Abstract
Scar formation occurs during the late stages of the inflammatory response but, when excessive, produces fibrosis that can lead to functional and structural damage of tissues. Here, we show that the profibrogenic agonist, transforming growth factor β1, transcriptionally decreases expression of Exchange protein activated by cAMP 1 (Epac1) in fibroblasts/fibroblast-like cells from multiple tissues (i.e., cardiac, lung, and skin fibroblasts and hepatic stellate cells). Overexpression of Epac1 inhibits transforming growth factor β1-induced collagen synthesis, indicating that a decrease of Epac1 expression appears to be necessary for the fibrogenic phenotype, an idea supported by evidence that Epac1 expression in cardiac fibroblasts is inhibited after myocardial infarction. Epac and protein kinase A, a second mediator of cAMP action, have opposite effects on migration but both inhibit synthesis of collagen and DNA by fibroblasts. Epac is preferentially activated by low concentrations of cAMP and stimulates migration via the small G protein Rap1 but inhibits collagen synthesis in a Rap1-independent manner. The regulation of Epac expression and activation thus appear to be critical for the integration of pro- and anti-fibrotic signals and for the regulation of fibroblast function.
Keywords: exchange protein activated by cyclic AMP, fibrosis, protein kinase A, tissue remodeling
Tissue fibrosis in the late stages of inflammation leads to organ damage and loss of function but is largely untreatable (1). Fibrosis results from the conversion of resting fibroblasts to profibrogenic myofibroblasts in response to certain growth factors, e.g., transforming growth factor (TGF) β1, and hormones, such as angiotensin II and endothelin (2).
The cAMP pathway is a potential target to blunt fibrosis: Increases in cAMP levels can decrease fibroblast function after cell injury and inhibit fibroblast-to-myofibroblast conversion (3), which accompanies repair after injury, including ischemic death (e.g., myocardial infarction) (2). The action of cAMP in eukaryotic cells was thought to occur exclusively via activation of protein kinase A (PKA) and PKA-mediated changes in protein expression and function (4, 5), but Epac, a guanine nucleotide exchange protein that regulates the activity of the G proteins Rap1 and Rap2, also mediates responses to cAMP (6–9). Epac has been implicated in a variety of cellular responses, including proliferation, differentiation, secretion, polarization and ion transport (6, 7, 10, 11). However, neither the regulation of Epac expression nor the contribution of Epac to pathophysiological processes is well defined.
In the heart, fibroblasts and extracellular matrix derived therefrom form a framework upon which cardiac myocytes contract and the conversion of fibroblasts to myofibroblasts is an important event in repair after myocardial infarction and other injuries (2, 12). Intracellular cAMP levels evoke numerous cellular responses after cardiac injury (13, 14). The role of Epac in cardiac injury and repair is not known, although Epac activation can influence other aspects of cardiac function (15). Here, we show that profibrotic agents inhibit Epac expression and that Epac plays a key role in the integration of pro- and anti-fibrotic responses.
Results
Profibrogenic Agonists Attenuate Epac1 Expression.
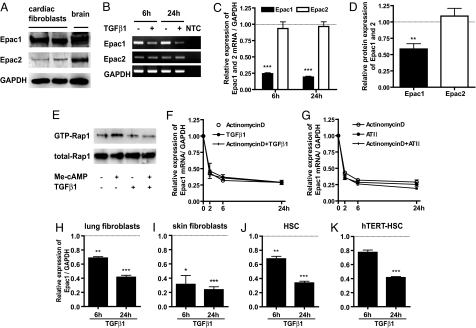
Two forms of Epac (Epac1 and Epac2) have been identified in a variety of cell types (8); passage 1 cardiac fibroblasts more abundantly express Epac1 than Epac2 (Fig. 1A). The fibrogenic agonist TGFβ1 promotes conversion of resting fibroblasts to profibrotic myofibroblasts (3), prominently decreasing expression of Epac1 (but not Epac2) mRNA and protein (Fig. 1 B–D and F) and not that of PKA catalytic subunits α and β mRNA (data not shown). Incubation with TGFβ1 negates an increase in Rap1 activity promoted by Me-cAMP [8-(4-chlorophenylthio)-2′-O-methyl-cAMP, an Epac-selective agonist] (Fig. 1E). The G protein-coupled receptor (GPCR) agonist angiotensin II also inhibits Epac1 mRNA expression (Fig. 1G). Actinomycin D, TGFβ1 and angiotensin II produce similar rates of decay in Epac1 mRNA expression, implicating inhibition of transcription, and not enhanced RNA degradation, in TGFβ1- and angiotensin II-promoted decrease in Epac1 mRNA (Fig. 1 F and G). TGFβ1 decreases Epac1 mRNA expression in multiple types of fibroblasts: rat cardiac, lung, and skin fibroblasts; mouse hepatic stellate cells; and a human hepatic stellate cell line (Fig. 1 H–K). Thus, inhibition of expression of Epac1 occurs in responses to profibrotic activation of fibroblasts from multiple tissues and represents a type of cross-talk between tyrosine kinase receptors (e.g., TGFβ1) and GPCR signaling via cAMP. Our subsequent experiments focused on cardiac fibroblasts.
Fig. 1.
