Abstract
In the event of an influenza pandemic, the most probable way in which the virus would be introduced to an isolated geographical area is by an infected traveller. We use a mathematical model, structured on the location at which infection occurs and based on published parameters for influenza, to describe an epidemic in a community of one million people. The model is then modified to reflect a variety of control strategies based on social distancing measures, targeted antiviral treatment and antiviral prophylaxis and home quarantine, and the effectiveness of the strategies is compared. The results suggest that the only single strategy that would be successful in preventing an epidemic (with 0=2.0) is targeted antiviral treatment and prophylaxis, and that closing schools combined with either closing work places or home quarantine would only prevent such an epidemic if these strategies were combined with a modest level of antiviral coverage.
Keywords: mathematical epidemiology, infectious diseases, exotic infections, pandemic influenza
1. Introduction
When a pandemic of an emerging infectious disease threatens, there are considerable advantages to isolated geographical regions. New Zealand is one example of such isolation, and this study addresses the threat to the country from pandemic influenza. Travel times from any other major land mass mean that the importation of infection through surface contamination is unlikely, and those arriving by sea would have had time to express symptoms en route. Hence, if pandemic influenza were to reach the country, the most probable scenario would be that an epidemic had already started elsewhere, and the virus would be introduced to New Zealand by an infected traveller. A response plan for dealing with such an event has been formulated (Jennings 2005; Coker & Mournier-Jack 2006). The major point of entry to the country is Auckland, which is also by far the biggest city and the most probable region for an epidemic to be initiated.
We presupposed a single unintentional introduction of a virus by an airline passenger, not detected by screening at the border. We used a structured Kermack–McKendrick integral equation model to describe the spread of a novel influenza-like virus in an isolated population. We used model parameters consistent with other published models of influenza, and investigated the ability of a range of control scenarios to contain the outbreak. It is anticipated that if and when an influenza pandemic begins, observations from other countries and regions will enable us to revise our parameters, and all our calculations will be repeated. In the meantime, it is believed that the model and parameters are based on reasonable estimates of what could happen, and these results are assisting the planning of a response.
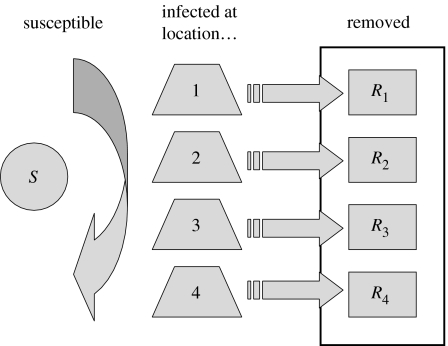
The model is summarized in figure 1, and described in appendix A. Similar models have previously been used to evaluate control policies for severe acute respiratory syndrome (SARS; Roberts 2004, 2006) and smallpox (Aldis & Roberts 2005).
Figure 1.
Schematic of the model structure. The locations at which infection occurs were as follows: 1, within the household; 2, at school; 3, within the workplace; 4, within the community.
A feature of the model is that susceptibles are treated as a homogeneous group, but the incidence of infection is structured according to the location where the infection takes place. The locations at which infection occurs are within the household, at school, within the workplace and within the community. Those that were infected at location ℓ infect others at location k at a rate proportional to the component Wkℓ of the mixing matrix W, which is scaled so that ‖W‖=1. It was proved by Roberts (submitted) that the final size equation for this model is the same as that for the unstructured model (Kermack & McKendrick 1927; Diekmann & Heesterbeek 2000 and references therein),
| (1.1) |
where P is the proportion of the population that becomes infected over the course of the epidemic and 0 is the basic reproduction number, defined as the expected number of secondary cases that would arise from a typical primary case introduced into a fully susceptible population (Diekmann et al. 1990; Diekmann & Heesterbeek 2000). It was also proved by Roberts (submitted) that the proportion of the population infected at each location is proportional to the components of the eigenvector of W that corresponds to the unit eigenvalue. These results are used here to assess the performance of control measures designed to reduce the impact of an influenza epidemic, in terms of the reduction in the total number of infections that result.
2. Method
We solved the model numerically for values of the basic reproduction number 0=2.0, 1.1 and 3.0, to determine the potential size and time-course of the epidemic in a region of one million people. The assumed parameter values are summarized in table 1. These are mostly taken from other published modelling exercises, and would be revised in the event of a pandemic as data become available. See the papers cited and their citations for details of the assumptions that led to these estimates. The exception is the assumed 95% uptake of antivirals. It was observed that 81% of passengers on a cruise ship took antiviral prophylaxis when offered during an outbreak of seasonal influenza (Miller et al. 2000). As pandemic influenza is a more serious threat to health, a higher uptake was assumed.
