-GFP sorting (Fig. S11). Many RNAi-treated cells transfected with HD-PTP Bro1-V showed a WT distribution of ubiquitin, and, in those cells with labeling of cytoplasmic structures, the phenotype was milder than that observed in untransfected cells (Table S3). The V domain alone did not rescue loss of HD-PTP (data not shown). Therefore, the Bro1-V domain is a minimal functional unit within HD-PTP, at least under these experimental conditions.
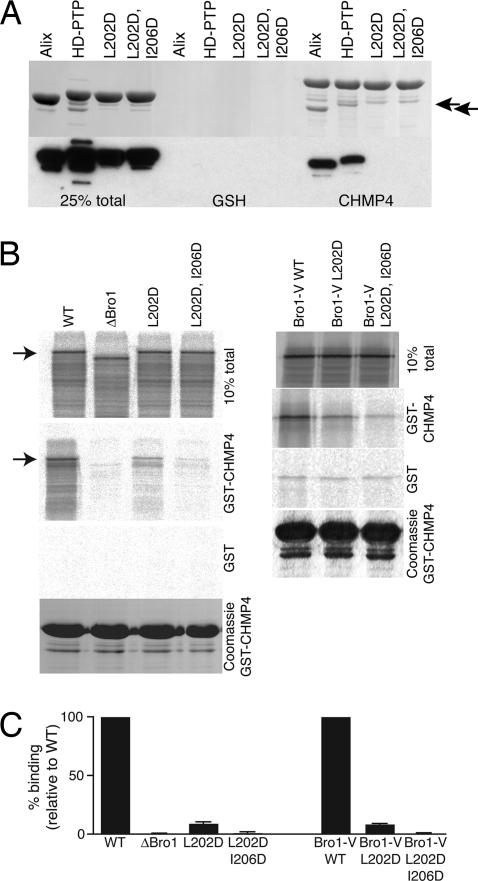
Having identified the importance of the Bro1 domain, we next addressed whether ESCRT-III binding is required for HD-PTP function. CHMP4B binds to a hydrophobic pocket within Alix Bro1, in which Ile-212 is essential (8). We mutated the equivalent hydrophobic residue in HD-PTP (Leu-202) to Asp. A neighboring conserved hydrophobic residue, Ile-206, was also mutated to Asp to generate a double mutant. As expected, the Bro1 domain of HD-PTP, like that of Alix Bro1, binds directly to GST-CHMP4B, and this binding is abolished in both HD-PTP Bro1(L202D) and HD-PTP Bro1(L202D, I206D) (Fig. 6A). To further establish that this region is the only CHMP4B binding site within HD-PTP, full-length protein and mutants were translated in vitro and used in binding assays (Fig. 6 B and C). These assays confirmed that the Bro1 domain is essential for CHMP4B binding. Surprisingly, however, HD-PTP(L202D) retained significant and reproducible CHMP4B binding activity (10% of WT), although this was reduced to 1% of WT in HD-PTP(L202D, I206D). This was also the case when binding was performed by using an in vitro translated Bro1-V construct (Fig. 6 B and C). It is possible that accessory factors within the reticulocyte lysate stabilize the interaction between HD-PTP and CHMP4B. Alternatively, the V domain may stabilize CHMP4B binding sufficiently to allow Bro1-V to tolerate a single amino acid change, whereas the isolated Bro1 domain cannot. The Bro1 and V domains within Alix form a structural unit, although the V domain is oriented at some distance from the CHMP4B binding site (8).
Fig. 6.
Molecular dissection of HD-PTP Bro1 binding. (A) His-tagged bacterially expressed Bro1 domains of Alix, HD-PTP or HD-PTP (L202D) or (L202D/I206D) mutants were incubated with GSH beads preloaded with GST-CHMP4B or control beads. After binding, samples were analyzed by Coomassie (Upper) or Western blot with anti-His (Lower). Arrows indicate positions of bound Alix and HD-PTP Bro1 domains. CHMP4B band and minor CHMP4B degradation products are present. (B) In vitro translated HD-PTP, the indicated mutants (Left), or the Bro1-V domain and indicated mutants (Right) were incubated with GSH beads preloaded with GST or GST-CHMP4B. Loading controls for GST-CHMP4B binding are shown below. Arrows indicate the position of full-length HD-PTP. (C) Data from binding translated constructs. Values for each mutant are expressed relative to the binding of either WT full-length HD-PTP or the WT Bro1-V construct, after subtracting binding to GST controls. Values are means of three experiments ± SD.
