Abstract
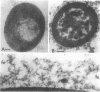
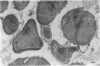
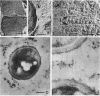
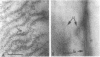
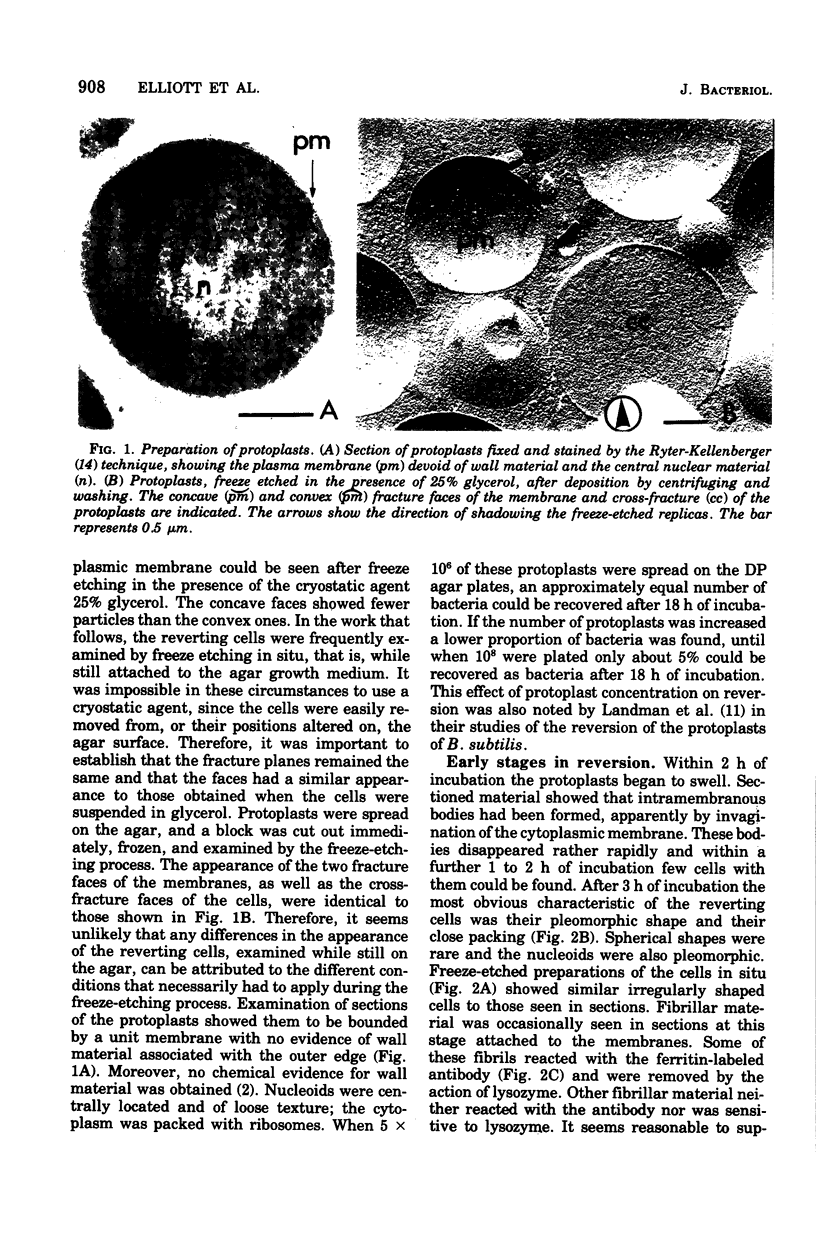
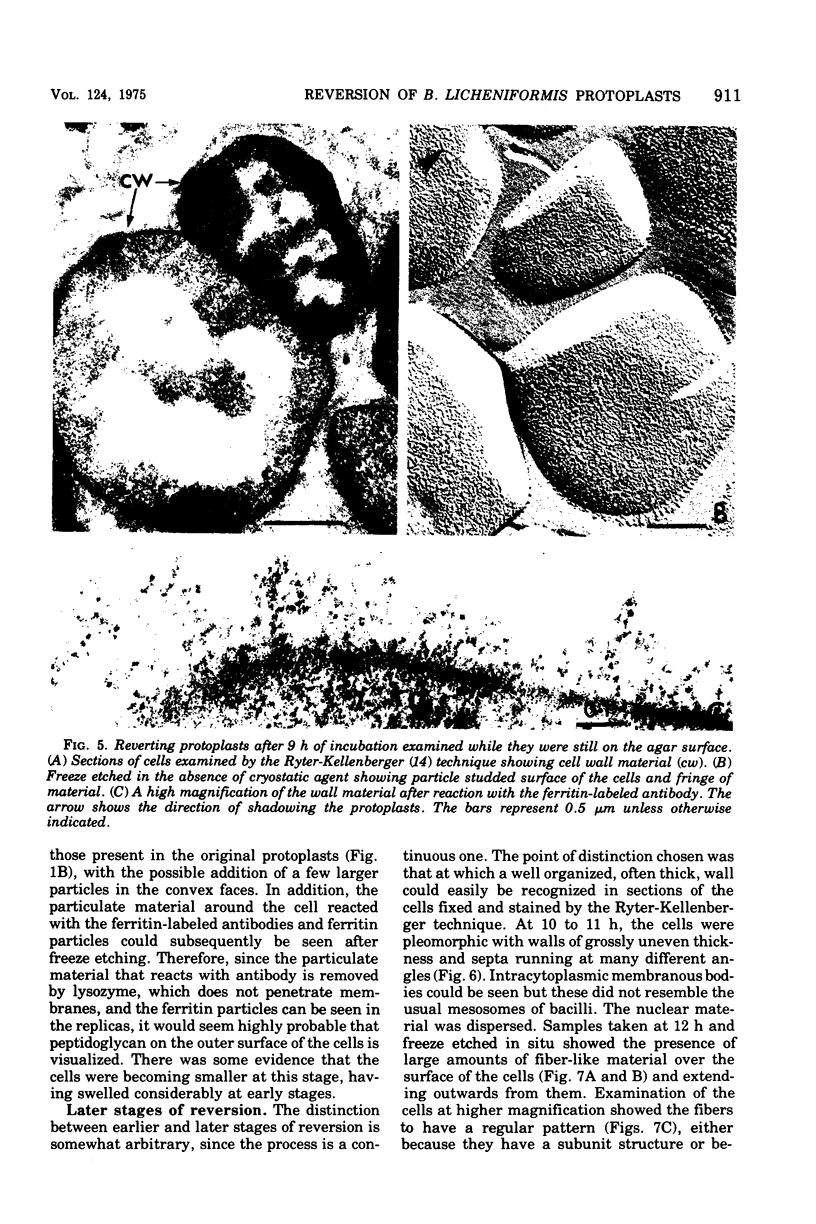
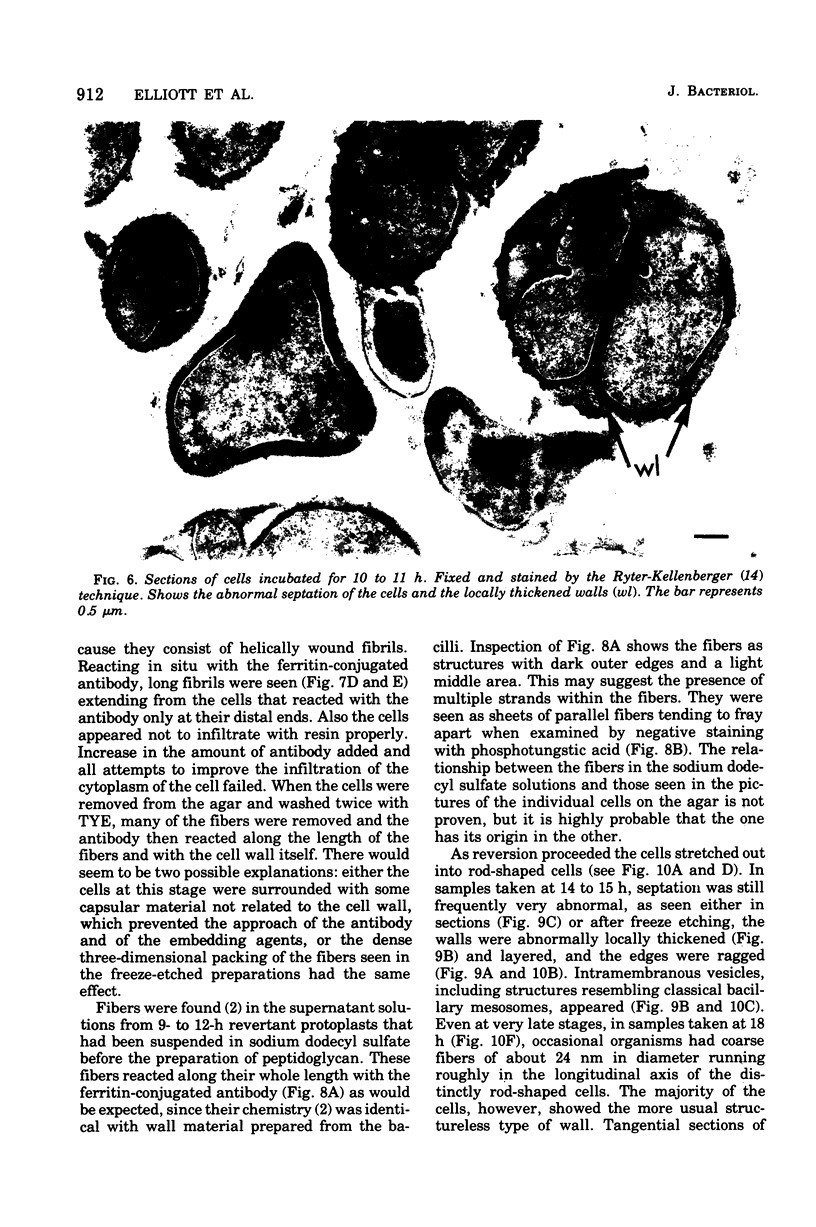
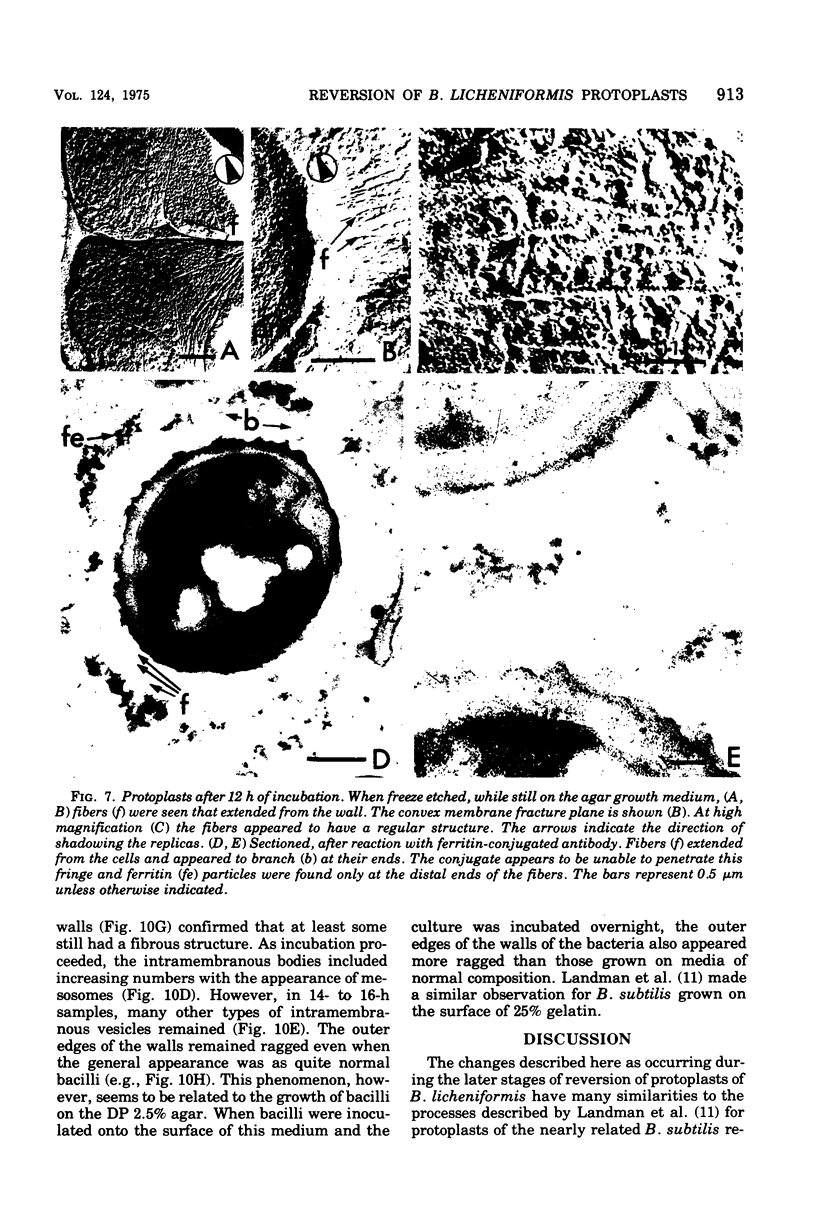
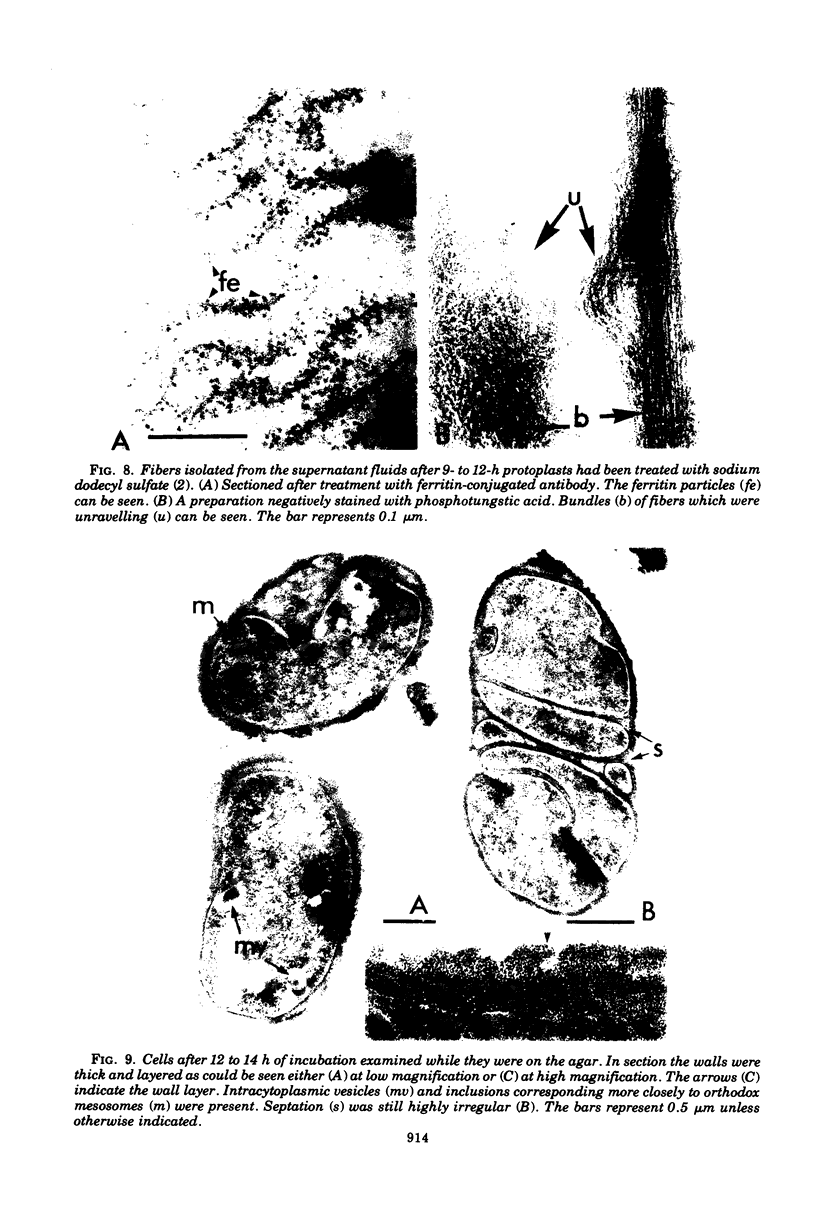
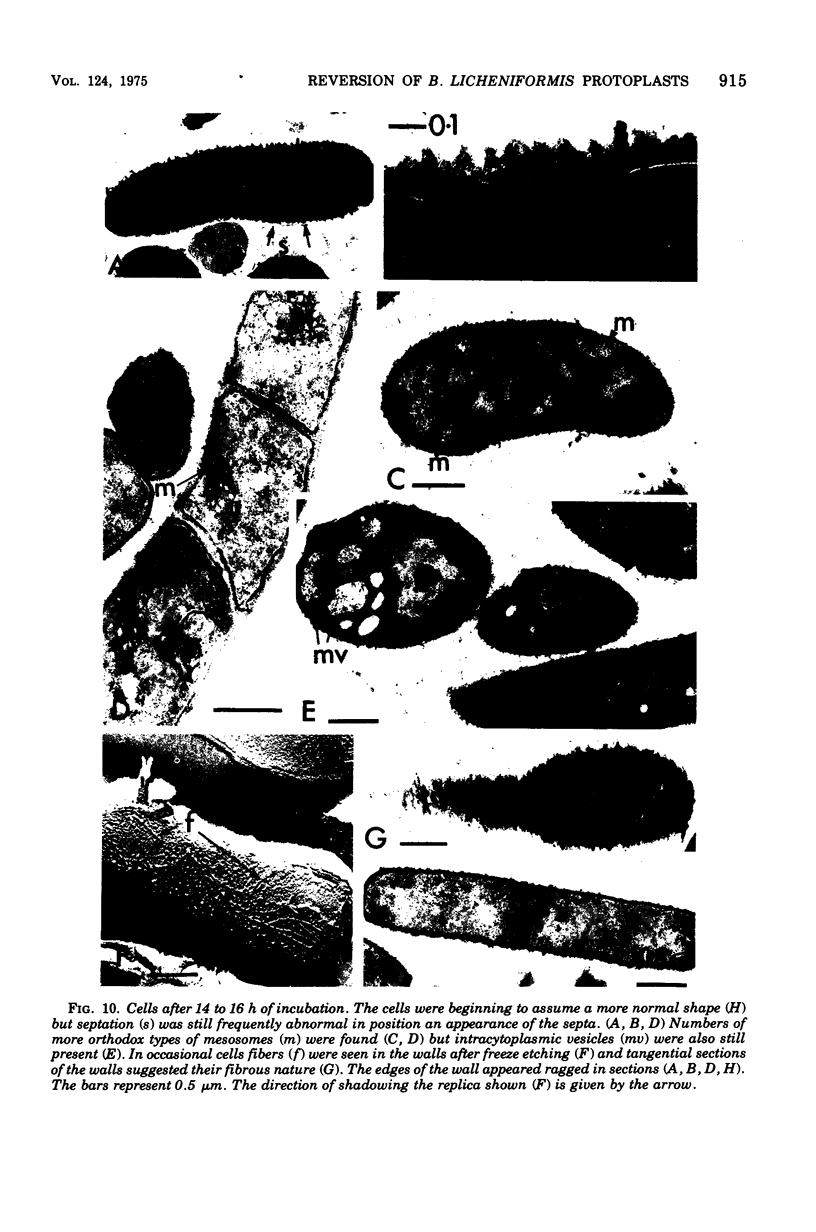
The reversion of protoplasts of Bacillus licheniformis 6346 His- on a medium containing 2.5% agar has been studied in sectioned material after reaction with a ferritin-conjugated antibody specific to the peptidoglycan isolated from the walls of the bacilli. Freeze etching has also been used. Fibrils of material reacting with the antibody have been detected emerging from isolated areas of the protoplasts after 3 h of incubation. This material gradually covers the cell and can eventually (at 6 h) be seen in freeze-etched