Table 1.
Summary of parameter values used in the model.
| parameter | value (range) |
|---|---|
| basic reproduction number (Ferguson et al. 2005, 2006; Longini et al. 2005; Germann et al. 2006) | 0=2.0(1.1, 3.0) |
| latent period (Longini et al. 2004, 2005; Mills et al. 2004; Gani et al. 2005) | 1.6 days (1.2, 2.0) |
| infectious period (Mills et al. 2004; Longini et al. 2005) | 4.1 days |
| proportion initially infected within the (Longini et al. 2005); | |
| household | 47% |
| school | 24% |
| workplace | 18% |
| community | 11% |
| proportion of infectives symptomatic (Longini et al. 2005) | 67% |
| relative infectivity of asymptomatics (Longini et al. 2005) | 50% |
| efficacy of antivirals (Ferguson et al. 2005; Longini et al. 2005) | 60% |
| uptake of antivirals | 95% |
We investigated the potential effects of control measures based on social distancing by determining how their implementation would modify the mixing matrix W to form a new matrix Wc. The basic reproduction number under control is then c=‖Wc‖0. Hence, we determined the ‘threshold’ control effort, which reduces c to 1. For example, reducing transmission in schools would multiply the second row of the matrix W by a factor c to obtain Wc. The threshold value determined was that value of c which led to ‖Wc‖=1/0 and hence c=1.
Finally, we postulated 12 control scenarios that combined social distancing, targeted antiviral treatment and antiviral prophylaxis (TATP) and home quarantine. For each, we calculated the value of c and the proportion of the population infected in an epidemic under the application of selected control scenarios. The scenarios are summarized as follows.
No intervention.
Close schools. Transmission at school ceases.
Close workplaces (70% compliance). Transmission at work is reduced to 30% of the no intervention level.
Close schools and workplaces. A combination of ii and iii above.
TATP. Antiviral treatment of cases prevents 45.6% of all transmission, and when combined with antiviral prophylaxis of home contacts, this leads to an overall reduction of 65.2% of within-household transmission. This is calculated as follows: 80% of transmission is due to symptomatic cases (see table 1) and 95% uptake of antivirals combined with 60% efficacy prevents 57% of this. Hence, out of the transmission that would occur without intervention, 20% is from asymptomatic cases, 45.6% is prevented by antiviral treatment and 34.4% continues from symptomatic cases. Antiviral prophylaxis of home contacts prevents a further 57% of the 34.4% of transmission due to symptomatic cases within the same household.
Home quarantine (70% compliance). Health authorities recommend that members of a household with a symptomatic case remain at home for 6 days and 70% comply. Hence, 56% (equal to 70% of 80%) of all transmission from those infected within their own household is prevented.
Home quarantine (50% compliance). Hence, 40% of transmission from household contacts is prevented.
Close schools and home quarantine. A combination of 2 and 4 above.
Close schools and TATP. A combination of 2 and 5 above.
TATP and home quarantine. A combination of 5 and 6 above. Hence, 66.9% (equal to 70% of the 80% of transmission that would result from symptomatic cases who comply with quarantine, plus 46.6% of the other 30% (of the 80%) prevented by antivirals) of transmission from those infected at home, 65.2% of within-household transmission and 45.6% of all other transmission (see v above) are prevented.
Close schools, TATP and quarantine. A combination of 2, 5 and 6 above.
Close schools and workplaces, TATP and quarantine. A combination of 4–6 above.
For all scenarios, it was assumed that if transmission were reduced at one location, there would be no compensatory increase in transmission elsewhere.
3. Results
3.1 The uncontrolled epidemic
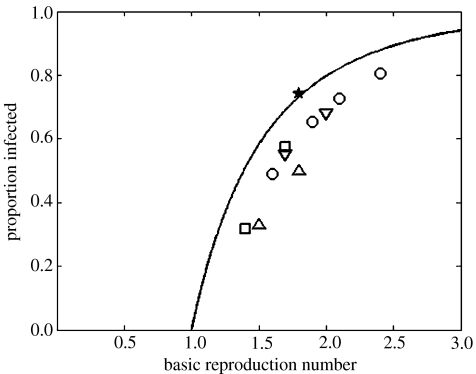
The solution of the final size equation (1.1) is shown in figure 2. When 0=2.0, P=0.7968, which implies that 79.7% of the population will be infected in an epidemic. The solutions for 0=1.1 and 0=3.0 imply that 17.6% or 94.0%, respectively, would be infected. The results from some recent simulation exercises for pandemic influenza are also presented in figure 2 for comparison.