To test the importance of ESCRT-III binding for HD-PTP function, RNAi-depleted cells were transfected with either HD-PTP(L202D) or HD-PTP(L202D, I206D). Surprisingly, both mutants were able to rescue EGF sorting, and ubiquitin distribution, close to WT levels, although minor accumulation of ubiquitinated proteins on cytoplasmic structures was found (Table 1 and Table S3). These data suggest that ESCRT-III binding is not obligatory for HD-PTP function in the context of the full-length protein, and indicate that the Bro1 domain has other essential function(s). The same mutations were then made within the Bro1-V domain fusion. In this case, Bro1-V(L202D) could restore HD-PTP function quite effectively, whereas Bro1-V(L202D, I206D) was substantially impaired (Table 1 and Table S3). Hence, in the absence of C-terminal residues that include ESCRT-I binding sites but that may also contain other compensating activities, HD-PTP function is lost when ESCRT-III binding is severely disrupted. None of the above HD-PTP mutants appeared to exert a dominant-negative effect on endosome function (data not shown).
The aim of this study was to establish whether mammalian proteins related to S. cerevisiae Bro1p are involved in MVB sorting. The most likely candidate was Alix, based on its pattern of molecular interactions and ability to support virus budding (6, 7). However, although attachment of a peptide capable of binding Alix diverts TfR to the MVB pathway, sorting of this chimera appears not to strictly depend on Alix. Depletion of Alix also affected EGF trafficking only moderately. Instead, transport of both cargoes was profoundly affected upon knockdown of HD-PTP. We do not know whether HD-PTP supports p6-TfR trafficking directly, because we have been unable to detect binding between HD-PTP and p6 [Alix has a moderate affinity for HIV p6 (8, 9), and HD-PTP may bind p6 with too low an affinity for us to detect.] However, it would be interesting to know whether HD-PTP influences retroviral budding. In conclusion, our findings suggest that HD-PTP is a key regulator of endocytic trafficking in which ESCRT-III binding is important but not strictly essential.
Materials and Methods
All experiments used standard procedures. The following protocols are provided in SI Materials and Methods: cell culture and transfection, generation of cDNA constructs and of all mutations, RT-PCR and Western blot analysis of RNAi depletions, the description of all membrane trafficking experiments and a description of all fluorescence and electron microscopical procedures, the generation of recombinant proteins and the generation of in vitro translation products, biochemical analysis of Bro1-CHMP4B binding.
Supplementary Material
Acknowledgments.
We thank our colleagues for their generous gifts of reagents and Martin Lowe for discussions and for reviewing the manuscript. This work was supported by Medical Research Council Grants G9722026 and G0001128.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. W.I.S. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/cgi/content/full/0707601105/DCSupplemental.
References
- 1.Hurley JH, Emr SD. The ESCRT complexes: Structure and mechanism of a membrane-trafficking network. Annu Rev Biophys Biomol Struct. 2006;35:277–298. doi: 10.1146/annurev.biophys.35.040405.102126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Odorizzi G, Katzmann DJ, Babst M, Audhya A, Emr SD. Bro1 is an endosome-associated protein that functions in the MVB pathway in Saccharomyces cerevisiae. J Cell Sci. 2003;116:1893–1903. doi: 10.1242/jcs.00395. [DOI] [PubMed] [Google Scholar]
- 3.Kim J, et al. Structural basis for endosomal targeting by the Bro1 domain. Dev Cell. 2005;8:937–947. doi: 10.1016/j.devcel.2005.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Richter C, West M, Odorizzi G. Dual mechanisms specify Doa4-mediated deubiquitination at multivesicular bodies. EMBO J. 2007;26:2454–2464. doi: 10.1038/sj.emboj.7601692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Morita E, Sundquist WI. Retrovirus budding. Ann Rev Cell Dev Biol. 2004;20:395–425. doi: 10.1146/annurev.cellbio.20.010403.102350. [DOI] [PubMed] [Google Scholar]
- 6.Strack B, Calistri A, Craig S, Popova E, Gottlinger HG. AIP1/ALIX is a binding partner for HIV-1 p6 and EIAV p9 functioning in virus budding. Cell. 2003;114:689–699. doi: 10.1016/s0092-8674(03)00653-6. [DOI] [PubMed] [Google Scholar]
