Abstract
Although Hox gene expression has been linked to motoneuron identity, a role of these genes in development of the spinal sensory system remained undocumented. Hoxb genes are expressed at high levels in the dorsal horn of the spinal cord. Hoxb8 null mutants manifest a striking phenotype of excessive grooming and hairless lesions on the lower back. Applying local anesthesia underneath the hairless skin suppressed excessive grooming, indicating that this behavior depends on peripheral nerve activity. Functional ablation of mouse Hoxb8 also leads to attenuated response to nociceptive and thermal stimuli. Although spinal ganglia were normal, a lower postmitotic neural count was found in the dorsalmost laminae at lumbar levels around birth, leading to a smaller dorsal horn and a correspondingly narrowed projection field of nociceptive and thermoceptive afferents. The distribution of the dorsal neuronal cell types that we assayed, including neurons expressing the itch-specific gastrin-releasing peptide receptor, was disorganized in the lumbar region of the mutant. BrdU labeling experiments and gene-expression studies at stages around the birth of these neurons suggest that loss of Hoxb8 starts impairing development of the upper laminae of the lumbar spinal cord at approximately embryonic day (E)15.5. Because none of the neuronal markers used was unexpressed in the adult dorsal horn, absence of Hoxb8 does not impair neuronal differentiation. The data therefore suggest that a lower number of neurons in the upper spinal laminae and neuronal disorganization in the dorsal horn underlie the sensory defects including the excessive grooming of the Hoxb8 mutant.
Keywords: anteroposterior patterning, mouse Hox genes, spinal cord, reaction to heat pain and itch
Hox transcription factors have been widely documented to be instructors of positional information in emerging embryonic structures during extension of the anteroposterior (A–P) axis. In vertebrates, this evolutionarily conserved function has been most extensively described during patterning of the vertebral column. Anteriorly expressed Hox genes have also been shown to have patterning functions in the hindbrain, where they control rhombomere development (1). One of these genes, Hoxa2, was recently shown to regulate a later event of rhombomere-derived neurogenesis, the generation of whisker somatosensory maps (2). Regarding the spinal cord, Hox gene expression has been documented to modulate the specification of motoneuron pools in the ventral horn. Particular mouse mutants in HoxC and HoxD genes exhibit aberrant motoneuron limb innervation (3, 4). In the chick, a hierarchy of regulatory interactions between Hox genes was found to underlie the specificity and topographic organization of motoneuron identity in the ventral horn (5, 6). Much less is known about how the vertebrate sensory neuron networks are laid down along the main axis and whether Hox genes play a role in the rostrocaudal specification of primary sensory neurons and the second-order neurons they project to in the most dorsal laminae of the spinal cord. In the hindbrain, the expression of 3′most Hox genes was shown to contribute to the generation of precursors of visceral sensory neurons (7). HoxB is unique among the Hox clusters in that its gene members are expressed in overlapping patterns at very high levels in the dorsal horn of the spinal cord (6, 8). Hoxb8, in particular, is expressed strongly in the dorsalmost spinal cord throughout development and in adults (ref. 9 and J.D., unpublished data). Loss of Hoxb8 function was reported earlier to lead to excessive lower back grooming, and to localized skin lesions in adult mutant mice (10–12). Here, we document that Hoxb8 mutant mice are also impaired in their thermal and nociceptive response. The number of neurons in the superficial layers of the dorsal horn is lower in the mutants than in controls, at birth and in adults, resulting in a mediolaterally narrowed dorsal horn in the lumbar region. This morphological change leads to a correspondingly narrowed primary afferent projection area, whereas cell counts and neurochemistry of the dorsal root ganglia (DRGs) are unchanged. Tracing the proliferation of the late born neurons, and the dorsal “inside out” migration of their descendants into the upper laminae suggests that loss of Hoxb8 starts impairing the development of organized laminae I and II of dorsal neurons at specific axial levels at approximately embryonic day (E)15.5.
Results
Hoxb8 Is Highly Expressed in Embryonic and Adult Dorsal Spinal Cord.
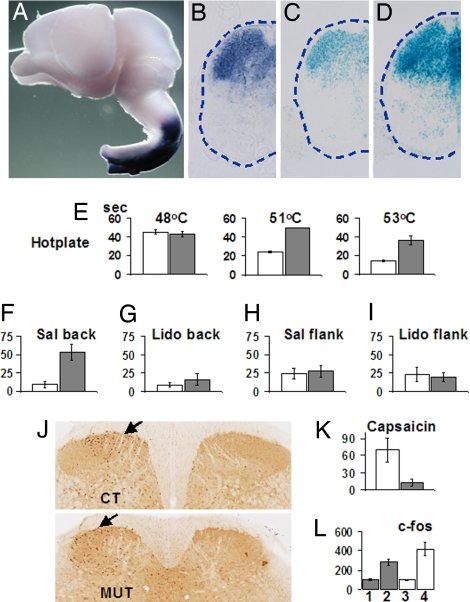
Hoxb genes and Hoxb8 in particular are strongly expressed in the dorsal horn of the spinal cord during embryonic development (8, 9, 13) (Fig. 1B), and expression remains high in newborn and adult mice. Along the dorsoventral (D/V) axis, strongest Hoxb8 expression is found in the superficial dorsal horn, corresponding to spinal laminae I and II (13), whereas a lower expression is also found in the deep dorsal horn, which mainly receives proprioceptive information (Fig. 1B). Because the Hoxb8 null allele is a lacZ“knockin” allele, Hoxb8-expressing cells can be followed by their lacZ expression in heterozygotes and homozygote embryos and mice (Fig. 1 C and D). Inspection of X-Gal-stained cross-sections of the mutant spinal cord revealed that Hoxb8lacZ-expressing neurons are present despite the loss of Hoxb8. Along the caudorostral axis, Hoxb8 transcripts are normally abundant in a domain that extends from caudal spinal cord levels up to a sharp boundary in the posterior hindbrain, later posterior medulla (Fig. 1A). No expression was detected, either by in situ hybridization or by X-Gal staining at positions more anterior than this sharp expression boundary (Fig. 1A).
