Abstract
Extremophiles are micro-organisms adapted to survive in ecological niches defined as ‘extreme’ for humans and characterized by the presence of adverse environmental conditions, such as high or low temperatures, extreme values of pH, high salt concentrations or high pressure. Biomolecules isolated from extremophiles possess extraordinary properties and, in particular, proteins isolated from extremophiles represent unique biomolecules that function under severe conditions, comparable to those prevailing in various industrial processes.
In this article, we will review some examples of recent applications of thermophilic proteins for the development of a new class of fluorescence non-consuming substrate biosensors for monitoring the levels of two analytes of high social interest, such as glucose and sodium.
Keywords: biosensor, fluorescence, extremophiles
1. Introduction
The discovery that many organisms, called extremophiles, live and thrive in environments considered extreme for human life (i.e. high temperatures, often above 100°C, extreme pH values, presence of high salt concentrations) led to a great interest in these organisms and their biomolecules. In fact, proteins and enzymes isolated from extremophiles are considered useful for a variety of applications, due to their extraordinary properties to work in hostile conditions. From a phylogenetic point of view, extremophiles belong to the kingdom of Archaea, one of the three domains of life, in addition to the domains of Bacteria and Eukarya. The discovery of extremophiles suggested a new hypothesis on the origin of life, due to the fact that the extreme conditions in which they live are considered to be very close to the ones present on Earth 4 billion years ago (Di Giulio 2003). As we learn more about the extreme conditions at which life can survive and thrive, more of these extremophiles are brought into culture and their genomes sequenced. Extremophiles are classified on the basis of the particular extreme conditions in which they live. In particular, there exist (i) thermophilic and hyperthermophilic organisms, living at high (up to 75°C) and very high temperatures (up to 115°C), respectively, (ii) psychrophilic organisms, living at very low temperatures, (iii) acidophiles and alcalophiles, living at extreme acidic or basic values of pH, respectively, and (iv) halophilic organisms, living in the presence of high salt concentrations (5–30%). There are also extremophilic organisms able to live in the presence of high metal ion concentrations (metallophiles), or high radiation levels (radiophiles), or in the absence of oxygen.
Many research laboratories have focused their attention on the study of the strategies adopted by extremophilic organisms to colonize such extreme environments. Psychrophiles, for example, adapted at low temperatures, are able to synthesize cold-adapted enzyme courses and produce molecules that reduce the freezing point of water, in order to ensure the normal course of cellular chemical processes at the cold temperatures of the environment. Furthermore, these enzymes have evolved a range of structural features that confer a high level of flexibility compared with thermostable homologues. High flexibility, particularly around the active site, is translated into low-activation enthalpy, low-substrate affinity and high specific activity at low temperatures (Cavicchioli 2006; Siddiqui & Cavicchioli 2006). Halophiles, organisms that survive in the presence of high salt concentration, are able to keep the concentration of ions constant in the cell by enhancing potassium content and taking sodium ions out of the cell (Rensing 2005). A comparison of the aminoacidic composition of proteins reveals that halophilic proteins are more acidic than their mesophilic counterpart. This is due to the presence of negative charges on their surface that allows a higher surface hydration, so that preventing high saline concentration causes protein aggregation phenomena. Acidophiles can live at extremely acid pH values (0–1), normally forbidden for cell life (Matin 1999). These organisms are able to keep their own cellular pH at a value near to neutrality, by two special regulation mechanisms. In addition, this class of organisms is able to fortify the membrane against the hostile outer environment, by a biofilm in order to prevent the diffusion into the cell, for example, or through the incorporation of fatty acids to protect the cell. Other strategy is represented by an active mechanism based on a pump that ejects hydrogen ions out of the cell, helping to keep constant the inner pH. Alcalophiles live at extremely basic pH values (10–12). Like acidophiles, they possess mechanisms of pH regulation to remain close to neutrality.
Extremophilic micro-organisms represent a potentially valuable resource in the development of novel biotechnological processes. Most applications involving extremophiles are based on the use of their biomolecules, in particular their enzymes. In fact, enzymes from extremophilic micro-organisms represent versatile tools for sustainable developments in a variety of industrial applications, as they show important environmental benefits due to their biodegradability, specific stability under extreme conditions, improved use of raw materials and decreased amount of waste products. These enzymes are already in use as biocatalysts in industrial processes (Sthal 1993; Jaenicke et al. 1996). Among the extremophiles, thermophilic and hyperthermophilic micro-organisms are probably the most studied organisms. The enzymes that have been isolated from these micro-organisms are extremely thermostable and usually resistant to the action of chemical denaturants, detergents, chaotropic agents, organic solvents as well as to the exposure to extreme values of pH (Nucci et al. 1995; D'Auria et al. 1996, 1998). As a consequence, they can be used as biomolecule models for designing and constructing proteins with new properties that are of interest for industrial applications (Cowan 1992). Moreover, the unusual properties showed by thermophilic enzymes prompted their use as protein models for addressing a number of fundamental problems in understanding the determinants of protein stability (Van den Burg et al. 1998).