Profibrogenic agents attenuate Epac1 expression. (A) Expression of Epac1 and Epac2 protein in rat cardiac fibroblasts (passage 1) grown for 48 h in serum-free media and examined by immunoblot analysis. Freshly isolated rat brain tissue was also blotted. GAPDH was used to calibrate protein loading. (B) Time-dependent changes in the mRNA expression of Epac1, Epac2, and GAPDH in cardiac fibroblasts treated with or without 10 ng/ml TGFβ1. Transcripts from quantitative RT-PCR were analyzed by 2% agarose gel electrophoresis and ethidium bromide staining and were detected as clear bands of the expected length. No template control (NTC) had no amplification. (C) Quantitative RT-PCR standardized to GAPDH was used to examine time-dependent changes in the mRNA expression of Epac1 and Epac2 in cardiac fibroblasts treated with 10 ng/ml TGFβ1 or 100 nM angiotensin II. Data are the fold-increase vs. control for each time-course. n = 4–6; ***, P < 0.001 compared with control for each time-course. (D) Densitometry of the blots for Epac1 and Epac2 protein normalized to GAPDH protein expression. Cardiac fibroblasts were incubated with TGFβ1 (10 ng/ml) for 16–24 h after 48 h serum-starvation. n = 4; **, P < 0.01. (E) Cardiac fibroblasts, serum-starved for 48 h, were incubated with or without TGFβ1 (10 ng/ml) for 24 h and then with Me-cAMP (50 μM) for 15min. Cells were lysed and assayed for Rap1 activation. Precipitates (Top) and total cell lysates (Middle) were analyzed by immunoblotting with an anti-Rap1 antibody. (F and G) Expression of Epac1 mRNA in cardiac fibroblasts treated with actinomycin D (5 μg/ml), TGFβ1 (10 ng/ml) or angiotensin II (ATII, 100 nM), using quantitative RT-PCR standardized to GAPDH. n = 4–6. (H–K) Time-dependent (6 h and 24 h) changes in the mRNA expression of Epac1 standardized to GAPDH in rat lung fibroblasts, rat skin fibroblasts, mouse hepatic stellate cells (HSC), and human hepatic stellate cells (hTERT-HSC) treated with 10 ng/ml TGFβ1. Data are the fold- increase vs. control for each time point. n = 6; *, P < 0.05; **, P < 0.01; ***, P < 0.001 compared with control for each time point.
Overexpression of Epac1 Inhibits TGFβ1 Induced Collagen Synthesis: Decrease in Epac Expression Is Necessary but Not Sufficient for the Profibrotic Phenotype.
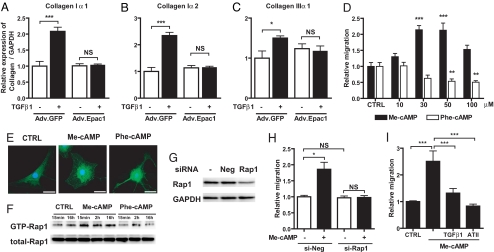
Adenovirus-mediated gene transfer of Epac1 dose-dependently increased expression of Epac1 mRNA and protein [supporting information (SI) Fig. S1] and negated TGFβ1-promoted enhancement of collagen type Iα1, Iα2, and IIIα1 mRNA expression (Fig. 2 A–C). Because mRNA expression of collagens type Iα1 and IIIα1 was not changed in cells treated with Epac1-targeted siRNA under conditions that selectively silenced Epac1 (data not shown), decrease of Epac1 expression is necessary, but not sufficient, for generation of the TGFβ1-promoted cardiac fibrotic phenotype.
Fig. 2.
Effect of Epac on collagen synthesis, migration and morphology in cardiac fibroblasts. (A–C) Fibroblasts were infected with Adv.GFP or Adv.Epac1 in serum-free media for 24 h and then with or without TGFβ1 (10 ng/ml) for 24 h. Cells were assayed by using real-time RT-PCR to quantify collagen Iα1, Iα2, and IIIα1; the data are normalized to GAPDH. n = 4. *, P < 0.05; ***, P < 0.001. (D) Fibroblast migration was examined by using a modified Boyden chamber method in the absence (CTRL) or presence of Me-cAMP, an Epac-activator, or Phe-cAMP, a PKA-activator. Data are shown as the fold-increase relative to control. n = 6–8. **, P < 0.01; ***, P < 0.001 compared with control (CTRL). (E) Immunocytochemistry was performed by using cardiac fibroblasts (passage 1) grown for 48 h in serum-free media and then for 1 h in serum-free media alone (CTRL) or together with Me-cAMP or Phe-cAMP. Cells were stained for Epac1 (green) and nuclear staining of DNA with DAPI (blue). (Scale bar, 30 μm.) (F) Time-dependent Rap1 activation in cardiac fibroblasts. Cells were serum-starved for 48 h, then incubated with Me-cAMP (50 μM) or Phe-cAMP (50 μM) up to 16 h and assayed for Rap1 activation. Precipitates (Upper) and total cell lysates (Lower) were analyzed by immunoblotting with an anti-Rap1 antibody. (G) siRNA-promoted silencing of Rap1 protein expression. Fibroblasts were incubated with Rap1-targeted siRNA for 24 h, lysed and subjected to immunoblot analysis with a Rap1 antibody. (H) Fibroblasts were incubated with Rap1-targeted siRNA for 24 h, treated with Me-cAMP (50 μM) and migration was assayed. n = 4–6; *, P < 0.05. (I) Migration was examined in cardiac fibroblasts treated with Me-cAMP (50 μM) alone, or together with TGFβ1 (10 ng/ml) or angiotensin II (100 nM). n = 4–7, ***, P < 0.001.