Fig. 1.
Hoxb8 and Hoxb8lacZ expression in the mouse spinal cord and sensory behavior of Hoxb8 null mice. (A) Hoxb8 expression was detected by whole-mount in situ hybridization in the dissected CNS of an E16.5 wild-type embryo. Only the rostral part of the spinal cord is shown here, with the sharp anterior expression boundary in the posterior medulla. (B) Hoxb8 expression after in situ hybridization on a transverse section of a wild-type E17.5 embryo.(C) Hoxb8lacZ expression assayed by X-Gal staining on a section of an E17.5 heterozygote mutant embryo. The expression domain is similar to that in B. (D) Hoxb8lacZ expression assayed by X-Gal staining on a section of an E17.5 homozygous Hoxb8 mutant embryo. Expression is similar to but stronger than that in C. (E) Latency (in seconds) before paw withdrawal in homozygous mutants (gray) versus wild types (white) on a plate at 48, 51, and 53°C, respectively. For each genotype, n = 10. (F) Number of groomings on the lower back per 30 min for a wild type (white, n = 3) and mutant (dark, n = 5) upon injection of saline under the skin at the hairless site and equivalent. (G) Number of groomings on the lower back per 30 min for a wild type (white, n = 3) and mutant (gray, n = 5) after s.c. injection of lidocaine at the hairless site and equivalent. (H and I) Number of groomings on the flank for a control (n = 3) and mutant (n = 5) after injection of saline (H) or lidocaine (I) under the flank lateral to the upper leg. (J) Sections of the dorsal spinal cord of a control (Upper) and a mutant (Lower) after immunocytochemistry against c-fos (arrows) 30 min after capsaicin injection in the left hind foot; controls are on the right. (K) Time (in seconds) spent licking the paw immediately after intraplantar injection of capsaicin in the hind foot. Control(white) and Hoxb8 mutants (gray). For each genotype, n = 4. (L) c-fos-positive neuron counts in the dorsal horn of the spinal cord 90 min after capsaicin injection in the left hind foot. For both wild types (white bars), and mutants (gray bars), the number of c-fos-positive neurons on the noninjected side (1 and 3) are taken as 100%. Counts on the injected side are 2 and 4. Data were analyzed with the unpaired t test. P < 0.005. For all graphs, data are expressed as means ± SEM, except in E, where the mutants at 51°C were taken off the hot plate after 50 s.
Examination of the expression pattern of Hoxb6 and Hoxb9 by whole-mount in situ hybridization in E11.5 Hoxb8 mutants and controls established that the mutation in Hoxb8 does not affect the regulation of its 3′ and 5′ Hox neighbors on cluster HoxB (data not shown).
Hoxb8-Deficient Mice Develop Localized Skin Lesions.
The Hoxb8 knockin mutant was described in ref. 10. Mice were initially kept in a 129 Ola × FVB background. Minor vertebral abnormalities and smaller appearance of the second cervical DRG of homozygous mutants in this background (10) were not observed once the mutation was brought onto C57BL/6J × CBA background. Abnormal grooming behavior and subsequent skin lesions were manifested in Hoxb8 null mice in both genetic backgrounds. Mice homozygous for the Hoxb8lacZ knockin allele initially exhibited skin lesions in the lower back and neck regions with a penetrance of 30% when in the 129Ola × FVB genetic background (10, 11). Once in the C57BL/6J × CBA background, homozygous mutants carrying the same allele developed very localized lower back lesions exclusively, with a penetrance of nearly100% (supporting information (SI) Fig. S1). Interestingly, when these mice were brought back to a 129-containing background, they again developed both neck and lower back wounds (A. Juan and F. Ruddle, personal communication). Mice carrying a different Hoxb8 null allele in the homozygous state bred in two genetic backgrounds (C57BL/6J × 129Sv and SWR/J) were reported to develop skin lesions at both back and neck levels (12) with a high penetrance. Loss of Hoxb8 thus always causes local excessive grooming leading to cutaneous lesions, with a body distribution within the Hoxb8 expression domain and with a penetrance depending on the genetic background.
Hoxb8 Null Mutants Groom Their Lower Backs Excessively and Are Prevented from Doing so by Application of a Local Anesthetic.
Observations of Hoxb8lacZ mutant and control animals in two genetic backgrounds (129 Ola × FVB and C57BL/6 J × CBA) indicated that the mutants groom their lower backs excessively. The detailed phenotypic and molecular analysis of the mutants was performed with the C57BL/6J × CBA mice, and the data presented here were obtained by using mice in this genetic background, unless explicitly mentioned otherwise. Video scoring of early postweaning mutants and controls during consecutive sessions of 9 h allowed us to document that homozygous Hoxb8 mutants groom their lower backs more frequently, and during longer periods of time, than controls (Fig. S1). Repeated intensive lower back grooming sessions were observed in mutants exclusively during periods up to 20 min. Mutants progressively developed hairless areas and, subsequently, skin wounds at the corresponding very localized lower back positions (Fig. S1). Subcutaneous (s.c.) application of the local anesthetic lidocaine underneath the hairless area completely eliminated the sustained grooming behavior (Fig. 1 F–I), whereas s.c. lidocaine injection in nearby normal skin areas had no effect on the excessive grooming behavior. These data suggest that excessive grooming in the mutant depends on activity in afferent fibers from the groomed skin area and that these afferent stimuli are aberrant or aberrantly processed in the spinal cord, causing the mutants to excessively groom their lower backs, leading to local hair loss and development of skin wounds. The time of first appearance of bald spots varied from just before weaning time (3 weeks) to ages after the animals have been weaned. Development of skin lesions on isolated animals indicated that they inflicted the lesions on themselves and were not hurt by cage mates. During the observation, mutants were seen to groom themselves but did not groom their cage mates more often than control mice did.