2. Applications of thermophilic enzymes in biotechnology
Biotechnological applications of enzymes are often hampered by their low stability to heat, pH, organic solvents and proteolysis (Sthal 1993, Shoichet et al. 1995). Many attempts have been made to improve the stability of current commercial enzymes, as well as to establish guidelines for improving the stability of proteins and enzymes (March et al. 1982; Rabinovitch et al. 1982; Amato 1992). Sensing systems that are more and more sensitive and simple are needed in various fields, such as clinical, environmental and food analysis. The development of such sensing systems requires that proteins be stable under a wide range of environmental conditions, as their replacement accounts for most of the operating costs. The use of proteins or enzymes from thermophilic organisms represents an interesting alternative to the efforts to improve stability properties of mesophilic biomolecules. Enzymes isolated from thermophilic sources are natural examples of stable biomolecules (Robinson et al. 1992). The most well-known example of a successful application of a thermophilic enzyme is Taq DNA polymerase isolated from Thermus aquaticus (Chien et al. 1976; Kaledin et al. 1980). The use of this enzyme allowed the automation of PCR technology, with a great advantage for research laboratories and industries. Other widespread biotechnological applications of thermophilic enzymes include the utilization of amilase for the production of glucose and xylanase to whiten paper. It is also important to name enzymes isolated from psychrophiles, such as lypases, proteases and cellulases, which have been used as additives for the preparation of detergents working at low temperatures, or as additives in the frozen food industry. Furthermore, thermophilic enzymes have been used for the construction of optical nanosensors, stable and non-consuming analyte. These innovative devices are based on the ability of thermophilic enzymes to bind the substrate at room temperature, without transforming it. (Staiano et al. 2005). The binding of substrate to thermophilic enzyme is monitored as fluorescence variations of the enzyme.
In fact, fluorescence detection, due to its simplicity and sensitivity, is the dominant analytical tool in medical testing, biotechnology and drug discovery. In the 1980s, fluorescence probes for specific analytes became available (Lakowicz 1995; Spichiger-Keller 1998; Wolfbeis 2000). Some of these sensing fluorophores are relatively simple, as illustrated by quinoline probes that are collisionally quenched by chloride (Verkman et al. 1989; Biwersi et al. 1994); however, the molecular complexity of the sensors quickly increases if analyte binding is required to cause a spectral change. For example, the fluorophores specific for calcium are structurally complex and only a few display spectral shifts upon binding calcium (Tsien et al. 1985). As a consequence, the development of specific sensors for social relevant analytes is even more challenging. Indeed, it is difficult to imagine how a fluorescent probe could be designed that specifically binds pyruvate, lactate or creatinine. Even if a suitable structure could be designed and synthesized, there is no guarantee that the final molecule will display a spectral change, adequate water solubility and a suitable affinity constant. A solution to this problem could be the use of proteins and enzymes as specific sensors for biochemical analytes (Gilardi et al. 1997; Miyawaki et al. 1997; Romoser et al. 1997). In the following sections some examples of the use of enzymes from thermophiles as probes for the realization of fluorescence biosensors are presented.
3. Glucose sensing
Close control of blood glucose is essential to avoid the long-term adverse consequences of elevated blood glucose, including neuropathies, blindness and other sequelae (The Diabetes Control & Complications Trial Research Group 1997; 1993). Non-invasive measurements of blood glucose have been a long-standing research goal. Such a capability would immediately allow the development of a variety of devices for diabetic health care, including continuous painless glucose monitoring, control of an insulin pump, and warning systems for hyper- and hypoglycemic conditions. At present, the only reliable method to measure blood glucose is by a finger stick and subsequent glucose measurement, typically by glucose oxidase (Ervin & Kiser 1999). This procedure is painful and even the most compliant individuals, with good understanding and motivation for glucose control, are not willing to finger stick themselves more than a few times per day. The use of manipulated enzymes as probes for the design of implantable and non-consuming glucose fluorescence biosensors represents a topic of high interest. Recently, we have demonstrated that the coenzyme-depleted glucose oxidase from Aspergillus niger is still able to bind glucose with the same efficiency than the native enzyme, but it is not able to transform the glucose. We have also showed that it is possible to monitor the binding of glucose to the coenzyme-depleted glucose oxidase by fluorescence spectroscopy methodologies (D'Auria et al. 1999, 2000a).
If a reliable fluorescence assay for glucose could be developed, then the robustness of lifetime-based sensing (Szmacinski & Lakowicz 1994, 1995) could allow development of a minimally invasive implantable glucose sensor or a sensor which uses extracted interstitial fluid. The lifetime sensor could be measured through the skin (Bambot et al. 1995) using a red laser diode or light-emitting diode (LED) device as the light source. These devices are easily powered with batteries and can be engineered into a portable device.
Unfortunately, the process of the Flavin Adenine Dinucleotide (FAD) depletion from the structure of the glucose oxidase from A. niger makes the coenzyme-depleted glucose oxidase not stable with respect to the storage conditions as well as its long-term utilization. As a consequence, the utilization of enzymes from thermophiles could solve the problem of long-term protein stability and allow the design of an implantable and non-consuming glucose sensor (D'Auria et al. 1999).
3.1 A thermostable glucokinase from the thermophilic organism Bacillus stearothermophilus
A thermostable glucokinase from the thermophilic organism Bacillus stearothermophilus (BSGK) was studied for use as a reversible glucose sensor (D'Auria et al. 2002). This protein has already been used as an active enzyme in glucose assays (Scott et al. 1990; Tomita et al. 1995). In this work, no ATP was added to the enzyme solution, so that it could not transform glucose.
The reaction catalysed by the enzyme is as follows:
The interaction with substrate was investigated by means of fluorescence polarization measurements. Polarization sensing provides a method by which a change in intensity is observed as a change in polarization. This can be accomplished using either a polarized reference film (Gryczynski et al. 1999; Lakowicz et al. 1999) or a protein sample in the absence of analyte as a reference (Dattelbaum et al. 1999). The principle of the measurement is shown in figure 1. Two solutions are placed side by side. The reference (R) solution observed through a vertical polarizer contains the protein without any glucose. The sample (S) observed through a horizontal polarizer contains the protein with various concentrations of glucose. The measured polarization depends on the intensity of the sample relative to the reference. If the sample intensity is very low, the polarization approaches 1.0. On the contrary, if the emission from the sample dominates, the polarization approaches −1.0. Hence, a wide range of polarization values can be available, resulting in a wide dynamic range for the sensor. It is important to note that polarization-based sensing can be accomplished without a change in the polarization of the sample. This result is obtained because the polarizers on the emission side of the sensor provide polarized light from sample and reference. For use as a sensor, the protein must display a good long-term stability. In order to check the stability properties of BSGK, a solution of the enzyme (enzyme concentration 1.0 mg ml−1) was incubated at room temperature. Enzyme aliquots were withdrawn, and the enzyme activity, as well as the fluorescence intensity, was monitored. As shown in figure 2, yeast hexokinase (YHK) lost the enzyme activity after few days of incubation at room temperature. In contrast, BSGK did not lose the enzyme activity over two weeks of incubation at room temperature (Tomita et al. 1990). The emission intensity of the indolic fluorescence of BSGK also remained constant, indicating no changes in the enzyme structure over two weeks of incubation at room temperature.