Activation of Epac Promotes Cardiac Fibroblast Migration via Rap1, Whereas Activation of PKA Inhibits Migration.
Migration of fibroblasts is a key step in their entry into sites of tissue damage. Treatment with cAMP analogs that selectively activate Epac (Me-cAMP and 8-(4-chlorophenylthio)-2′-O-methyl-cAMPS, Sp- isomer [Sp-Me-cAMP]), or PKA [N6-phenyl cAMP (Phe-cAMP)] (7, 16) revealed that Epac activation promotes (by 2- to 3-fold), but PKA activation inhibits (by ≈50%) fibroblast migration (Fig. 2D and Fig. S2A). Immunocytochemistry indicated that, akin to the observations in ref. 17, PKA activation (Phe-cAMP) rapidly (within 1 h) produces long branching processes that yield a stellate pattern, whereas Epac activation (Me-cAMP) results in rounded cells without such processes (Fig. 2E). Epac1 is primarily cytoplasmic under resting conditions but localizes to perinuclear and nuclear regions after activation by Me-cAMP (Fig. 2E).
We assessed the role of Rap1, a downstream target of Epac (8, 9), on Epac-promoted migration of fibroblasts. Epac activation by Me-cAMP increased GTP-bound (activated) Rap1; PKA activation by Phe-cAMP had negligible effects on such binding (Fig. 2F). Silencing by Rap1-targeted siRNA (Fig. 2G) negated Me-cAMP-induced fibroblast migration (Fig. 2H). Pretreatment with TGFβ1 and angiotensin II inhibited Me-cAMP-induced migration (Fig. 2I), consistent with their ability to decrease Epac1 expression (Fig. 1). Thus, Epac activation promotes fibroblast migration via Rap1, whereas activation of PKA inhibits such migration.
Low Concentrations of cAMP Promote Fibroblast Migration, Whereas Higher cAMP Concentrations Inhibit Such Migration.
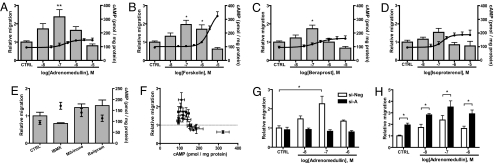
Several cAMP-elevating agents, including stimulants of cAMP synthesis [e.g., adrenomedullin, beraprost (an IP receptor agonist), isoproterenol (a β-adrenergic receptor agonist), and the diterpene forskolin) (Fig. 3 A–D)] and inhibitors of cAMP hydrolysis [cyclic nucleotide phosphodiesterase (PDE), e.g., a nonselective PDE inhibitor, 3-isobutyl-1-methylxanthine (200 μM), a PDE3 inhibitor, milrinone (10 μM) or a PDE4 inhibitor, rolipram (10 μM) (Fig. 3E)] stimulated fibroblast migration at lower concentrations but decreased migration at higher concentrations, as summarized in Fig. 3F. Lower and higher concentrations of these agents produce morphologic changes akin to those elicited by activation of Epac and PKA, respectively (Fig. S2B and Fig. 2E). Cell viability was maintained, as demonstrated by trypan blue staining.
Fig. 3.
Lower concentrations of cAMP promote cardiac fibroblast migration via Epac. (A–E) Fibroblast migration and cAMP concentrations in response to various agents were measured by a modified Boyden chamber method and RIA, respectively. Cardiac fibroblasts were grown for 48 h in serum-free media and then incubated for 16 h to assess migration or stimulated for 3–10 min for assay of cAMP concentrations after treatment with the indicated concentrations of each drug. The bar graph and the left y axis indicate migration, and the line graph and the right y axis indicate cellular cAMP concentrations. Values represent mean ± SEM of at least three independent experiments. Data are the fold-increase relative to control. n = 6–8. *, P < 0.05; **, P < 0.01 compared with control (CTRL). (F) The relation between migration and cAMP production by cAMP-elevating drugs. (G) The effect of Epac1-targeted siRNA (si-A) on adrenomedullin-induced migration was examined as in B. n = 4–8; *, P < 0.05. (H) Effect of the PKA inhibitor (PKI) Wiptide on adrenomedullin-induced migration. Cardiac fibroblasts were incubated with 10 μM PKI 20 min before assessment of migration. n = 4–7; *, P < 0.05.
Treatment with Epac1-targeted siRNA selectively decreases expression of Epac1 mRNA and protein for at least 24–48 h (Fig. S3A and data not shown). Epac1-targeted siRNA, but not Epac2-targeted siRNA (data not shown), negated the enhancement of migration induced by the GPCR agonist adrenomedullin (Fig. 3G and Fig. S3B). Thus, Epac1 appears to mediate the enhancement of fibroblast migration by cAMP-elevating agonists.
Adrenomedullin also activates PKA in cardiac fibroblasts (Fig. S3C); treatment with the PKA inhibitor Wiptide (10 μM) (Fig. S3D) enhances adrenomedullin-promoted migration (Fig. 3H), implying that Epac activation still occurs in the presence of Wiptide. Data obtained with Wiptide, together with other data (Fig. 3 and Figs. S2 and S3), lead us to conclude that lower cAMP concentrations preferentially promote fibroblast migration via Epac.