Hoxb8 Null Mutants Show Reduced Avoidance Reactions to Noxious Stimuli Such as Heat and Capsaicin.
Homozygous Hoxb8 mutant mice in the two genetic backgrounds (129 Ola × FVB and C57BL/6 J × CBA) exhibited a strongly increased limb withdrawal latency in the hot plate test (ref. 11 and Fig. 1E, respectively), which was completely penetrant. Heterozygous animals reacted comparably with wild types in this test. Reaction to chemical nociception was impaired as well, because intraplantar capsaicin injection in the hind paw gave rise to much shorter licking times in homozygous mutants than observed in both heterozygous and nonmutant littermates (Fig. 1K). Thus, Hoxb8 mutant mice are much less sensitive to heat or chemical nociceptive inputs. Transganglionic labeling of primary afferent fibers in the superficial dorsal horn after wheat germ agglutinin–horseradish peroxidase (WGA–HRP) injection in the hind paw showed effective transport in primary afferent fibers and showed intact connectivity between the area of injection and the corresponding area in the superficial dorsal horn in homozygous Hoxb8 mutants (Fig. S2). These connections were further shown to be functional because they effectively activated neurons in the superficial dorsal horn, as shown by the increased number of neurons expressing c-fos after peripheral stimulation with capsaicin compared with the contralateral nonstimulated side (Fig. 1 J and L).
The attenuated behavioral response of Hoxb8 mutant mice to noxious stimuli was the result of a diminished sensory perception, reflected by the decreased duration of time spent licking upon capsaicin injection, rather than the result of a motor deficit in these mice. Animals freely moved their four legs without difficulty when walking and grooming. There was no significant difference between the number and distribution of motoneurons in the ventral horn of the lumbar spinal cord of the mutants and controls (data not shown).
DRG Neurons Are Not Participating in Causing the Hoxb8 Mutant Phenotype.
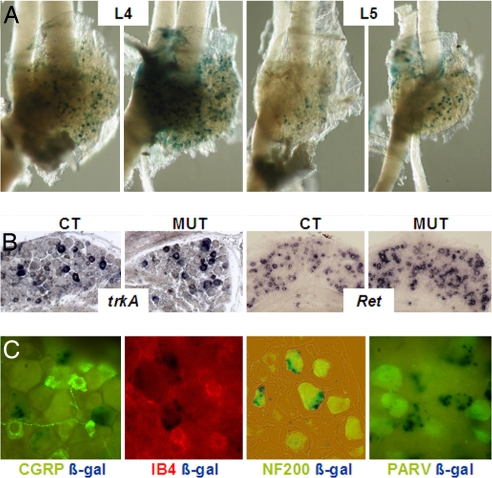
Hoxb8 that is expressed in the neural crest is expressed in the DRGs from the time of their formation at approximately E9.5. Expression levels are lower than in the spinal cord and further decrease after E 13.5 to remain detectable only in a subpopulation of DRG neurons after birth. The neuronal circuitry transmitting sensory signals develops at the embryonic stage between E10 and birth (14, 15). We examined the sensory neurons in the DRGs corresponding to hindlimb and lower back sensory innervation, lumbar DRG L4 and L5, in Hoxb8 mutant mice and controls. The size of the DRGs in Hoxb8 mutant and controls appeared to be indistinguishable in embryos, at postnatal day (P)11 (Fig. 2A) and at other postnatal ages (data not shown). Detailed cell counts in the L4 and L5 ganglia of adult wild type and mutants showed that the number of ganglion cells and the number of CGRP-labeled neurons were the same (data not shown). The proportion of cells expressing Hoxb8lacZ was comparable in heterozygous and homozygous mutant DRGs.
Fig. 2.
Expression of Hoxb8lacZ and neuronal markers in the DRGs of heterozygote and homozygote Hoxb8 mutant mice. (A) Whole-mount DRGs L4 and L5 from a heterozygote (CT) and a homozygote (MUT) at P11, stained with X-Gal. (B) In situ detection of trkA and Ret transcripts in sections of paraffin-embedded DRG L4 and L5 of control and Hoxb8 mutant mice at P28. (C) Representative sections of DRG L4 from a heterozygote Hoxb8lacZ at P11, doubly stained for β-galactosidase and CGRP, β-Gal and IB4, β-Gal and NF200, and β-Gal and parvalbumin, respectively. Only NF200 imunoreactivity colabeled with some lacZ-positive DRG neurons.
At late embryonic stages, trkA+ sensory neurons in the DRGs represent a crucial population for nociception. During postnatal development, diversification of this population into trkA+ and trkA− Ret+ sensory neurons is required for differentiation of the two types of sensory neurons involved in nociception (14). In situ hybridization of DRG sections from P28 animals with trkA and Ret probes allowed us to conclude that the proportion of both types of neurons was similar in mutants and controls (Fig. 2B), showing that diversification of nociceptive sensory neurons occurs normally in the mutants.