preparations as a fringe of up to 400 nm around the cells and covering the surfaces with particles that can be removed by lysozyme. At later stages the wall begins to take on a compact, well-defined appearance that can be seen in sections; however, the cells are still grossly deformed. A transitory emergence, beyond the wall of long fibers of 6 nm in diameter, takes place after about 12 h of incubation. These fibers react with the conjugated antibody and after freeze etching show a regular banded structure. They are probably indentical with the fibers isolated elsewhere (Elliott et al., 1975) and shown to contain all the wall constituents (i.e., peptidoglycan, teichoic acid, and teichuronic acid). These fibers are not detectable in the final stages of reversion.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Elliott T. S., Ward J. B., Rogers H. J. Formation of cell wall polymers by reverting protoplasts of Bacillus licheniformis. J Bacteriol. 1975 Nov;124(2):623–632. doi: 10.1128/jb.124.2.623-632.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsberg C. W., Ward J. B. N-acetylmuramyl-L-alanine amidase of Bacillus licheniformis and its L-form. J Bacteriol. 1972 Jun;110(3):878–888. doi: 10.1128/jb.110.3.878-888.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes R. C., Stokes E. Cell wall growth in Bacillus licheniformis followed by immunofluorescence with mucopeptide-specific antiserum. J Bacteriol. 1971 May;106(2):694–696. doi: 10.1128/jb.106.2.694-696.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes R. C., Tanner P. J. The action of dilute alkali on some bacterial cell walls. Biochem Biophys Res Commun. 1968 Oct 10;33(1):22–28. doi: 10.1016/0006-291x(68)90248-9. [DOI] [PubMed] [Google Scholar]
- Hughes R. C., Thurman P. F., Salaman M. R. Antigenic properties of Bacillus licheniformis cell wall components. Eur J Biochem. 1971 Mar 1;19(1):1–8. doi: 10.1111/j.1432-1033.1971.tb01281.x. [DOI] [PubMed] [Google Scholar]