Both Epac and PKA Activation Inhibit Collagen Type I and III Expression and DNA Synthesis.
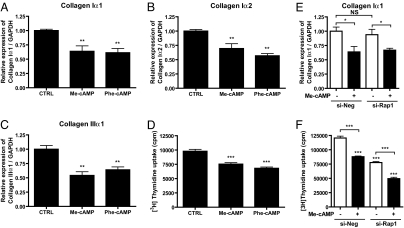
Despite their differing effects on fibroblast migration, Me-cAMP and Phe-cAMP both inhibit collagen Iα1, Iα2, and IIIα1 mRNA expression (Fig. 4 A–C) and DNA synthesis (Fig. 4D). Incubation with Rap1-targeted siRNA did not affect Me-cAMP-induced inhibition of collagen and DNA synthesis (Fig. 4 E and F). Activation of PKA inhibits expression of α-smooth muscle actin (αSMA), a measure of fibroblast-to-myofibroblast conversion (2, 3); Epac activation does not alter αSMA expression (data not shown).
Fig. 4.
Epac and PKA activation inhibit collagen I and III expression and DNA synthesis of cardiac fibroblasts. (A–C) Cardiac fibroblasts were incubated in serum-free media for 48 h and then with either Me-cAMP (50 μM) or Phe-cAMP (50 μM) for 24 h. Cells were assayed by using real-time RT-PCR to quantify collagens Iα1, Iα2, and IIIα1; the data are normalized to GAPDH. n = 4. **, P < 0.01; ***, P < 0.001 compared with CTRL. (D) Cells were serum-starved for 48 h and then incubated with either Me-cAMP (50 μM) or Phe-cAMP (50 μM) in presence of [3H]thymidine for 24 h and processed to quantify DNA synthesis. n = 6. ***, P < 0.01 compared with CTRL. (E) Cells were incubated with Rap1-targeted siRNA for 24 h and then stimulated with Me-cAMP for 24 h. Quantitative RT-PCR was used to analyze collagen type Iα1 mRNA expression. n = 4–6. *, P < 0.05. (F) Cells were incubated with Rap1-targeted siRNA for 24 h, then for 24 h with Me-cAMP and [3H]thymidine. n = 4. ***, P < 0.001.
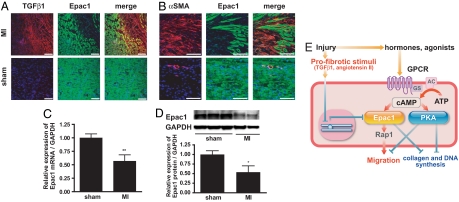
Epac1 Expression Decreases After Myocardial Infarction.
Using a myocardial infarction model, we assessed TGFβ1 and Epac1 expression by immunostaining and assessed images of the border zone that abuts on regions of infarction. TGF β1 (red) was highly expressed in the area of infarction (Fig. 5A Upper Right); TGF β1-expressing cells had less Epac1 (green) expression than did TGF β1-negative cells. αSMA (red) was expressed in the area of infarction and in vessels in sham-operated animals; Epac1 expression was decreased in αSMA-expressing cells (Fig. 5B). Fibroblasts isolated from the intraventricular septa 1 week after myocardial infarction had decreased Epac1 mRNA and protein expression (Fig. 5 C and D). We also found decreased expression of Epac1 and high levels of TGFβ1 in the border zone of mice with myocardial infarction (Fig. S4). Thus, a decrease in Epac1 expression parallels the increase in expression of TGF β1 after myocardial infarction, results consistent with those we observed with isolated fibroblasts (Fig. 1).
Fig. 5.
Epac1 expression is decreased after myocardial infarction in vivo. (A) Sections of cardiac intraventricular septa from rats subjected to myocardial infarction (MI) or sham operation (sham) were stained for TGFβ1 (red), Epac1 (green) and nuclei with DAPI (blue). The images of the border zone that abuts on areas of infarction are shown. (Scale bar, 100 μm.) (B) Sections of intraventricular septa of rats subjected to myocardial infarction (MI) or sham operation (sham) were stained for αSMA (red), Epac1 (green), and DNA with DAPI (blue). The images of the border zone of infarction are shown. (Scale bar, 100 μm.) (C) Cardiac fibroblasts isolated from intraventricular septa of postmyocardial infarction and sham-operated rats were cultured for 16 h to eliminate other cell types. RNA was isolated and subjected to quantitative RT-PCR. GAPDH was used as standard. n = 4–5. **, P = 0.0085 compared with the sham-operated group. (D) Expression of Epac1 protein. (Upper) Representative images of immunoblots. (Lower) Densitometry of the blots for Epac1 protein normalized to GAPDH protein expression is shown in the graph. n = 3, **, P = 0.0367. (E) Model for the role of Epac and PKA in fibroblasts after injury. After injury, levels of cAMP-elevating hormones increase, activating cognate GPCR. The enhanced synthesis of cAMP by adenylyl cyclase activation stimulates Epac and PKA. Epac promotes fibroblast migration, whereas PKA inhibits migration and fibroblast-to-myofibroblast transformation. Both Epac and PKA inhibit collagen synthesis and fibroblast proliferation. Profibrotic stimuli inhibit Epac expression and stimulate collagen synthesis.