To determine which type of DRG neurons express Hoxb8, we performed double labeling on sections through spinal ganglion L4 and L5 of P11 heterozygous Hoxb8lacZ mice with X-Gal and with markers characteristic of specific classes of DRG neurons. We used immunofluorescence and immunocytochemistry to detect CGRP, a neuropeptide mainly characterizing the trkA+ group of nociceptive neurons with axons terminating in laminae I and II outer of the spinal dorsal horn. We also used binding of IB4, a lectin characterizing the Ret-expressing group of nociceptive neurons, terminating in lamina II inner and parvalbumin, characterizing large nonnociceptive neurons terminating in the deeper laminae of the dorsal horn (Fig. 2C). Absence of double labeling revealed that the Hoxb8lacZ-positive cells are neither nociceptive (CGRP and IB4 reacting) nor parvalbumin-positive DRG neurons. This indicates that DRG neurons expressing Hoxb8 are not neurons involved in transmitting nociceptive and/or thermal peripheral information. Costaining with X-Gal and with an antibody against NF 200 revealed that at least some of the Hoxb8-expressing DRG neurons are large myelinated neurons belonging to a parvalbumin-negative class, possibly involved in proprioception. This might correlate with a transient abnormal locomotion feature of some of the mutants, who were sometimes seen to pull their hindlimbs higher or extend them more than normally when walking (data not shown).
Taken together, the results provide strong evidence that the DRG neurons involved in thermo- and nociception are not compromised in the Hoxb8 mutant mice. This strongly suggests that the behavioral abnormalities observed are due to the changes that we observe in the dorsal spinal cord, as described below.
Dorsal Spinal Neurons Are Abnormally Distributed at and After Birth in Hoxb8 Mutants.
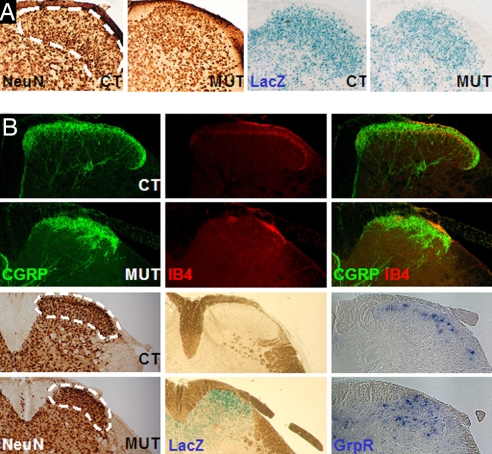
We labeled dorsal horn neurons with the panneuronal marker NeuN (16) on lumbar sections of Hoxb8 homozygous mutant and control spinal cords (L4/L5 level) around birth and at postnatal stages. From a certain stage on, laminae I and II are recognizable in separate domains in the dorsal spinal cord (17, 18) (Fig. 3A). At P1 and P2, the morphologically narrower laminae I and II in the mutants, corresponding to the domain of Hoxb8 and Hoxb8lacZ expression, were found to contain 35% less neurons than in the controls (Fig. 3 and Fig. S3). These lower numbers, corresponding to a mediolateral reduction of the upper spinal laminae in Hoxb8 null mice, remained the same at P2, P6, P13, and P28, when the phenotype of excessive grooming was first manifested in the mutants and in adults (P90) (Fig. S3). Apoptosis detection assays (activated caspase 3 and TUNEL labeling) did not reveal more apoptosis in mutants than in controls at any of the stages that were analyzed (each day between E15.5 and P13, data not shown).
Fig. 3.
Organization of the dorsal horn of the spinal cord in controls and Hoxb8 null mice. (A) Distribution of NeuN- (Left) and lacZ- (Right) positive neurons in the dorsal spinal cord of heterozygotes (CT) and homozygotes (MUT) at P2. (B) (Upper) Labeling of the dorsalmost spinal cord of control and mutant mice (P28) with anti-CGRP and anti-IB4. The outside IB4 signal aligned with the contour of the gray matter corresponds to the incoming fibers of the afferents and not to their end projection inside lamina II. (Lower Left) Staining of consecutive sections of the lumbar spinal cord of a wild-type (WT) and a Hoxb8 null animal (MUT) at P28 with NeuN and X-Gal (the wild type is not stained). (Lower Right) Expression of the itch-specific marker GrpR in a subpopulation of dorsal horn spinal neurons of laminae I in wild-type (CT) and Hoxb8 null (MUT) adult mice at P28.
As a control for the observed lower neuron counts in the mutant dorsal horn, ventral spinal cord neurons were scored at L4 levels. No difference was seen between controls and mutants at birth and in adults (data not shown). Isl1 staining and motoneuron identification using immunocytochemistry for choline acetyl transferase (ChAT) did not reveal any change in the number or distribution of the motoneurons in the lumbar spinal cord of Hoxb8 null mutants compared with wild types (data not shown).
The lower NeuN-positive neuron number and the narrowed Hoxb8lacZ territory in the dorsal spinal cord at lumbar levels in the mutants suggested a change in the cytoarchitecture of this area. This was confirmed at later, adult stages after staining with galanin, a marker of neurons in lamina I and II outer, calretinin and calbinding, markers of neurons in lamina I and II, and PKCγ, a marker of lamina II inner. All differentiation markers tested were expressed in the dorsal horn, although in territories less extended mediolaterally in mutants compared with controls (Fig. S3), indicating that Hoxb8 is not required for the differentiation of these neurons but for their organization in the dorsal horn.