Figure 2.
The proportion of the population infected in an epidemic, as a function of the basic reproduction number 0, and as predicted by the final size equation (1.1). The symbols are the results from models of pandemic influenza obtained by the following authors: uptriangles, Ferguson et al. (2005); downtriangles, Ferguson et al. (2006); squares, Longini et al. (2005); circles, Germann et al. (2006); star, Wu et al. (2006).
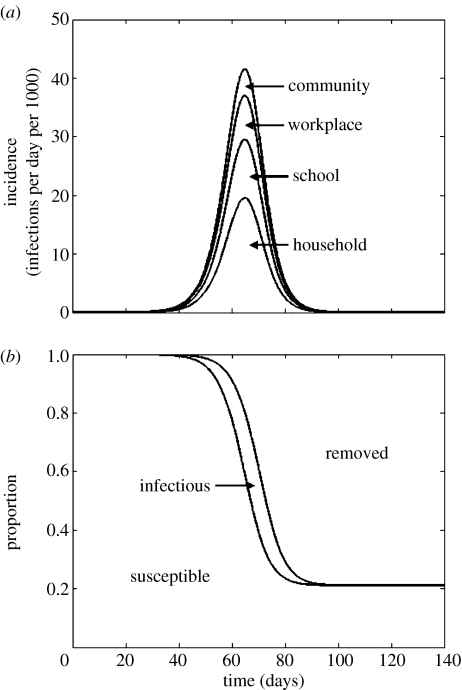
Numerically solving the structured epidemic model without any control intervention for 0=2.0 resulted in an epidemic lasting approximately 100 days (figure 3). The other numerical results obtained (not shown) resulted in incidence curves of a similar shape, except that for 0=3.0, the epidemic was completed in approximately 80 days and for 0=1.1, the epidemic lasted over 600 days. It was confirmed that the final size equation (1.1) estimates the proportion of the population infected in an epidemic, and that the distribution of cases among infection locations (see table 1) is determined by the eigenvector of W that corresponds to the unit eigenvalue.
Figure 3.
Results from the structured model when 0=2.0 and no control is applied. (a) The incidence of infection. Curves are (from bottom to top) as follows: ı1(t), incidence in the household; ı1(t)+ı2(t), incidence in the household and schools; ı1(t)+ı2(t)+ı3(t), incidence in the household, schools and workplaces; and ı1(t)+ı2(t)+ı3(t)+ı4(t), total incidence. (b) Curves are (from bottom to top) as follows: S(t)/N, the proportion susceptible; and 1−R(t)/N, the proportion susceptible plus the proportion infected.
3.2 The controlled epidemic
The threshold values for the level of control required to prevent epidemics are presented in table 2. These are the values at which c=1.0. These show, for example, that if 0 were 1.1 then transmission in schools would need to be reduced to 63% of its previous (no control) level to prevent epidemics, but this could also be achieved by reducing transmission to 45% in workplaces or to 80% in both schools and workplaces. Note that these values are the percentage of the pre-control value, hence the figure of 80% translates to a 20% reduction in transmission.
Table 2.
The threshold quantity by which transmission at specified locations should be multiplied to prevent an epidemic if 0 were equal to 1.1, 2.0 or 3.0. Note: the long dash signifies not achievable, as eliminating all transmission at that location would not reduce c below 1.