Discussion
The current findings indicate that profibrotic agonists inhibit Epac1 expression in fibroblasts from multiple tissues and that overexpression of Epac1 inhibits TGFβ1-induced collagen synthesis, implying that a decrease in Epac expression is required for profibrotic response. Moreover, PKA and Epac, the two major effectors of cAMP action, differentially regulate fibroblast migration and morphology: Epac promotes migration via Rap1 and expression of a rounded morphology, whereas PKA inhibits migration, decreases fibrogenicity, and produces a stellate appearance. Findings obtained with cAMP derivatives that selectively activate Epac or PKA and with cAMP-elevating agonists indicate that lower cAMP concentrations preferentially promote migration via Epac. Activation of Epac or PKA has opposite effects on fibroblast migration, but both cAMP effectors inhibit collagen and DNA synthesis.
Our results, which show that TGFβ1 decreases Epac1 expression in fibroblasts from multiple tissues, are akin to data indicating a similar decrease in Epac1 mRNA expression in U937 monocytic cells (18): TGFβ-mediated inhibition of Epac expression may thus be a widespread mechanism for controlling responses to mitogens. Smad3 is required for TGFβ-induced gene expression in fibroblasts (19); its loss prevents interstitial fibrosis in the heart and attenuates cardiac remodeling (20), suggesting a role for Smad3 in the decrease of Epac1 by TGFβ. Epac1 protein can interact with type I TGFβ receptor and inhibit transcriptional activation and phosphorylation of Smad2 (21). Functionally, the decrease in Epac expression would facilitate localization of fibroblasts at sites where fibrogenesis is required and contribute to this process by blunting the negative impact of Epac activation on collagen synthesis. The ability of overexpression of Epac to block profibrotic response (Fig. 2) suggests that such an approach has the therapeutic potential to inhibit or perhaps reverse tissue fibrosis.
Epac-promoted fibroblast migration appears to occur via Rap1, although PKA may also phosphorylate and regulate Rap1 (22, 23). The Epac target Rap1-RAPL, a Rap1-binding molecule, can move to a cell's leading edge and activate integrins to promote cell polarization and migration (24). Our data regarding localization of Epac1 are consistent with evidence that Epac1 localizes perinuclearly in migrating cells, suggesting that Epac1 activates Rap1 in the location where activated Rap1 may associate with RAPL (24). Perhaps the ability of cAMP-elevating agonists to regulate cell migration to varying degrees results from increases in cAMP that activate compartmentalized PKA isoforms and Epac (25). By contrast with our data, in fibroblasts, Epac activation inhibits migration of endothelial cells (26), implying that Epac activation has cell type-differences in its effect on migration.
Our data (Fig. 3) implicate Epac as the preferential effector (vs. PKA) in fibroblast migration in response to increases in cAMP. Previous studies have reached inconsistent conclusions regarding which effector is preferentially involved in cAMP actions (7, 27, 28). The physiologically relevant cAMP affinities for Epac vs. PKA have not been clearly defined, in part because of limitations in the assays currently available and the difficulty in knowing the contribution of other cellular proteins and constituents to Epac activity in intact cells.
Plasma and tissue levels of cAMP-elevating agonists, including catecholamines and adrenomedullin, rapidly increase in many organs, including the heart, after injury (13). TGFβ1 expression increases at wound sites 1–3 days after myocardial infarction (29). Our data indicate that TGFβ1 inhibits fibroblast migration via inhibition of Epac expression and suggest a mechanism for facilitation of TGFβ1 action by promotion of the synthesis of extracellular matrix at sites of injury (3, 29) (Fig. 5E). This idea is consistent with our in vivo data showing that TGFβ1 is increased and Epac1 expression is decreased in the border zone after myocardial infarction. The ability of TGFβ1 to inhibit expression of Epac1 provides an example of cross-talk between tyrosine kinase receptors and GPCR that occurs at a more distal site in the GPCR signaling pathway that is different than previously noted (30).
In conclusion, the current data show that inhibition of Epac expression and action contributes to responses induced by profibrotic agents and that increases in intracellular cAMP concentrations (produced by activators of adenylyl cyclase or inhibitors of PDE) preferentially promote migration of fibroblasts via the Epac–Rap1 pathway. Decreased fibroblast migration in response to profibrotic agents occurs via inhibition of Epac1 expression; prevention of this decrease in Epac expression can inhibit profibrotic response. Because Epac appears to integrate pro- and anti-fibrotic signals, treatments that enhance Epac expression have the potential to blunt and perhaps reverse tissue fibrosis.
Materials and Methods
Antibodies and Regents.
Forskolin, adrenomedullin, isoproterenol, TGFβ1, and antibody to α-smooth muscle actin were purchased from Sigma. 8-(4-chlorophenylthio)-2′-O-methyladenosine-3′,5′-cyclic monophosphate, 8-pCPT-2′-O-Me-cAMP, (Me-cAMP), N6-phenyladenosine-3′,5′-cyclic monophosphate (Phe-cAMP), and 8-(4-chlorophenylthio)-2′-O-methyl-cAMPS Sp-isomer (Sp-Me-cAMP) were purchased from the BIOLOG Life Science Institute. The PKA inhibitor (5–22) (Wiptide) and beraprost were purchased from Anaspec and Cayman Chemical, respectively. Antibodies to Epac1 and Epac2 were purchased from Santa Cruz Biotechnology. Antibody to TGFβ1 was purchased from R&D Systems.