The late differentiation step of late born dorsal neurons leading to the appearance of inhibitory, GABAergic neurons was shown to take place in the mutant as well, because mutant dorsal horn expressed glutamic acid decarboxylase (Gad67) mRNA and enkephalin mRNA (data not shown).
Altered Projection Field of Thermo- and Nociceptive Sensory Afferents in the Dorsal Spinal Cord of the Mutants.
Labeling of the projections of CGRP- and IB4-positive neurons in the dorsal spinal cord made it clear that the projection field of the thermo- and nociceptive sensory afferents from the DRG neurons is reduced and altered in shape in the mutants (Fig. 3B) in correlation with the alteration in laminae I and II. Whereas the CGRP-labeled domain is narrower mediolaterally, and less well dorsally restricted than in controls, the IB4-labeled area is both narrower and much more reduced in mutants than in controls (Fig. 3B). Accordingly, the dorsal spinal neurons receiving the IB4-positive afferent fibers, marked by their expression of PKCγ in lamina II inner are also apparently reduced in numbers and distributed in a narrower domain (Fig. S3). Double staining for CGRP and IB4 showed that the termination areas of these nociceptive afferents remained separated. The IB4-labeled territory mapped directly ventrally to the CGRP domain, in both controls and mutants. Detection of nociception-associated markers such as the Sub P receptor NK1 in the spinal dorsal laminae (data not shown) confirmed the reduction and disorganization of these dorsal horn neurons. These data clearly point to defects in the target territories of nociceptive sensory projections in the dorsal spinal cord of Hoxb8 null mice. It seems likely that the observed changes correlate with the increased nociceptive thresholds observed in the mutants.
Disorganization of Spinal Neurons Mediating Itch Sensations in Hoxb8 Mutants.
A separate perception channel involving lamina I dorsal horn neurons has been proposed to process itch-inducing stimuli. A subpopulation of dorsal lamina I neurons expressing the gastrin-related peptide receptor (GrpR) was recently identified to specifically mediate itch sensation independently of nociceptive signals in the mouse spinal cord (19). Hybridization of lumbar spinal cord sections of 4-week-old Hoxb8 mutants and controls with a GRPR probe revealed that the itch-mediating dorsal neurons are highly disorganized in the mutant, compared with their nicely aligned dorsal distribution in controls (Fig. 3). Future work will tell whether the disorganization of these neurons and their resulting abnormal relative location are causally related to the abnormal grooming behavior of the mutants.
Early Establishment of Laminae I and II of the Dorsal Spinal Cord Is Affected in Hoxb8 Mutants.
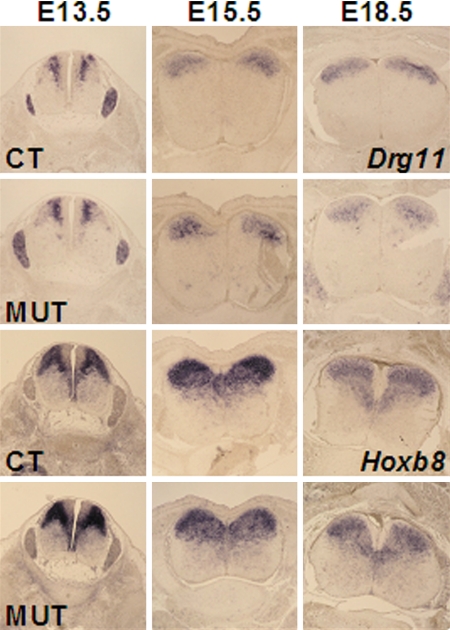
Virtually all neurons of dorsalmost laminae at the level of L4/L5, which receive nociceptive inputs from the hindlimbs and lower back region, express Hoxb8 (see Fig. 3A). Lamina I and II neurons are born at approximately E12. 5 from the middle region of the dorsal ventricular zone, become postmitotic, and migrate dorsally and “inside out,” reaching the upper dorsal horn layers at approximately E14.5 (14, 20). We first examined the proliferation activity of the progenitors of lamina I and II neurons in the ventricular zone of the L4/L5 lumbar region of mutant and control embryos, after in utero labeling of E12.25 embryos with BrdU and analysis of the number and localization of BrdU-labeled neurons 2 h later. Labeled dorsal neurons were in similar number in homozygous Hoxb8 mutants and littermate controls (data not shown). Because the transcription factor-encoding Drg11 is also required for correct processing of cutaneous sensory information (15, 21) and because Drg11, like Hoxb8, is strongly expressed in the upper dorsal laminae at E15.5 and later (Fig. 1) (13, 21), we compared the evolution of the expression of these genes with the behavior of the precursors of laminae I and II neurons of the dorsal spinal cord. The expression of Hoxb8lacZ and Drg11 at E13.5 was similar in mutants and controls, with the only difference being that the expression level of Hoxb8lacZ is higher in the homozygous Hoxb8 mutant than in its heterozygous control, as expected (Fig. 4). Strikingly, the expression patterns of Hoxb8lacZ and Drg11 nicely overlap with the distribution pattern of the precursors of laminae I and II neurons, revealed after BrdU incorporation between E12.25 and E13.5 (Fig. 4 and Fig. S4). The expression domains of both Hoxb8lacZ and Drg11 at E18.5 had an altered shape in mutants, being narrower mediolaterally, and wider dorsoventrally (Fig. 4). BrdU labeling at E12.25 and subsequent analysis of the labeled cell populations at E14.5 revealed that the distribution of BrdU-labeled dorsal neurons was similar in controls and mutants (Fig. S4). However, the mutants seem to already exhibit a slight mediolateral narrowing and a less strong dorsal restriction of Drg11 expression at E15.5 (Fig. 4). This suggests that an impairment in the establishment of well organized laminae I and II begins at approximately E15.5 and is more evident at E18.5. These changes thus foreshadow the clear differences between mutants and controls at and after birth, documented by narrower, less densely packed, and less well layered domains of neurons expressing laminae I and II markers at postnatal stages (Fig. S3).