| location control applied | 0=1.1 | 0=2.0 | 0=3.0 |
|---|---|---|---|
| household | 0.74 | 0.055 | — |
| schools | 0.63 | — | — |
| workplaces | 0.45 | — | — |
| schools and workplaces | 0.80 | 0.15 | — |
| community | 0.39 | — | — |
| all locations | 0.90 | 0.50 | 0.33 |
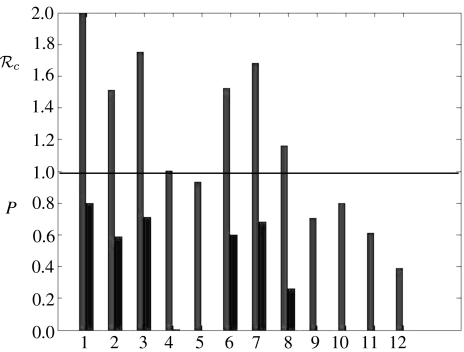
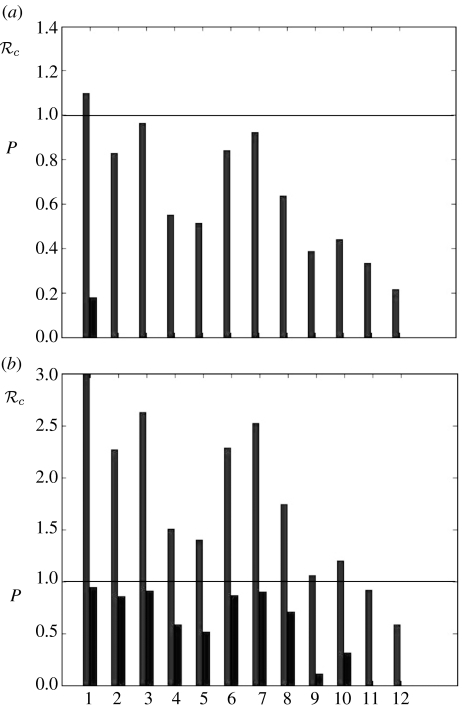
The values of c, the basic reproduction number under control, calculated for 12 specific control interventions and 0=2.0 are presented in figure 4. If c>1.0 for an intervention, then the final size equation (1.1) was used to compute P, the proportion of the population that would be infected if those control measures were in place throughout the epidemic. These are also shown in figure 4. The equivalent results for 0=1.1 and 0=3.0 are shown in figure 5.
Figure 4.
Results from control interventions when 0=2.0. The basic reproduction number under control (c) and the proportion of the population infected in an epidemic (P) if Rc>1. The numbers on the horizontal axis specify the control scenarios: 1. no control; 2. close schools; 3. close workplaces; 4. 2 and 3 combined; 5. TATP; 6. quarantine (70%); 7. quarantine (50%); 8. 2 and 6 combined; 9. 2 and 5 combined; 10. 5 and 6 combined; 11. 2, 5 and 6 combined; 12. 2, 3, 5 and 6 combined. For further details see text.
Figure 5.
Results from control interventions when (a) 0=1.1 and (b) 0=3.0. The basic reproduction number under control (c) and the proportion of the population infected in an epidemic (P) if c>1. The numbers on the horizontal axis specify the control scenarios, see the legend to figure 4.
4. Discussion
We have constructed and analysed a model for the transmission of a virus, using only the parameter values in table 1. These are based on those in the literature, but they are still only educated guesses. Until a form of pandemic influenza transmitted between humans emerges, we can only base the parameters on previous influenza epidemics in different social conditions and due to different strains of the virus. However, the model has been constructed so that the parameters may be changed and the results revised should a pandemic eventuate. As the results are sensitive to the parameter values, they should only be taken to indicate what could happen should these parameters prove to be approximately correct.
The final size of the epidemic, as computed, shows that should the basic reproduction number (0) be equal to 2.0 we could expect nearly 80% of the population to become infected. The population here is a homogeneously mixing community of one million people, motivated by the City of Auckland (population approx. 1.2 million). The epidemic would peak at approximately 65 days, with peak incidence of approximately 43 000 new cases per day. Increasing 0 to 3.0 results in a faster epidemic infecting more than 90% of the population. For different size populations the proportion infected is determined only by 0, but a larger population size results in an epidemic that takes longer to complete.
The proportion of the population that is infected in an epidemic, as determined by equation (1.1), is plotted in figure 2, where it is compared with the results obtained by other authors from simulation models for outbreaks of pandemic influenza. The results suggest that the final size equation (1.1) overestimates the proportion infected in an epidemic as determined by the more complex models, except in the case of Wu et al. (2006). This could be due, in part, to several reasons, apart from the obvious one that the structure of all these models is more complicated and the equation does not necessarily apply. For example, with a stochastic, spatially structured, individual-based model, local exhaustion of susceptible contacts will occur. In addition, the analytic final size equation is for the proportion infected over an infinite time-scale, whereas numerical simulations often cease when the incidence becomes small and the epidemic ‘is largely over’ (Ferguson et al. 2005). For complicated models, it is not always clear how the value of 0 relates to the parameters in the model, and the supplementary information to Germann et al. (2006) provides a discussion of this point. Hence, there are reasons for uncertainty about the points plotted in figure 2, in both the vertical and horizontal directions.