Isolation and Culture of Adult Rat Cardiac, Lung, and Skin Fibroblasts.
Cardiac fibroblasts were isolated from adult Sprague–Dawley rats (250–300 g, male) and cultured as described in ref. 3. To obtain lung and skin fibroblasts, lung and skin were isolated from anesthetized/heparinized adult Sprague–Dawley rats (250–300 g, male). Isolated tissues were placed in Ca2+- and Mg2+-free PBS, rinsed, minced, and placed into a 60-mm dish and allowed (10 min) to adhere to the dish. Tissue explants were then incubated in medium (DMEM supplemented with 10% FBS, 5% NCS, l-glutamine, sodium pyruvate, and antibiotic-antimycotic), and allowed to incubate in a humidified 5% CO2–95% atmospheric air incubator at 37°C. Tissue fragments were removed by aspiration; fibroblasts were washed twice with Ca2+- and Mg2+-free PBS, trypsinized, and passaged once over a 5-day period. All animals were cared for in compliance with the guiding principles of the American Physiologic Society and as approved by the UCSD Institutional Animal Care and Use Committee.
Isolation and Culture of Adult Mouse and Human Hepatic Stellate Cells.
Mouse hepatic stellate cells were isolated as described in ref. 31. Passage 1 cells were used. The immortalized human hepatic stellate cell line was used as described in ref. 32.
Adenovirus Construction.
Full-length cDNA-encoding human Epac1 (kindly provided by J. L. Bos) was cloned into a shuttle vector by using an AdenoX adenovirus construction kit (Clontech) kindly provided by Y. Ishikawa (Yokohama City University, Yokohama, Japan). A control adenovirus vector with GFP was used at the same multiplicity of infection. All experiments were performed 24 h after infection.
Quantitative RT-PCR.
Total RNA was isolated by using TRIzol (Invitrogen) according to the manufacturer's instructions. Generation of cDNA and RT-PCR analysis were done as described in ref. 33. Primers for PCR amplification were designed based on the nucleotide sequences of Epac1; Epac2; collagen Iα1, I α2, III α1; and GAPDH. Each forward and reverse primer set was designed between multiple exons, and PCR products were confirmed by sequencing (the information of primer sequences is in Table S1). For quantitative RT-PCR analysis, each template was tested at least three times to confirm reproducibility. mRNA abundance was determined relative to that of GAPDH.
Immunoblot Analysis.
Proteins from whole cells were analyzed by immunoblotting as described in ref. 3.
[3H]thymidine Incorporation.
[3H]thymidine incorporation was used to measure DNA synthesis as described in ref. 33. Cells (1 × 105 per well) were seeded into a 12-well culture plate and incubated with 1 μCi [methyl-3H]thymidine with or without drug for 24 h at 37°C.
Rap1 Pull-Down Assay.
Rap1 activity was assayed with a Rap1 activation assay kit (Upstate) according to the manufacturer's instructions (SI Methods).
cAMP Production.
Cardiac fibroblasts were serum-starved for 48 h and assayed for cAMP production by RIA after 3–10 min incubation with drugs of interest (3).
PKA Activity.
PKA activity was measured by using an assay kit (StressGen Biotechnologies) according to the manufacturer's instructions (SI Methods).
Immunocytochemistry.
Immunocytochemistry was performed as described in ref. 9 and in SI Methods.
RNA Interference (siRNA).
Double-stranded siRNAs to the selected regions of Epac1, Rap1, and negative siRNA used as a control were purchased from Ambion. Epac2-targeted siRNA was purchased from Qiagen. The siRNA sequences are shown in Table S2. Cells were transfected with siRNA (300 pmol), using Lipofectamine RNAiMAX (Invitrogen).
Cell Migration Assay.
Migration was assayed by using the Boyden chamber method, as described in ref. 34. The cells were maintained in serum-free conditions for 48 h before the experiments and suspended in serum-free DMEM at a density of 1 × 105 cells per 100 μl in the inserts without coating. Basal migration was assessed by adding 600 μl of serum-free DMEM to the lower chambers. Cells were then allowed to migrate. After 16 h, the cells were fixed in Accustain (Sigma) and stained with Hema 3 (Fisher Scientific). Cells on the upper surface of the membrane were mechanically removed with a cotton swab. Migrated cells were identified microscopically and counted from three different fields (0.5 mm2 per field).
Rat and Mouse Myocardial Infarction Models.
We used a slightly modified version of a procedure published in ref. 35 for inducing myocardial infarction. Briefly, animals were intubated and anesthetized with 1–2% isoflurane (anesthetic mixed with 1 liter of oxygen per minute). The proximal left coronary artery was ligated with a 7-0 prolene suture (BV-1 needle). The hearts were harvested 1 week after myocardial infarction.
Immunofluorescence Microscopy of Cardiac Tissue.
Cardiac ventricles were harvested, frozen, and then mounted on a cryostat to cut 10-μm semithin sections. Sections were fixed in cold acetone, blocked with 4% BSA in 0.1% Tween and PBS, and incubated with primary antibodies (1:100) in 4% BSA in 0.1% Tween and PBS. After incubation with Alexa-conjugated secondary antibody (Molecular Probes) (1:250), staining of nuclei with DAPI (1:5,000), samples were mounted in gelvatol. Specificity of staining was determined by omission of the primary antibody. Images were obtained by using a Zeiss LSM510 Laser Scanning Confocal Microscope and Zeiss Image Examiner software.