Figure 1.
Polarization sensing with a reference solution.
Figure 2.
Stability of BSGK and YHK at room temperature. Fluorescence measurements were performed at room temperature. Ex=290 nm; Em=340 nm.
BSGK has a single cysteine residue located near the active site (D'Auria et al. 2002). This residue was labelled with the sulphhydryl-reactive fluorophore 2-(4-(iodoacetamido)anilino)naphthalene-6-sulphonic acid, IA-ANS. The emission of the labelled protein was near 460 nm. The emission intensity of the ANS-labelled protein decreased upon addition of glucose. The decreased intensity was interpreted as the displacement of the water sensitive ANS into the aqueous phase upon binding glucose. The change in intensity occurred near 3 mM, which is comparable to the concentration of glucose in the blood. From these conclusions it has been possible to affirm that BSGK binds glucose in the absence of ATP and can thus serve as a non-consuming glucose sensor. For highly accurate glucose measurements, the fluorescence lifetimes were examined to determine if a change occurred upon glucose binding. Unfortunately, ANS-labelled BSGK displayed no change in lifetime upon glucose binding. Hence, an alternative method to use BSGK as a glucose sensor was considered. Resonance Energy Transfer (RET) reliably occurs whenever fluorescent donors and acceptors are in close proximity (Lakowicz 1999). A method to use RET to develop a competitive glucose assay was studied. To demonstrate the feasibility of a competitive glucose assay, the unmodified protein and its intrinsic tryptophan emission as the donor were used. As the acceptor glucose containing the absorbing nitrophenyl group, o-nitrophenyl-β-d-glucopyranoside (ONPG; figure 3) was used.
Figure 3.
o-Nitrophenyl-β-d-glucopyranoside (ONPG).
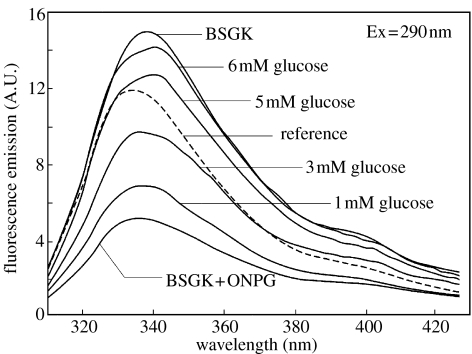
Figure 4 shows the intrinsic tryptophan emission of BSGK. The addition of ONPG (3 μM) resulted in an approximate 80% decrease in the tryptophan intensity. The addition of glucose resulted in the recovery of the fluorescence intensity. At approximately 6 mM glucose concentration, fluorescence intensity returned to its initial value before the addition of ONPG. Further addition of glucose did not change the fluorescence signal. The fact that the intensity was sensitive to glucose demonstrated that the intensity changes were due to a binding event and not to trivial inner filter effects from ONPG. Polarization sensing is accomplished by constructing a sensor, such that a stable intensity reference is observed through one polarizer and the sample is observed through a second orthogonal polarizer. One such configuration is shown in figure 1. In this case, the reference we used was BSGK solution, which could be expected to display similar temperature-, time- or illumination-dependent changes as the sample. To optimize the sensor response, the reference intensity was approximately 65% of the sample response, as calculated for an expected two- to threefold intensity change (data not shown). This reference was observed through a horizontally oriented polarizer. The sample contained BSGK, ONPG and various concentrations of glucose, and it was observed through a vertically oriented polarizer. The emission from both sides of the sensor was then observed through a vertically and horizontally oriented polarizer in order to measure polarization of the system. Figure 5 shows the observed polarization of the system for BSGK–ONPG at different concentrations of glucose. An advantage of polarization measurements for sensing is that they are self-normalized and thus independent of the overall intensity of the sensor. The results of this study demonstrated that a thermostable glucokinase can serve as a glucose sensor. Additional studies are needed to obtain a BSGK-based sensor that displays larger spectral changes. For example, it would be worth to check the use of fluorophores other than IA-ANS to obtain larger intensity changes, spectral shifts or changes in lifetime. The results in the competitive RET were especially interesting, because RET is a through-space interaction that occurs whenever the donor and acceptor are within the Forster distance (R0) and does not require a conformational change and/or a change in the probe environment. Since the measurements through the skin can be easily performed by using a red laser diode or a LED as an excitation source, one may envision a polarization-based device with an external calibrated standard (Gryczynski et al. 1999, 2000) that will allow non-invasive glucose determinations. The main advantage of using this method is the obtainment of ratiometric polarization measurements that are not influenced by light instability and sample perturbation.
Figure 4.
Effect of glucose on the intensity emission of BSGK in the presence of ONPG. The excitation was at 290 nm and the emission was recorded at 340 nm. [BSGK]=3 μM.
Figure 5.
Effect of glucose on the polarization spectra of BSGK in the presence of ONPG. Excitation was at 290 nm. [BSGK]=3 μM.