Fig. 4.
Expression of Drg11 and Hoxb8 or Hoxb8lacZ in the dorsal horns of the spinal cords of mutant and control embryos. Shown is expression of Drg11 (Upper) and Hoxb8lacZ (Lower) in the lumbar spinal cords of controls and mutants at E13.5, E15.5, and E18.5.
In agreement with the restriction of the phenotype to the lower back area in these mice, changes in dorsal laminae I and II in the Hoxb8 mutants were observed in the lumbar area, whereas there was hardly any difference the cervical region (Fig. S5).
Discussion
Grooming Phenotype and Dorsal Horn Defects Involve Hoxb Genes in Sensory Perception at the Spinal Cord Level.
The Hoxb8 loss-of-function mutants do not die at birth and therefore present the valuable advantage of being amenable to behavioral experimentation. We sought to understand the cellular and molecular genetic basis for the localized excessive grooming phenotype exhibited by the Hoxb8 null mutant mice.
The fact that Hoxb8 is expressed at particularly high levels in the dorsal spinal cord throughout embryogenesis and after birth warranted further investigation of the spinal sensory system in the mutant phenotype. The superficial dorsal horn area, receiving peripheral sensory information, was found to be abnormal in Hoxb8 null animals. A decreased lateral extension of the superficial neuronal layers in mutants relative to controls was accompanied by less well dorsally packed laminae I and II neurons in the lumbar region. In particular, the disorganized distribution of the recently discovered itch-specific GrpR-positive neurons in lamina I of the dorsal spinal cord (19) opens the possibility that the defect in dorsal laminae in the Hoxb8 mutant leads to irrelevant itch perception, and elicits the localized excessive lower back grooming of the animals. Suppression of the excessive lower back grooming by locally anesthetizing the groomed skin area proves that the mutant phenotype depends on peripheral inputs and must result from abnormal spinal processing of external stimuli. This functional evidence implies that the cause of the abnormal Hoxb8 null mutant behavior involves the relay of sensory transmission by dorsal spinal neurons. The disorganized lamina I neurons might secondarily aberrantly contact their projection targets in the brain, indirectly involving higher brain centers in the phenotype, thus in agreement with the conclusion by Greer and Capecchi (12) in their study of another Hoxb8 mutant allele.
Altered Dorsal Laminae and Defective Nociceptive and Thermoceptive Response of Hoxb8 Mutants.
Hoxb8 null mice exhibit an aberrant reaction to noxious stimuli. An attenuated response to pain and heat was observed with a penetrance of nearly 100% in the mutants. The mutant dorsal horn showed narrowed upper laminae, the neuronal territories receiving the primary afferent projections from DRG sensory neurons transmitting pain and heat signals. Accordingly, a significant, 35% lower number of neurons in the superficial laminae was documented around birth and in adult Hoxb8 mutant mice. The changes in interneuronal organization that this decrease must cause may well account for the reduced response of the Hox mutant mice to nociceptive and thermal stimuli observed in our behavioral experiments.
Different Genetic Pathways Lead to Similar Sensory Phenotypes.
The reduced response to pain and heat, together with changes in the afferent projection field of corresponding sensory neurons, reduced dorsal horn neuron numbers, and appearance of skin lesions on the lower back of Hoxb8 null mutants, is reminiscent of the phenotype of Drg11 knockout mice. The aberrant projection of the cutaneous sensory fibers in Drg11 mutants was attributed partly to defects of the dorsal spinal cord neurons (13, 21). However, Drg11 mutants exhibit a more dramatic dorsal neuron loss than Hoxb8 mutants, partially accounted for by enhanced cell death at E17.5. In addition, Drg11 was shown to be required for the survival of CGRP- and IB4-positive sensory neurons in the DRGs (15), which was not the case for Hoxb8. Hoxb8 is expressed in the dorsal spinal cord from its emergence before E8 (22) and, thus, long before Drg11 starts to be transcribed at E12 (21). The expression of Drg11 was not changed in early Hoxb8 mutants (Fig. 4, E13.5), and expression of Hoxb8 was unaltered in Drg11 null mice at early stages after Drg11 normally starts to be expressed (Z. F. Chen, personal communication). Therefore, the genes are not likely to directly regulate each other. However, the abnormally low dorsal neuron counts in the postnatal Hoxb8 and Drg11 mutants and the resulting lower expression of Drg11 and Hoxb8, respectively, in the dorsal horn, may underlie the similarity in sensory phenotypes.
A recent study reported sensory deficiency and defects in the dorsal spinal cord in mouse mutants missing Pbx3 function in the nervous system posterior to the hindbrain (23). Because Pbx proteins act as Hox transcriptional cofactors, it is not unlikely that this mutant and the Hoxb8 mutant affect partly overlapping developmental pathways. The present work on Hoxb8 mutants, together with the work on Pbx3 and experiments showing altered sensory response in transgenic mice ectopically expressing another Hox gene (24), strongly suggests that Hox gene expression has to be well balanced at the different A–P levels in the dorsal spinal neurons relaying sensory information.
Strain Dependence of the Hox Mutant Phenotype.