Statistical Analysis.
Data shown are the mean ± SEM of n independent experiments. Statistical analysis was performed by unpaired Student t test or one-way ANOVA followed by Newman–Keuls multiple comparison test, using GraphPad Prism software, Version 4.0. P < 0.05 was considered significant.
Supplementary Material
Acknowledgments.
We thank Drs. J. L. Bos (University Medical Centre Utrecht, Utrecht, The Netherlands), Y. Ishikawa, D. A. Brenner (University of California, San Diego), and E. Seki, who kindly provided the Epac1 construct, adenovirus Epac1, hTERT-HSC, and mouse HSC, respectively; M. Jennings for technical support; and M. Mukoyoshi for assistance with illustrations. This work was supported by National Institutes of Health Grants 2P01HL066941 (to P.A.I. and D.M.R.) and HL081400 (to D.M.R.), the Ellison Medical Foundation (P.A.I.), and American Heart Association Grant 060039N (to H.H.P.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0801490105/DCSupplemental.
References
- 1.Wynn TA. Common and unique mechanisms regulate fibrosis in various fibroproliferative diseases. J Clin Invest. 2007;117:524–529. doi: 10.1172/JCI31487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tomasek JJ, Gabbiani G, Hinz B, Chaponnier C, Brown RA. Myofibroblasts and mechano-regulation of connective tissue remodelling. Nat Rev Mol Cell Biol. 2002;3:349–363. doi: 10.1038/nrm809. [DOI] [PubMed] [Google Scholar]
- 3.Swaney JS, et al. Inhibition of cardiac myofibroblast formation and collagen synthesis by activation and overexpression of adenylyl cyclase. Proc Natl Acad Sci USA. 2005;102:437–442. doi: 10.1073/pnas.0408704102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cohen P. Protein kinases—the major drug targets of the twenty-first century? Nat Rev Drug Discov. 2002;1:309–315. doi: 10.1038/nrd773. [DOI] [PubMed] [Google Scholar]
- 5.Zambon AC, et al. Gene expression patterns define key transcriptional events in cell-cycle regulation by cAMP and protein kinase A. Proc Natl Acad Sci USA. 2005;102:8561–8566. doi: 10.1073/pnas.0503363102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Roscioni SS, Elzinga CR, Schmidt M. Epac: Effectors and biological functions. Naunyn Schmiedebergs Arch Pharmacol. 2008 doi: 10.1007/s00210-007-0246-7. [DOI] [PubMed] [Google Scholar]
- 7.Bos JL. Epac: A new cAMP target and new avenues in cAMP research. Nat Rev Mol Cell Biol. 2003;4:733–738. doi: 10.1038/nrm1197. [DOI] [PubMed] [Google Scholar]
- 8.Kawasaki H, et al. A family of cAMP-binding proteins that directly activate Rap1. Science. 1998;282:2275–2279. doi: 10.1126/science.282.5397.2275. [DOI] [PubMed] [Google Scholar]
- 9.de Rooij J, et al. Epac is a Rap1 guanine-nucleotide-exchange factor directly activated by cyclic AMP. Nature. 1998;396:474–477. doi: 10.1038/24884. [DOI] [PubMed] [Google Scholar]
- 10.Holz GG, Kang G, Harbeck M, Roe MW, Chepurny OG. Cell physiology of cAMP sensor Epac. J Physiol. 2006;577:5–15. doi: 10.1113/jphysiol.2006.119644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bos JL. Epac proteins: Multi-purpose cAMP targets. Trends Biochem Sci. 2006;31:680–686. doi: 10.1016/j.tibs.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 12.Leask A. TGFbeta, cardiac fibroblasts, and the fibrotic response. Cardiovasc Res. 2007;74:207–212. doi: 10.1016/j.cardiores.2006.07.012. [DOI] [PubMed] [Google Scholar]
- 13.Rabinowitz B, et al. Myocardial and plasma levels of adenosine 3′:5′-cyclic phosphate. Studies in experimental myocardial ischemia. Chest. 1975;68:69–74. doi: 10.1378/chest.68.1.69. [DOI] [PubMed] [Google Scholar]
- 14.Thiemermann C, Zacharowski K. Selective activation of E-type prostanoid(3)-receptors reduces myocardial infarct size. A novel insight into the cardioprotective effects of prostaglandins. Pharmacol Ther. 2000;87:61–67. doi: 10.1016/s0163-7258(00)00069-3. [DOI] [PubMed] [Google Scholar]
- 15.Schmidt M, Sand C, Jakobs KH, Michel MC, Weernink PA. Epac and the cardiovascular system. Curr Opin Pharmacol. 2007;7:193–200. doi: 10.1016/j.coph.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 16.Christensen AE, et al. cAMP analog mapping of Epac1 and cAMP kinase. Discriminating analogs demonstrate that Epac and cAMP kinase act synergistically to promote PC-12 cell neurite extension. J Biol Chem. 2003;278:35394–35402. doi: 10.1074/jbc.M302179200. [DOI] [PubMed] [Google Scholar]