3.2 Glucose dehydrogenase from the thermoacidophilic archaeon Thermoplasma acidophilum
The potential application of glucose dehydrogenase (GD) from the thermoacidophilic archaeon Thermoplasma acidophilum for glucose sensing was investigated by D'Auria and co-workers (D'Auria et al. 2000a). This enzyme catalyses the following reaction:
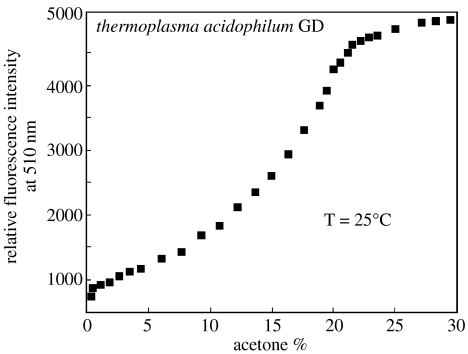
The study was based on the use of the thermophilic enzyme under conditions where no reaction occurs and no substrate is consumed. To prevent the glucose oxidation, no NAD(P) was added to the enzyme solution. In these conditions, the enzyme is still able to bind the substrate with an affinity comparable to that of the holo-enzyme. 8-Anilino-1-naphthalene sulphonic acid (ANS) is known to be a polarity-sensitive fluorophore, which is able to bind proteins with an increase in fluorescence emission intensity. The effects of GD on the emission intensity of ANS were examined. A moderate enhancement was found, but the ANS intensity remained low compared with other ANS-protein complexes. Also, the addition of glucose to this GD–ANS complex did not change upon addition of glucose. Being a thermophilic protein, GD is rigid under mesophilic conditions; while it displays increased activity at higher temperatures or in the presence of non-polar solvents (D'Auria et al. 1998), conditions are expected to increase the protein dynamics. The addition of acetone to the solution containing ANS and GD resulted in a dramatic increase in the ANS intensity (figure 6) as well as in a blue shift of the emission maximum (data not shown). To be useful as a glucose sensor, the ANS-labelled GD must display usefully large spectral changes in the presence of glucose. The addition of glucose to ANS–GD in the presence of 3% acetone resulted in an approximate 25% decrease in intensity (figure 7). This seemed to be the optimal acetone concentration because smaller spectral changes were seen at lower and higher acetone concentrations. The observed behaviour was studied to understand if the glucose-dependent decrease in intensity would be accompanied by a similar change in the ANS decay time. The frequency-domain intensity decays of ANS–GD are shown in figure 8. Glucose induced a modest shift in the response to higher frequencies, which indicated a decrease in the mean decay time. In the presence and absence of glucose, the data analysis indicated that the decay was dominated by a sub-nanosecond component whose contribution is increased by glucose. However, the changes in the intensity decay, or the equivalent phase and modulation, were not adequate for lifetime-based sensing. Polarization sensing was also investigated to explain the observed changes. The polarization decreased at higher glucose concentrations, because the emission from this solution was observed through the horizontal polarizer. Moreover, the change in polarization was larger at shorter wavelengths, and this is due to the differences in the emission spectra of reference (ANS in buffer) and sample (ANS–GD). The wavelength-dependent changes in polarization were used to create a calibration curve for glucose (figure 9). This curve shows that the present ANS–GD system can yield glucose concentrations of approximately ±2.5 mM, at a glucose concentration near 20 mM. In spite of the difficulties in realizing a glucose sensor by using GD, due to the non-covalent binding of the enzyme with ANS which can lead to calibration curve alteration in cases of concentration changes, and to the presence of an organic solvent such as acetone, this study demonstrated that enzymes which use glucose as their substrate can be used as reversible and non-consuming glucose sensors in the absence of required cofactors. It is worth highlighting that there are over 400 reactions catalysed by dehydrogenase enzymes. Usually, these reactions are catalysed by coenzyme–enzyme complex. It is of high interest to check if some of these reactions can also take place in the absence of coenzyme. This could open a new perspective in the biosensor design.
Figure 6.
ANS-labelled GD fluorescence intensity in the presence of different concentrations of acetone. [GD]=3 μM; [ANS]=4 μM. The excitation was at 370 nm and the emission was monitored at 510 nm.
Figure 7.
Emission spectra of ANS-labelled GD in the presence of 3% acetone and at different concentrations of glucose. [GD]=3 μM; [ANS]=4 μM. Increase of glucose concentration over 70 mM does not introduce further changes in fluorescence intensity.
Figure 8.
Frequency-domain intensity decay of ANS-labelled GD with 3% acetone in the absence and presence of glucose.
Figure 9.
Effect of glucose on the polarization of GD in the presence of 3% acetone. The excitation was at 370 nm and the emission was recorded at 470 nm. [GD]=3 μM; [ANS]=4 μM.