Hoxb8 null mice in the C57BL/6J × CBA background develop skin wounds on the lower back exclusively, whereas the same Hoxb8 null allele gives rise to lesions at lumbar and scapular levels when brought into the C57BL/6J × 129 background. Losing Hoxb8 function thus caused the appearance of cutaneous lesions in specific axial regions, scapular in some genetic backgrounds and pelvic in all cases. The functional requirement of Hoxb8 may be stronger at girdle positions, where an increment of somatosensory information from the limbs has to be processed. Variable Hoxb8 requirement depending on the genetic background may reflect slight variations in the expression levels of the other Hox gene complement in discrete axial regions all along the axis, resulting in a different contribution of Hoxb8 to the combinatorial Hox code at specific positions in different strains. The extent of redundancy between Hox gene family members at given axial positions might possibly vary with the genetic constitution of the strain. The genetic components of this variation may be interesting to identify, because they potentially represent quantitative trait loci (QTLs) underlying the strain-specific sensory phenotype.
Hoxb8 and Dorsal Horn Neuronal Distribution and Differentiation at the Lumbar Level.
Our data demonstrate A–P-restricted defects in the neuronal populations of laminae I and II in Hoxb8 null mice. These late born neurons seem to begin their dorsal “inside out” migration normally, but the data are compatible with the possibility that not all of them end up populating the dorsalmost layers of the spinal cord, accounting for the observation of a reduced number of laminae I and II neurons and of an altered organization of these neurons in the lumbar region of the Hoxb8 mutants at and after birth.
Despite the lower number of laminae I and II dorsal neurons and their abnormal organization, differentiation of these second-order neurons was found to have occurred normally in adults, as judged by their expression of a number of specific markers. None of the markers we assayed was unexpressed in mutant dorsal horn neurons. Loss of Hoxb8 function therefore does not seem to prevent dorsal horn neuron differentiation but rather the organization of neuron distribution within the dorsalmost laminae I and II at lumbar axial levels.
These findings reveal a cross-talk between the genetic network underlying Hox-associated A–P positional information and the laying down of functionally defined neuronal populations in the sensory system in the spinal cord. Because processing of sensory information in the dorsal horn is a necessary relay in the pain and itch pathways (25, 19), its understanding from studying mutants such as the Hoxb8 loss-of-function mice should help shed light on the development and modulation of pathological pain and itch states in humans.
Materials and Methods
Mice.
The Hoxb8lacZ heterozygote mice used in this work were in the C57BL/6J × CBA genetic background (but see Results for data on different backgrounds). They were intercrossed to produce homozygote embryos. Mice and embryos were genotyped by PCR as described in ref. 10. For the grooming observations, mice of the same age and gender were videotaped while alone in their cages. For all behavioral experiments, no selection was made of either males or females, but it was verified retrospectively that both male and female mutants were present among the experimental animals in all cases. Wild-type littermates of the Hoxb8 mutants were taken as controls. The day of the plug was taken as E0.5, and the day of birth was P1.
Hot Plate, Lidocaine, and Capsaicin Tests.
These procedures are detailed in SI Text.
Dissection of Spinal Cords and DRGs.
Spinal cord segments and DRGs were dissected from embryos and mice of the different genotypes as described in SI Text.
Immunocytochemistry and Immunofluorescence.
The source of antibodies used is described in SI Text.
In Situ Hybridization and X-Gal Staining.
Documentation of gene expression by in situ hybridization with nonradioactive riboprobes was performed according to standard protocols. See SI Text for details and for probes.
Neuron Counting and Statistics.
Dorsal spinal cord neurons were counted in an area corresponding to laminae I and II as indicated in the Fig. 3 legend. Counting was done on NeuN-stained sections by using the SIS software. See SI Text for details. Statistical analysis used the nonparametric Mann–Whitney test and the software program SPSS (version 15). The P values were determined and indicated in the figure legends.
Supplementary Material
Acknowledgments.
We thank Qiufu Ma (Dana–Farber Cancer Institute, Boston) for the Ret and TrkA probes, Zhou-Feng Chen (Washington University, St. Louis) for the Drg11 probe, Frank Hamers for help with the hot plate tests in the initial series of experiments, Kevin de Smet and Daniel de Waard for technical help, Linda van Laake for help with the statistical analysis, Frits Meijlink for reading the manuscript, and Filippo Rijli and Jeremy Dasen for discussions. J.D. received support from the Netherlands Organisation for Scientific Research Earth and Life Sciences, and J.D. and W.d.G. were supported by a grant from the Dutch government (BSIK Program 03038 “Stem Cells in Development and Disease” and by the European Network of Excellence (FP6) “Cells into Organs.”
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0802176105/DCSupplemental.