- 17.Pelletier S, Julien C, Popoff MR, Lamarche-Vane N, Meloche S. Cyclic AMP induces morphological changes of vascular smooth muscle cells by inhibiting a Rac-dependent signaling pathway. J Cell Physiol. 2005;204:412–422. doi: 10.1002/jcp.20308. [DOI] [PubMed] [Google Scholar]
- 18.Basoni C, et al. Inhibitory control of TGF-beta1 on the activation of Rap1, CD11b, and transendothelial migration of leukocytes. FASEB J. 2005;19:822–824. doi: 10.1096/fj.04-3085fje. [DOI] [PubMed] [Google Scholar]
- 19.Leask A, Abraham DJ. TGF-beta signaling and the fibrotic response. FASEB J. 2004;18:816–827. doi: 10.1096/fj.03-1273rev. [DOI] [PubMed] [Google Scholar]
- 20.Bujak M, et al. Essential role of Smad3 in infarct healing and in the pathogenesis of cardiac remodeling. Circulation. 2007;116:2127–2138. doi: 10.1161/CIRCULATIONAHA.107.704197. [DOI] [PubMed] [Google Scholar]
- 21.Conrotto P, Yakymovych I, Yakymovych M, Souchelnytskyi S. Interactome of transforming growth factor-beta type I receptor (TbetaRI): Inhibition of TGFbeta signaling by Epac1. J Proteome Res. 2007;6:287–297. doi: 10.1021/pr060427q. [DOI] [PubMed] [Google Scholar]
- 22.Li J, et al. Cyclic adenosine 5′-monophosphate-stimulated neurotensin secretion is mediated through Rap1 downstream of both Epac and protein kinase A signaling pathways. Mol Endocrinol. 2007;21:159–171. doi: 10.1210/me.2006-0340. [DOI] [PubMed] [Google Scholar]
- 23.Hochbaum D, Hong K, Barila G, Ribeiro-Neto F, Altschuler DL. Epac, in synergy with PKA, is required for cAMP-mediated mitogenesis. J Biol Chem. 2008;283:4464–4468. doi: 10.1074/jbc.C700171200. [DOI] [PubMed] [Google Scholar]
- 24.Kinashi T, Katagiri K. Regulation of lymphocyte adhesion and migration by the small GTPase Rap1 and its effector molecule, RAPL. Immunol Lett. 2004;93:1–5. doi: 10.1016/j.imlet.2004.02.008. [DOI] [PubMed] [Google Scholar]
- 25.Fischmeister R, et al. Compartmentation of cyclic nucleotide signaling in the heart: The role of cyclic nucleotide phosphodiesterases. Circ Res. 2006;99:816–828. doi: 10.1161/01.RES.0000246118.98832.04. [DOI] [PubMed] [Google Scholar]
- 26.Hong J, et al. Anthrax edema toxin inhibits endothelial cell chemotaxis via Epac and Rap1. J Biol Chem. 2007;282:19781–19787. doi: 10.1074/jbc.M700128200. [DOI] [PubMed] [Google Scholar]
- 27.Dao KK, et al. Epac1 and cAMP-dependent protein kinase holoenzyme have similar cAMP affinity, but their cAMP domains have distinct structural features and cyclic nucleotide recognition. J Biol Chem. 2006;281:21500–21511. doi: 10.1074/jbc.M603116200. [DOI] [PubMed] [Google Scholar]
- 28.DiPilato LM, Cheng X, Zhang J. Fluorescent indicators of cAMP and Epac activation reveal differential dynamics of cAMP signaling within discrete subcellular compartments. Proc Natl Acad Sci USA. 2004;101:16513–16518. doi: 10.1073/pnas.0405973101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Khan R, Sheppard R. Fibrosis in heart disease: Understanding the role of transforming growth factor-beta in cardiomyopathy, valvular disease and arrhythmia. Immunology. 2006;118:10–24. doi: 10.1111/j.1365-2567.2006.02336.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Natarajan K, Berk BC. cross-talk coregulation mechanisms of G protein-coupled receptors and receptor tyrosine kinases. Methods Mol Biol. 2006;332:51–77. doi: 10.1385/1-59745-048-0:51. [DOI] [PubMed] [Google Scholar]
- 31.Bataller R, et al. NADPH oxidase signal transduces angiotensin II in hepatic stellate cells and is critical in hepatic fibrosis. J Clin Invest. 2003;112:1383–1394. doi: 10.1172/JCI18212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schnabl B, Choi YH, Olsen JC, Hagedorn CH, Brenner DA. Immortal activated human hepatic stellate cells generated by ectopic telomerase expression. Lab Invest. 2002;82:323–333. doi: 10.1038/labinvest.3780426. [DOI] [PubMed] [Google Scholar]
- 33.Yokoyama U, et al. Multiple transcripts of Ca2+ channel alpha1-subunits and a novel spliced variant of the alpha1C-subunit in rat ductus arteriosus. Am J Physiol Heart Circ Physiol. 2006;290:H1660–1670. doi: 10.1152/ajpheart.00100.2004. [DOI] [PubMed] [Google Scholar]
- 34.Yokoyama U, et al. Chronic activation of the prostaglandin receptor EP4 promotes hyaluronan-mediated neointimal formation in the ductus arteriosus. J Clin Invest. 2006;116:3026–3034. doi: 10.1172/JCI28639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bayat H, et al. Progressive heart failure after myocardial infarction in mice. Basic Res Cardiol. 2002;97:206–213. doi: 10.1007/s003950200013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.