We have modelled control measures in two ways. We represented social distancing by reducing the amount of transmission occurring at one or more locations. If this were applied uniformly across all locations and at a level sufficient to prevent a major outbreak, then transmission would need to be multiplied by 1/0 to achieve c=1. The results in table 2 also show the effects of social distancing applied to specific locations, for example if 0 were equal to 2.0, it would be necessary to eliminate 85% of transmission that takes place in schools and workplaces to prevent a major epidemic. This assumes that there is no compensatory increase in transmission, for example within the household. One can envisage that closing schools could result in increased household transmission, as the children would spend more time at home, and possibly the parents too. However, there could be a considerable educational component associated with taking this action, leading to improved hygiene and decreased transmission within the household. Hence, we have taken a neutral position, assuming no change in the model. If 0 were less than 2.0, then the prospects for control would be more optimistic.
Some specific control strategies have been investigated, with the results presented in figures 4 and 5. All of the strategies successful in preventing an epidemic, with 0=2.0 (c<1), involved TATP, with the assumption of 95% uptake and 60% efficacy. However, the strategy of closing schools (100% compliance) combined with either closing workplaces or home quarantine (70% compliance; no. 4 and 8 in figure 4) resulted in values of c just over 1. These strategies, combined with a modest amount of antiviral coverage would be sufficient to reduce c below 1. Under the low basic reproduction number assumption (0=1.1), all strategies considered apart from closing schools were sufficient to contain epidemics. Under the high basic reproduction number assumption (0=3.0), the only strategies sufficient to contain epidemics involved the use of antivirals, school closure and home quarantine. These results are derived assuming that there is no delay from the start of the epidemic until putting the strategy in place. As the scenario is that a pandemic has started elsewhere and is subsequently imported, it is reasonable to assume that the authorities have a response plan in place and the delay is minimal. Hence, the proportion infected will be approximately as calculated. This caveat does not affect the basic reproduction number under control (c) for each strategy.
Experiments with different contact structures in the model have shown the overall results to be quite robust, depending only on 0 for total numbers infected, and on the latent and infectious periods, and therefore the generation time, for the incidence curve for the epidemic. The number of infections that take place within the household, within school or the workplace, or within the wider community is determined by the assumed percentages at the beginning of an epidemic (table 1). As these weights also determine to a great extent the success or otherwise of control measures, they should be a prime target for scrutiny should a pandemic eventuate.
Acknowledgments
This project was commissioned and funded by the New Zealand Ministry of Health, and the material was contained in an unpublished report to the Ministry. The authors are very grateful for the information and comments provided by staff in the Public Health Directorate, Ministry of Health (including Alyson Baker, Martin Bonné, John Boyd, Andreya Brown, Andrea Forde, Alison Roberts, David Sinclair and Paul White). They are also grateful for the input of the external peer reviewers, Angela McLean and Patricia Priest. The views expressed are those of the authors, and do not necessarily reflect the views of the New Zealand Ministry of Health.
Appendix A
The model as described in the text and figure 1 may be represented by a set of integral equations, which are as follows:
where N is the population size (assumed constant), S(t) is the number in the population that are susceptible (immunologically naive to the emerging virus and assumed to equal the whole population prior to the epidemic), the components of the vector ı(t) are equal to the incidence of infection at each location and R(t)=N|r(t)| is the expected number removed from the epidemic (those that have been infected and are no longer infectious). The function g(τ) is the probability that an individual infected at time zero has ceased to be infectious by time τ. The vector e is a unit vector, with its only non-zero component that which signifies the location community, hence δ(t)e is the incidence of the index case. The matrix W weights the contacts between the infection groups and is scaled so that ‖W‖=1. For a vector, |…| signifies the sum of the components, for a matrix, ‖…‖ signifies the largest eigenvalue (spectral radius). We assume that those infected within their own household (location 1) do not go on to infect others within the same household, hence W11=0. That is, secondary within-household infections may occur, but we neglect tertiary within-household infections. We also assume that individuals may attend school (location 2) or work (location 3) but not both, hence W23=W32=0. In the absence of any other information, and in the absence of control measures, we have assumed that contact rates at each location are independent of the location at which an individual is infected (Roberts 2004, 2006). Hence, the non-zero entries in each row of W are set equal and
The infectivity function is defined by
This is a suitable approximation to an infectivity function, where nobody is infectious before τa days or after τd days post-exposure, maximum infectivity occurs between τb and τc days after exposure and contact rates are constant. It is consistent with a mean latent period of TE=(τa+τb)/2 days and a mean infectious period of TI=(τd+τc−τb−τa)/2 days. Hence, we set (τa, τb, τc, τd)=(1.2, 2.0, 5.3, 6.1) days.