4. Cation sensing: a thermostable enzyme as A probe for sodium sensing
Measurements of sodium and potassium in the blood are a routine part of clinical blood analysis. Fluorescent probes for sodium are known (Minta & Tsien 1989; Oguz & Akkaya 1998; Smith et al. 1998). It would be valuable to have simple optical methods for rapid point-of-care testing especially for potassium, which is measured during hypertensive screening. A variety of fluorescence probes have been developed that respond to sodium and/or potassium. Most of these responses are based on partially selective binding of these cations to crown ethers (Pedersen 1988). These probes typically display association constants suitable for intracellular measurements when sodium and potassium concentrations are near 6 and 120 mM, respectively. In the blood, the extracellular concentrations of sodium and potassium are near 140 and 4 mM, respectively. Consequently, measurements of these ions in the blood are particularly difficult, given the 25-fold excess of chemically similar sodium ions. The sensing of sodium was investigated using the enzyme pyruvate kinase (PK) from Bacillus acidocaldarius (D'Auria et al. 2000b). This enzyme (ATP–pyruvate 2-O-phosphotransferase, EC 2.7.1.40) catalyses the essentially irreversible transphosphorylation from phosphoenolpyruvate to ADP, a reaction that requires magnesium and potassium ions (Gupta et al. 1976; Gupta & Mildvan 1977; Baek & Nowak 1982). However, although the vast majority of pyruvate kinases studied to date requires activation by monovalent cations, some microbial pyruvate kinases lack this property (Benziman 1969; Ozaki & Shjio 1969; Liao & Atkinson 1971; Waygood et al. 1975; Jetten et al. 1994). As demonstrated by Larsen et al., the pyruvate kinase from Corynebacterium glutanicum and Escherichia coli do not require K+ for activation. These enzymes are characterized by the replacement of Glu 117–Lys 117 in the cation-binding site. The proximity of Glu 117 to the potassium-binding site in the rabbit pyruvate kinase, and the conservation of the binding site in the two bacterial enzymes, which lack a dependence on monovalent cations, suggested that a protonated ϵ-amino group of Lys 117 in these bacterial enzymes may provide an internal monovalent cation (Larsen et al. 1997). This suggested that pyruvate kinases from other thermophilic organisms may show specific binding of sodium or potassium. PK was isolated from Bacillus acidocaldarius and its affinity to sodium was studied by means of fluorescence experiments. The steady-state emission spectrum of PK (data not shown) displays a maximum characteristic of partially shielded tryptophan residues. The steady-state intensity of pyruvate kinase decreases by approximately 25% upon saturation with sodium. The cation-dependent intensities were used to determine the apparent cation-binding constants of pyruvate kinase, as shown in figure 10 and table 1. By mathematically fitting data, it was clear that K+ binding is very weak. This result is in agreement with the probable substitution of a lysine at position 117, which serves as an internal cation (Larsen et al. 1997). Mg2+ and Ca2+ also caused a decrease in the emission intensity of pyruvate kinase. Comparison of typical cation concentrations in the blood (table 2) revealed that only sodium is present in concentrations adequate to alter the emission intensity of pyruvate kinase. Hence, this pyruvate kinase, or this pyruvate kinase labelled with an extrinsic fluorophore, may provide a protein biosensor specific for sodium. The intrinsic tryptophan decay of pyruvate kinase change upon binding of cations was also investigated. The frequency responses with Na+ shifted to higher frequencies were also investigated, which reflected a decrease in the mean lifetime upon sodium binding (table 3). The frequency response of PK in the presence of potassium was also examined. In this case, the intensity decay was essentially unchanged at 200 mM K+ (data not shown) in agreement with the absence of an intensity change, as shown in figure 10. The effects of sodium binding on the intensity decay of PK were observed by examining the Na+-dependent amplitudes in the presence of K+. In all cases, a Na+-dependent decrease in the amplitudes of the two longer decay times of 2.6 and 6.7 ns and an increased amplitude of the short component with a 0.47 ns decay time were observed. The apparent Na dissociation constants for PK were calculated. The dissociation constant was found to be near 15 mM, and it decreased on increasing the potassium concentration. The absence of a strong effect of potassium over a wide range of concentrations indicated that PK can be used as a sodium sensor without significant interference from potassium. The utilization of this thermostable enzyme is of fundamental importance for the development of an optical method for rapid point-of-care testing for these analytes, which are measured during hypertensive screening.
Figure 10.
Steady-state fluorescence titration curves of pyruvate kinase from Bacillus acidocaldarius.
Table 1.
Apparent cation dissociation constants and Hill coefficients for pyruvate kinase from Bacillus acidocaldarius.
| cation | KD (mM) | na |
|---|---|---|
| Na | 27 | 1.08 |
| K | — | — |
| Ca | 2.1 | 1.44 |
| Mg | 2.1 | 1.97 |
Determined from the steady-state intensities at 350, 295 nm excitation.
Table 2.
Typical concentrations of cations inside cells and in the whole blood.
| cation | intracellular (mM) | whole blood, extracellular (mM) |
|---|---|---|
| Na | 4–10 | 135–148 |
| K | 100–140 | 3.5–4.5 |
| Ca | 50–200 | 4.5–5.5 |
| Mg | 0.5–2 | – |
Table 3.
Fluorescence anisotropy decay of pyruvate kinase from Bacillus acidocaldarius in the absence and presence of Na+ ions. Emission and excitation were set at 340 and 290 nm, respectively.
| Na+ | 0 mM | 80 mM | |
|---|---|---|---|
| 0.47 (0.02)a | |||
| τ1 (ns)b | 2.6 (0.1)a | ||
| τ2 (ns)b | 6.7(0.2)a | ||
| τ3 (ns)b | |||
| α1 | 0.314 (0.009)a | 0.567 (0.008, 0.007)a | |
| α2 | 0.47 (0.01)a | 0.347 (0.007)a | |
| α3 | 0.21 (0.02)a | 0.086 (0.008, 0.009)a | |
| ϕ1 (ns) | 0.038 (0.003)a | 0.056 (0.003)a | |
| ϕ2 (ns) | 26.5 (1.1)a | 26.2 (1.5)a | |
| r0c | 0.28 | 0.28 | |
| g2 | 0.428 (0.002)a | 0.399 (0.003)a | |
| Χ2dR | 1.05 | 1.57 |
Standard deviations were estimated according to D'Auria et al. (2000b).
Global parameters from fitting of 15 frequency-domain decay curves.
A fixed parameter for λexc=297 nm.
For δθ=0.2° and δm=0.005.