References
- 1.Trainor PA, Krumlauf R. Patterning the cranial neural crest: Hindbrain segmentation and Hox gene plasticity. Nat Rev Neurosci. 2000;1:116–124. doi: 10.1038/35039056. [DOI] [PubMed] [Google Scholar]
- 2.Oury F, et al. Hoxa2- and rhombomere-dependent development of the mouse facial somatosensory map. Science. 2006;313:1408–1413. doi: 10.1126/science.1130042. [DOI] [PubMed] [Google Scholar]
- 3.Tiret L, Le Mouellic H, Maury M, Brulet P. Increased apoptosis of motoneurons and altered somatotopic maps in the brachial spinal cord of Hoxc-8 deficient mice. Development. 1998;125:279–291. doi: 10.1242/dev.125.2.279. [DOI] [PubMed] [Google Scholar]
- 4.Tarchini B, Huynth TH, Cox GA, Duboule D. HoxD cluster scanning deletions identify multiple defects leading to paralysis of the mouse mutant Ironside. Genes Dev. 2005;19:2862–2876. doi: 10.1101/gad.351105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dasen JS, Liu JP, Jessell TM. Motoneuron columnar fate imposed by sequential phases of Hox-c activity. Nature. 2003;425:926–933. doi: 10.1038/nature02051. [DOI] [PubMed] [Google Scholar]
- 6.Dasen JS, Tice BC, Brenner-Morton S, Jessell TM. A Hox regulatory network establishes motoneuron pool identity and target-muscle connectivity. Cell. 2005;123:363–365. doi: 10.1016/j.cell.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 7.Gaufo GO, Wu S, Capecchi MR. Contribution of Hox genes to the diversity of the hindbrain sensory system. Development. 2004;131:1251266. doi: 10.1242/dev.01029. [DOI] [PubMed] [Google Scholar]
- 8.Graham A, Maden M, Krumlauf R. The murine Hox-2 genes display dynamic dorsoventral patterns of expression during central nervous system development. Development. 1991;112:255–264. doi: 10.1242/dev.112.1.255. [DOI] [PubMed] [Google Scholar]
- 9.Charité J, de Graaff W, Vogels R, Meijlink F, Deschamps J. Regulation of the Hoxb-8 gene: Synergism between multimerized cis-acting elements increases responsiveness to positional information. Dev Biol. 1995;171:294–305. doi: 10.1006/dbio.1995.1282. [DOI] [PubMed] [Google Scholar]
- 10.Van den Akker E, et al. Targeted inactivation of Hoxb8 affects survival of a spinal ganglion and causes aberrant limb reflexes. Mech Dev. 1999;89:103–114. doi: 10.1016/s0925-4773(99)00212-9. [DOI] [PubMed] [Google Scholar]
- 11.Van den Akker E. Utrecht, The Netherlands: Univ of Utrecht; 2001. Patterning functions of mouse Hox genes; pp. 53–68. ISDN 90-393-2623-1. PhD thesis. [Google Scholar]
- 12.Greer JM, Capecchi MR. Hoxb8 is required for normal grooming behavior in mice. Neuron. 2002;33:23–34. doi: 10.1016/s0896-6273(01)00564-5. [DOI] [PubMed] [Google Scholar]
- 13.Ding YQ, et al. Lmx1b controls the differentiation and migration of the superficial dorsal horn neurons of the spinal cord. Development. 2004;131:3693–36703. doi: 10.1242/dev.01250. [DOI] [PubMed] [Google Scholar]
- 14.Fitzgerald M. The development of nociceptive circuits. Nat Rev Neurosci. 2005;6:507–520. doi: 10.1038/nrn1701. [DOI] [PubMed] [Google Scholar]
- 15.Rebelo S, Chen ZF, Anderson DJ, Lima D. Involvement of DRG11 in the development of the primary afferent nociceptive system. Mol Cell Neurosci. 2006;33:236–246. doi: 10.1016/j.mcn.2006.07.013. [DOI] [PubMed] [Google Scholar]
- 16.Mullen RJ, Buck CR, Smith AM. NeuN, a neuronal specific protein in vertebrates. Development. 1992;115:201–211. doi: 10.1242/dev.116.1.201. [DOI] [PubMed] [Google Scholar]
- 17.Todd AJ, Spike RC, Polgar E. A quantitative study of neurons which express neurokinin-1 or somatostatin sst2a receptor in rat spinal dorsal horn. Neuroscience. 1998;85:459–473. doi: 10.1016/s0306-4522(97)00669-6. [DOI] [PubMed] [Google Scholar]
- 18.Polgar E, Gray S, Riddell JS, Todd AJ. Lack of evidence for significant neuronal loss in laminae I–III of the spinal dorsal horn of the rat in the chronic constriction injury model. Pain. 2004;111:144–150. doi: 10.1016/j.pain.2004.06.011. [DOI] [PubMed] [Google Scholar]
- 19.Sun YG, Chen ZF. A gastrin-related peptide receptor mediates the itch sensation in the spinal cord. Nature. 2007;448:700–703. doi: 10.1038/nature06029. [DOI] [PubMed] [Google Scholar]
- 20.Caspary T, Anderson KV. Patterning cell types in the dorsal spinal cord: What the mouse mutants say. Nat Rev Neurosci. 2003;4:289–297. doi: 10.1038/nrn1073. [DOI] [PubMed] [Google Scholar]
- 21.Chen ZF, et al. The paired homeodomain protein DRG11 is required for the projection of cutaneous sensory afferent fibers to the dorsal spinal cord. Neuron. 2001;31:59–73. doi: 10.1016/s0896-6273(01)00341-5. [DOI] [PubMed] [Google Scholar]
- 22.Deschamps J, Wijgerde M. Two phases in the establishment of HOX expression domains. Dev Biol. 1993;156:473–480. doi: 10.1006/dbio.1993.1093. [DOI] [PubMed] [Google Scholar]
- 23.Rottcamp CA, Lobur KJ, Wladyka CL, Lucky AK, O'Gorman S. Pbx3 is required for normal locomotion and dorsal horn development. Dev Biol. 2008;314:23–29. doi: 10.1016/j.ydbio.2007.10.046. [DOI] [PubMed] [Google Scholar]
- 24.Abbott MA, Joksimovic M, Tuggle CK. Ectopic HOXA5 expression results in abnormal differentiation, migration and p53-independent cell death of superficial dorsal horn neurons. Dev Brain Res. 2005;159:87–97. doi: 10.1016/j.devbrainres.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 25.Zeilhofer HU. Synaptic modulation in pain pathways. Rev Physiol Biochem Pharmacol. 2005;154:73–100. doi: 10.1007/s10254-005-0043-y. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.