References
- Aldis G.K, Roberts M.G. An integral equation model for the control of a smallpox outbreak. Math. Biosci. 2005;195:1–22. doi: 10.1016/j.mbs.2005.01.006. [DOI] [PubMed] [Google Scholar]
- Coker R.J, Mournier-Jack S. Pandemic influenza preparedness in the Asia-Pacific region. Lancet. 2006;368:886–889. doi: 10.1016/S0140-6736(06)69209-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diekmann O, Heesterbeek J.A.P. Wiley; Chichester, UK: 2000. Mathematical epidemiology of infectious diseases: model building, analysis and interpretation. [Google Scholar]
- Diekmann O, Heesterbeek J.A.P, Metz J.A.J. On the definition and computation of the basic reproduction ratio R0 in models for infectious diseases in heterogeneous populations. J. Math. Biol. 1990;28:365–382. doi: 10.1007/BF00178324. [DOI] [PubMed] [Google Scholar]
- Ferguson N, Cummings D.A.T, Cauchemez S, Fraser C, Riley S, Meeyai A, Iamsirithaworn S, Burke D.S. Strategies for containing an emerging influenza pandemic in Southeast Asia. Nature. 2005;437:209–214. doi: 10.1038/nature04017. [DOI] [PubMed] [Google Scholar]
- Ferguson N, Cummings D.A.T, Fraser C, Cajka J.C, Cooley P.C, Burke D.S. Strategies for mitigating an influenza pandemic. Nature. 2006;442:448–452. doi: 10.1038/nature04795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gani R, Hughes H, Fleming D, Griffin T, Medlock J, Leach S. Potential impact of antiviral drug use during influenza pandemic. Emerg. Infect. Dis. 2005;11:1355–1362. doi: 10.3201/eid1109.041344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germann T.C, Kadau K, Longini I.M, Macken C.A. Mitigation strategies for pandemic influenza in the United States. Proc. Natl Acad. Sci. USA. 2006;103:5935–5940. doi: 10.1073/pnas.0601266103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennings L. New Zealand's preparedness for the next influenza pandemic. N.Z. Med. J. 2005;118:1–3. [PubMed] [Google Scholar]
- Kermack, W. O. & McKendrick, A. G. 1927 Contributions to the mathematical theory of epidemics. I. Proc. R. Soc. A 115, 700–721. (Reprinted in Bull. Math. Biol 53, 33–55 (1991).) [DOI] [PubMed]
- Longini I.M, Halloran M.E, Nizam A, Yang Y. Containing pandemic influenza with antiviral agents. Am. J. Epidemiol. 2004;159:623–633. doi: 10.1093/aje/kwh092. [DOI] [PubMed] [Google Scholar]
- Longini I.M, Nizam A, Xu S, Ungchusak K, Hanshaoworakul W, Cummings D.A.T, Halloran M.E. Containing pandemic influenza at the source. Science. 2005;309:1083–1087. doi: 10.1126/science.1115717. [DOI] [PubMed] [Google Scholar]
- Miller J.M, Tam T.W, Maloney S, Fukuda K, Cox N, Hockin J, Kertesz D, Klimov A, Cetron M. Cruise ships: high-risk passengers and the global spread of new influenza viruses. Clin. Infect. Dis. 2000;31:433–438. doi: 10.1086/313974. [DOI] [PubMed] [Google Scholar]
- Mills C.E, Robins J.M, Lipsitch M. Transmissibility of 1918 pandemic influenza. Nature. 2004;432:904–906. doi: 10.1038/nature03063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts M.G. Modelling strategies for minimizing the impact of an imported exotic infection. Proc. R. Soc. B. 2004;271:2411–2415. doi: 10.1098/rspb.2004.2865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts M.G. Modeling strategies for containing an invading infection. Math. Popul. Stud. 2006;13:205–214. doi: 10.1080/08898480600950473. [DOI] [Google Scholar]
- Roberts, M. G. Submitted. The final size equation for a structured Kermack-McKendrick epidemic model.
- Wu J.T, Riley S, Fraser C, Leung G.M. Reducing the impact of the next influenza pandemic using household-based public health interventions. PLoS Med. 2006;3:1532–1540. doi: 10.1371/journal.pmed.0030361. [DOI] [PMC free article] [PubMed] [Google Scholar]