5. Conclusions
The steady increase in the number of newly isolated extremophilic micro-organisms and the related discovery of their enzymes document their enormous potential within the scientific field. Extremophilic enzymes have become model systems to study enzyme evolution, enzyme stability and activity mechanisms, protein structure–function relationships and biocatalysis under extreme conditions. In particular, enzymes from thermophiles and hyperthermophiles possess a great potential for biotechnological applications, due to their high resistance not only to temperature, but also to chemical, organic solvents and extreme pH values. Analyses of clinical interest, such as the determination of analytes in the blood, could find an answer by the use of thermophilic enzymes as the recognition element of the sensing system. Particularly, relevant in this field of research are the glucose concentration determination in the blood of diabetic patients and the sodium sensing in the blood, without potassium interference. The binding between the enzyme, inactivated by depletion of the cofactor required for the catalytic activity to avoid substrate consumption, and the substrate can be monitored by sensitive and simple analytical techniques, such as fluorescence spectroscopy. Although many fluorescent probes have been synthesized ad hoc for the sensing of analytes of interest, the sensitivity, substrate specificity and stability required to realize a working sensing system often are not easily achievable through chemical synthesis of simple molecules and require the utilization of thermostable biomolecules as probes for the development of biosensors.
In conclusion, the utilization of coenzyme-depleted enzymes, when labelled with suitable fluorophores, has high potential in biotechnology, opening new perspectives in clinical diagnosis as well as in all the applications requiring the use of sensors with high specificity and/or a non-consuming substrate. The use of the possibility of thermostable apoenzymes for reversible sensors greatly expands the range of social relevant analytes, which can be easily measured. In fact, a survey of the genomes of thermophiles looking for e.g. oxidases could highlight the availability of new stable enzymes for its use in the design of innovative biosensors.
Acknowledgments
This project was realized in the frame of the CNR Commessa ‘Diagnostica Avanzata ed Alimentazione’. This work was also supported by the ASI project MoMa no. 1/014/06/0 and by a grant from the Ministero degli Affari Esteri, Direzione Generale per la Promozione e la Cooperazione Culturale (S.D.).
Footnotes
This article is dedicated to Prof. Dr Karl O. Stetter (University of Regensburg, Germany) for his outstanding contribution to the world of extremophiles.
References
- Amato I. In search of the human touch. Science. 1992;258, 5087:1436–1437. doi: 10.1126/science.1439836. [DOI] [PubMed] [Google Scholar]
- Baek Y.H, Nowak T. Kinetic evidence for a dual cation role for muscle pyruvate kinase. Arch. Biochem. Biophys. 1982;217:491–497. doi: 10.1016/0003-9861(82)90529-X. [DOI] [PubMed] [Google Scholar]
- Bambot S.B, Rao G, Romauld M, Carter G.M, Lakowicz J.R. Sensing oxygen through skin using a red diode laser and fluorescence lifetimes. Biosens. Bioelectron. 1995;10:643–652. doi: 10.1016/0956-5663(95)96941-Q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benziman M. Factors affecting the activity of pyruvate kinase of Acetobacter xylinum. Biochem. J. 1969;112:631–636. doi: 10.1042/bj1120631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biwersi J, Tulk B, Verkman A.S. Long-wavelength chloride-sensitive fluorescent indicators. Anal. Biochem. 1994;219:139–143. doi: 10.1006/abio.1994.1242. [DOI] [PubMed] [Google Scholar]
- Cavicchioli R. Cold-adapted archaea. Nat. Rev. Microbiol. 2006;4:331–343. doi: 10.1038/nrmicro1390. [DOI] [PubMed] [Google Scholar]
- Chien A, Edgar D.B, Trela J.M. Deoxyribonucleic acid polymerase from the extreme thermophile Thermus aquaticus. J. Bacteriol. 1976;127:1550–1557. doi: 10.1128/jb.127.3.1550-1557.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowan D.A. Enzymes from thermophilic archaebacteria: current and future applications in biotechnology. Biochem. Soc. Symp. 1992;58:149–169. [PubMed] [Google Scholar]
- D'Auria S, Rossi M, Nucci R, Barone G, Catanzano F, Del Vecchio P, Graziano G. Temperature-induced denaturation of β-glycosidase from the archeon Sulfolobus solfataricus. J. Biochem. 1996;120:292–300. doi: 10.1093/oxfordjournals.jbchem.a021412. [DOI] [PubMed] [Google Scholar]
- D'Auria S, Moracci M, Febbraio F, Tanfani F, Nucci R, Rossi M. Structure-function studies on β-glycosidase from Sulfolobus solfataricus. Molecular bases of thermostability. Biochimie. 1998;80:949–957. doi: 10.1016/S0300-9084(00)88892-6. [DOI] [PubMed] [Google Scholar]
- D'Auria S, Herman P, Rossi M, Lakowicz J.R. The fluorescence emission of the apo-glucose oxidase from Aspergillus niger as probe to estimate glucose concentrations. Biochem. Biophys. Res. Commun. 1999;263:550–553. doi: 10.1006/bbrc.1999.1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Auria S, Di Cesare N, Gryczynski Z, Rossi M, Lakowicz J.R. A thermophilic apoglucose dehydrogenase as nonconsuming glucose sensor. Biochem. Biophys. Res. Commun. 2000a;274:727–731. doi: 10.1006/bbrc.2000.3172. [DOI] [PubMed] [Google Scholar]