- 7.von Schwedler UK, et al. The protein network of HIV budding. Cell. 2003;114:701–713. doi: 10.1016/s0092-8674(03)00714-1. [DOI] [PubMed] [Google Scholar]
- 8.Fisher RD, et al. Structural and biochemical studies of ALIX/AIP1 and its role in retrovirus budding. Cell. 2007;128:841–852. doi: 10.1016/j.cell.2007.01.035. [DOI] [PubMed] [Google Scholar]
- 9.Lee S, Joshi A, Nagashima K, Freed EO, Hurley JH. Structural basis for viral late-domain binding to Alix. Nat Struct Mol Biol. 2007;14:194–199. doi: 10.1038/nsmb1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cabezas A, Bache KG, Brech A, Stenmark H. Alix regulates cortical actin and the spatial distribution of endosomes. J Cell Sci. 2005;118:2625–2635. doi: 10.1242/jcs.02382. [DOI] [PubMed] [Google Scholar]
- 11.Schmidt MHH, et al. Alix/AIP1 antagonizes epidermal growth factor receptor downregulation by the Cbl-SETA/CIN85 complex. Mol Cell Biol. 2004;24:8981–8993. doi: 10.1128/MCB.24.20.8981-8993.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bowers K, et al. Degradation of endocytosed epidermal growth factor and virally-ubiquitinated MHC class I is independent of mammalian ESCRTII. J Biol Chem. 2006;281:5094–5105. doi: 10.1074/jbc.M508632200. [DOI] [PubMed] [Google Scholar]
- 13.Welsch S, et al. Ultrastructural analysis of ESCRT proteins suggests a role for endosome-associated tubular-vesicular membranes in ESCRT function. Traffic. 2006;7:1551–1566. doi: 10.1111/j.1600-0854.2006.00489.x. [DOI] [PubMed] [Google Scholar]
- 14.Benharouga M, Haardt M, Kartner N, Lukacs GL. COOH-terminal truncations promote proteasome-dependent degradation of mature cystic fibrosis transmembrane conductance regulator from post-Golgi compartments. J Cell Biol. 2001;153:957–970. doi: 10.1083/jcb.153.5.957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mobius W, et al. Recycling compartments and the internal vesicles of multivesicular bodies harbor most of the cholesterol found in the endocytic pathway. Traffic. 2003;4:222–231. doi: 10.1034/j.1600-0854.2003.00072.x. [DOI] [PubMed] [Google Scholar]
- 16.White IJ, Bailey LM, Aghakhani MR, Moss SE, Futter CE. EGF stimulates annexin 1-dependent inward vesiculation in a multivesicular endosome subpopulation. EMBO J. 2006;25:1–12. doi: 10.1038/sj.emboj.7600759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Deneka M, Pelchen-Matthews A, Byland R, Ruiz-Mateos E, Marsh M. In macrophages, HIV-1 assembles into an intracellular plasma membrane domain containing the tetraspanins CD81, CD9, and CD53. J Cell Biol. 2007;177:329–341. doi: 10.1083/jcb.200609050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Babst M, Wendland B, Estapa EJ, Emr SD. The Vps4p AAA ATPase regulates membrane association of a Vps protein complex required for normal endosome function. EMBO J. 1998;17:2982–2993. doi: 10.1093/emboj/17.11.2982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Garrus JE, et al. Tsg101 and the vacuolar protein sorting pathway are essential for HIV-1 budding. Cell. 2001;107:1–20. doi: 10.1016/s0092-8674(01)00506-2. [DOI] [PubMed] [Google Scholar]
- 20.Strack B, Calistri A, Accola MA, Palu G, Gottlinger HG. A role for ubiquitin ligase recruitment in retrovirus release. PNAS. 2000;97:13063–13068. doi: 10.1073/pnas.97.24.13063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Razi M, Futter CE. Distinct roles for Tsg101 and Hrs in multivesicular body formation and inward vesiculation. Mol Biol Cell. 2006:3469–3483. doi: 10.1091/mbc.E05-11-1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Doyotte A, Russell MRG, Hopkins CR, Woodman PG. Depletion of TSG101 forms a mammalian “Class E” compartment: A multicisternal early endosome with multiple sorting defects. J Cell Sci. 2005;118:3003–3017. doi: 10.1242/jcs.02421. [DOI] [PubMed] [Google Scholar]
- 23.Toyooka S, et al. HD-PTP: A novel protein tyrosine phosphatase gene on human chromosome 3p21.3. Biochem Biophys Res Commun. 2000;278:671–678. doi: 10.1006/bbrc.2000.3870. [DOI] [PubMed] [Google Scholar]
- 24.Ichioka F, et al. HD-PTP and Alix share some membrane-traffic related proteins that interact with their Bro1 domains or proline-rich regions. Arch Biochem Biophys. 2007;457:142–149. doi: 10.1016/j.abb.2006.11.008. [DOI] [PubMed] [Google Scholar]
- 25.Bishop N, Horman A, Woodman P. Mammalian class E vps proteins recognize ubiquitin and act in the removal of endosomal protein-ubiquitin conjugates. J Cell Biol. 2002;157:91–102. doi: 10.1083/jcb.200112080. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.