- King J. R., Gooder H. Induction of enterococcal L-forms by the action of lysozyme. J Bacteriol. 1970 Sep;103(3):686–691. doi: 10.1128/jb.103.3.686-691.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King J. R., Gooder H. Reversion to the streptococcal state of enterococcal protoplasts, spheroplasts, and L-forms. J Bacteriol. 1970 Sep;103(3):692–696. doi: 10.1128/jb.103.3.692-696.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LANDMAN O. E., HALLE S. ENZYMICALLY AND PHYSICALLY INDUCED INHERITANCE CHANGES IN BACILLUS SUBTILIS. J Mol Biol. 1963 Dec;7:721–738. doi: 10.1016/s0022-2836(63)80119-9. [DOI] [PubMed] [Google Scholar]
- Landman O. E., Forman A. Gelatin-induced reversion of protoplasts of Bacillus subtilis to the bacillary form: biosynthesis of macromolecules and wall during successive steps. J Bacteriol. 1969 Aug;99(2):576–589. doi: 10.1128/jb.99.2.576-589.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landman O. E., Ryter A., Fréhel C. Gelatin-induced reversion of protoplasts of Bacillus subtilis to the bacillary form: electron-microscopic and physical study. J Bacteriol. 1968 Dec;96(6):2154–2170. doi: 10.1128/jb.96.6.2154-2170.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Necas O., Svoboda A. Effect of proteases, phospholipases and polysaccharide-splitting enzymes on plasma membrane particles and on the synthesis of the fibrillar cell wall component in yeast protoplasts. Folia Microbiol (Praha) 1974;19(2):81–87. doi: 10.1007/BF02872839. [DOI] [PubMed] [Google Scholar]
- RYTER A., KELLENBERGER E., BIRCHANDERSEN A., MAALOE O. Etude au microscope électronique de plasmas contenant de l'acide désoxyribonucliéique. I. Les nucléoides des bactéries en croissance active. Z Naturforsch B. 1958 Sep;13B(9):597–605. [PubMed] [Google Scholar]
- Roth G. S., Shockman G. D., Daneo-Moore L. Balanced macromolecular biosynthesis in "protoplasts" of Streptococcus faecalis. J Bacteriol. 1971 Mar;105(3):710–717. doi: 10.1128/jb.105.3.710-717.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehrli E., Mühlethaler K., Moor H. Membrane structure as seen with a double replica method for freeze fracturing. Exp Cell Res. 1970 Feb;59(2):336–339. doi: 10.1016/0014-4827(70)90609-9. [DOI] [PubMed] [Google Scholar]
- Wyrick P. B., McConnell M., Rogers H. J. Genetic transfer of the stable L form state to intact bacterial cells. Nature. 1973 Aug 24;244(5417):505–507. doi: 10.1038/244505a0. [DOI] [PubMed] [Google Scholar]
- Wyrick P. B., Rogers H. J. Isolation and characterization of cell wall-defective variants of Bacillus subtilis and Bacillus licheniformis. J Bacteriol. 1973 Oct;116(1):456–465. doi: 10.1128/jb.116.1.456-465.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]