- D'Auria S, Rossi M, Herman P, Lakowicz J.R. Pyruvate kinase from the thermophilic eubacterium Bacillus acidocaldarius as probe to monitor the sodium concentrations in the blood. Biophys. Chem. 2000b;84:167–176. doi: 10.1016/S0301-4622(00)00110-1. [DOI] [PubMed] [Google Scholar]
- D'Auria S, DiCesare N, Staiano M, Gryczynski Z, Rossi M, Lakowicz J.R. A novel fluorescence competitive assay for glucose determinations by using a thermostable glucokinase from the thermophilic microorganism Bacillus stearothermophilus. Anal. Biochem. 2002;303:138–144. doi: 10.1006/abio.2001.5544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dattelbaum J.D, Lakowicz J.R, Gryczynski I, Gryczynski Z, Tolosa L, Rao G. Polarization-based sensing with a self-referenced sample. Appl. Spectrosc. 1999;53:1149–1157. doi: 10.1366/0003702991947964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Giulio M. The universal ancestor and the ancestor of bacteria were hyperthermophiles. J. Mol. Evol. 2003;57:721–730. doi: 10.1007/s00239-003-2522-6. [DOI] [PubMed] [Google Scholar]
- Ervin K.R, Kiser E.J. Issues and implications in the selection of blood glucose monitoring techniques. Diabet. Technol. Ther. 1999;1:3–11. doi: 10.1089/152091599317512. [DOI] [PubMed] [Google Scholar]
- Gilardi G, Mei G, Rosato N, Finazzi-Agro’ A.F, Cass A.E.G. Spectroscopic properties of an engineered maltose-binding protein. Prot. Eng. 1997;10:479–486. doi: 10.1093/protein/10.5.479. [DOI] [PubMed] [Google Scholar]
- Gryczynski I, Gryczynski Z, Lakowicz J.R. Polarization sensing with visual detection. Anal. Chem. 1999;71:1241–1251. doi: 10.1021/ac981301i. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gryczynski Z, Gryczynski I, Lakowicz J.R. Simple apparatus for polarization sensing of analytes. Opt. Eng. 2000;39:2351–2358. doi: 10.1117/1.1286521. [DOI] [Google Scholar]
- Gupta R.K, Mildvan A.S. Structure of enzyme-bound metal-nucleotide complexes in the phosphoryl transfer reaction of muscle pyruvate kinase 31D NMR studies with magnesium and kinetic studies with chromium nucleotides. J. Biol. Chem. 1977;252:5967–5976. [PubMed] [Google Scholar]
- Gupta R.K, Oesterling R.M, Mildvan A.S. Dual divalent cation requirement for activation of pyruvate kinase essential of both enzyme and nucleotide-bound metal ions. Biochemistry. 1976;15:2881–2887. doi: 10.1021/bi00658a028. [DOI] [PubMed] [Google Scholar]
- Jaenicke R, Schuring H, Beaucamp N, Ostendorp R. Structure and stability of hyperstable proteins: glycolytic enzymes from hyperthermophilic bacterium Thermotoga maritima. Adv. Protein Chem. 1996;48:181–269. doi: 10.1016/s0065-3233(08)60363-0. [DOI] [PubMed] [Google Scholar]
- Jetten M.S.M, Gubler M.E, Lee S.H, Sinksey A.J. Structural and functional analysis of pyruvate kinase from Corynebacterium glutaminum. Appl. Environ. Microbiol. 1994;60:2501–2507. doi: 10.1128/aem.60.7.2501-2507.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaledin A.S, Sliusarenko A.G, Gorodetskii S.I. Isolation and properties of DNA polymerase from extreme thermophylic bacteria Thermus aquaticus YT-1. Biokhimiya. 1980;45:644–651. [PubMed] [Google Scholar]
- Lakowicz J.R. Advances in fluorescence sensing technology II. Proc. SPIE. 1995;2388:159–170. doi:full_text [Google Scholar]
- Lakowicz, J. R. 1999 Principles of fluorescence spectroscopy, II edn. New York, NY: Kluwer Academic/Plenum Publishers.
- Lakowicz J.R, Gryczynski I, Gryczynski Z, Dattelbaum J.D. Anisotropy-based sensing with reference fluorophores. Anal. Biochem. 1999;267:397–405. doi: 10.1006/abio.1998.3029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen T.M, Benning M.M, Esenberg G.E, Rayment I, Reed G.H. Ligand-induced domain movement in pyruvate kinase: structure of the enzyme from rabbit muscle with Mg2+, K+, and l-phospholactate at 2.7 Å resolution. Arch. Biochem. Biophys. 1997;348:262–267. doi: 10.1006/abbi.1997.0448. [DOI] [PubMed] [Google Scholar]
- Liao C.-L, Atkinson D.E. Regulation at the phosphenolpyruvate branchpoint in Azotobacter inelandii: pyruvate kinase. J. Bacteriol. 1971;106:37–44. doi: 10.1128/jb.106.1.37-44.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- March W.F, Rabinovitch B, Adams R, Wise J.R, Melton M. Ocular glucose sensor. Trans. Am. Soc. Artif. Intern. Organs. 1982;28:232–235. [PubMed] [Google Scholar]
- Matin A. pH homeostasis in acidophiles. Novartis Found Symp. 1999;221:152–163. doi: 10.1002/9780470515631.ch10. discussion 163–166. [DOI] [PubMed] [Google Scholar]
- Minta A, Tsien R.Y. Fluorescent indicator for cytosolic sodium. J. Biol. Chem. 1989;264:19 449–19 457. [PubMed] [Google Scholar]
- Miyawaki A, Llopis J, Heim R, McCaffery J.M, Adams J.A, Ikura M, Tsien R.Y. Fluorescent indicators for Ca++ based on green fluorescent proteins and calmodulin. Nature. 1997;388:882–887. doi: 10.1038/42264. [DOI] [PubMed] [Google Scholar]
- Nucci R, D'Auria S, Febbraio F, Vaccaro C, Morana A, De Rosa M, Rossi M. A thermostable β-glycosidase from Sulfolobus solfataricus: temperature and SDS effects on its functional and structural properties. Biotechnol. Appl. Biochem. 1995;21:265–274. [Google Scholar]
- Oguz U, Akkaya E.U. A squaraine-based sodium selective fluorescent chemosensor. Tetrah. Lett. 1998;39:5857–5860. doi: 10.1016/S0040-4039(98)01165-4. [DOI] [Google Scholar]
- Ozaki I, Shjio I. Regulation of the TCA and glyoxylate cycles in Brevibacterium flavum. II. Regulation of phosphoenolpyruvate carboxylase and pyruvate kinase. J. Biochem. 1969;66:297–311. doi: 10.1093/oxfordjournals.jbchem.a129148. [DOI] [PubMed] [Google Scholar]
- Pedersen C.J. The discovery of crown ethers. Science. 1988;241:536–540. doi: 10.1126/science.241.4865.536. [DOI] [PubMed] [Google Scholar]
- Rabinovitch B, March W.F, Adams R.L. Non-invasive glucose monitoring of the aqueous humour of the eye, Part I. Diabetes Care. 1982;5:254–258. doi: 10.2337/diacare.5.3.254. [DOI] [PubMed] [Google Scholar]
- Rensing C. Review of “adaptation to life at high salt concentrations in Archaea, Bacteria, and Eukarya”. Saline Systems. 2005;1:6. doi: 10.1186/1746-1448-1-6. [DOI] [Google Scholar]
- Robinson M.R, Eaton R.P, Haaland D.M, Koepp G.W, Thomas E.V, Stallard B.R, Robinson P.L. Noninvasive glucose monitoring in diabetic patients: a preliminary evaluation. Clin. Chem. 1992;38:1618–1622. [PubMed] [Google Scholar]
- Romoser V.A, Hinkle P.M, Persechini A. Detection of living cells of Ca++ dependent changes in the fluorescence emission of an indicator composed of two green fluorescent protein variants linked by a calmodulin-binding sequence. J. Biol. Chem. 1997;272:13 270–13 274. doi: 10.1074/jbc.272.20.13270. [DOI] [PubMed] [Google Scholar]
- Scott D.A, Goward C.R, Scawen M.D, Atkinson T, Price C.P. Colorimetric glucose assay using thermostable glucokinase. Annu. Clin. Biochem. 1990;1:33–37. doi: 10.1177/000456329002700107. [DOI] [PubMed] [Google Scholar]
- Shoichet B.K, Baase W.A, Kuroki R, Matthews W. A relationship between protein stability and protein function. Proc. Natl Acad. Sci. USA. 1995;92:452–456. doi: 10.1073/pnas.92.2.452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddiqui K.S, Cavicchioli R. Cold-adapted enzymes. Annu. Rev. Biochem. 2006;75:403–433. doi: 10.1146/annurev.biochem.75.103004.142723. [DOI] [PubMed] [Google Scholar]
- Smith G.A, Hesketh T.R, Metcalfe J.C. Design and properties of a fluorescent indicator of intracellular free Na+ concentrations. Biochem. J. 1998;250:227–232. doi: 10.1042/bj2500227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spichiger-Keller U.E. Wiley-VCH; New York, NY: 1998. Chemical sensors and biosensors for medical and biological applications; pp. 313–328. [Google Scholar]
- Staiano M, Bazzicalupo P, Rossi M, D'Auria S. Glucose biosensors as models for the development of advanced protein-based biosensors. Mol. Biosyst. 2005;1:354–362. doi: 10.1039/b513385h. [DOI] [PubMed] [Google Scholar]
- Sthal S. Thermostability of enzymes. In: Gupta M.N, editor. Springer; Berlin, Germany: 1993. pp. 45–74. [Google Scholar]
- Szmacinski H, Lakowicz J.R. In: Topics in fluorescence spectroscopy. Lakowicz J.R, editor. Vol. 4. Plenum Press; New York, NY: 1994. pp. 295–334. [Google Scholar]
- Szmacinski H, Lakowicz J.R. Fluorescence lifetime-based sensing and imaging. Sens. Actuators B. 1995;29:15–24. doi: 10.1016/0925-4005(95)01658-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N. Engl. J. Med. 1993;329:977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- The Diabetes Control and Complications Trial Research Group. Hypoglycemia in the diabetes control and complications trial. Diabetes. 1997;4:271–286. [PubMed] [Google Scholar]
- Tomita K, Nagata K, Kondo H, Shiraishi T, Tsubota H, Suzuki H, Ochi H. Thermostable glucokinase from Bacillus stearothermophilus and its analytical application. Ann. NY Acad. Sci. 1990;613:421–425. doi: 10.1111/j.1749-6632.1990.tb18191.x. [DOI] [PubMed] [Google Scholar]
- Tomita K, Nomura K, Kondo H, Nagata K, Tsubota H. Stabilized enzymatic reagents for measuring glucose, creatine kinase and gamma-glutamyltransferase with thermostable enzymes from a thermophile, Bacillus stearothermophilus. J. Pharm. Biomed. Anal. 1995;13:477–481. doi: 10.1016/0731-7085(95)01338-L. [DOI] [PubMed] [Google Scholar]
- Tsien R.Y, Rink T.J, Poenie M. Measurements of cytosolic free Ca++ in individual small cells using fluorescence microscopy with dual excitation wavelengths. Cell Calcium. 1985;6:145–157. doi: 10.1016/0143-4160(85)90041-7. [DOI] [PubMed] [Google Scholar]
- Van den Burg B, Vriend G, Veltman O.R, Vemma G, Eijsink V.G.H. Engineering an enzyme to resist boiling. Proc. Natl Acad. Sci. USA. 1998;95:2056–2060. doi: 10.1073/pnas.95.5.2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verkman A.S, Sellers M.C, Chao A.C, Leung T, Ketcham R. Synthesis and characterization of improved chloride-sensitive fluorescent indicators for biological applications. Anal. Biochem. 1989;178:355–361. doi: 10.1016/0003-2697(89)90652-0. [DOI] [PubMed] [Google Scholar]
- Waygood E.B, Rayman M.K, Sanwal B.D. The control of pyrvuate kinase of Escherichia coli. II. Effectors and regulatory properties of the enzyme activated by ribose 5-phosphate. Can. J. Biochem. 1975;53:444–454. doi: 10.1139/o75-061. [DOI] [PubMed] [Google Scholar]
- Wolfbeis O.S. Fiber-optic chemical sensors and biosensors. Anal. Chem. 2000;72:81R–89R. doi: 10.1021/a1000013k. [DOI] [PubMed] [Google Scholar]