Abstract
Individuals are thought to have their own distinctive scent, analogous to a signature or fingerprint. To test this idea, we collected axillary sweat, urine and saliva from 197 adults from a village in the Austrian Alps, taking five sweat samples per subject over 10 weeks using a novel skin sampling device. We analysed samples using stir bar sorptive extraction in connection with thermal desorption gas chromatograph–mass spectrometry (GC–MS), and then we statistically analysed the chromatographic profiles using pattern recognition techniques. We found more volatile compounds in axillary sweat than in urine or saliva, and among these we found 373 peaks that were consistent over time (detected in four out of five samples per individual). Among these candidate compounds, we found individually distinct and reproducible GC–MS fingerprints, a reproducible difference between the sexes, and we identified the chemical structures of 44 individual and 12 gender-specific volatile compounds. These individual compounds provide candidates for major histocompatibility complex and other genetically determined odours. This is the first study on human axillary odour to sample a large number of subjects, and our findings are relevant to understanding the chemical nature of human odour, and efforts to design electronic sensors (e-nose) for biometric fingerprinting and disease diagnoses.
Keywords: individual odour, chemical communication, volatile biomarkers, metabolomics, chemometric pattern recognition
1. Introduction
An individual's odour can change due to a variety of factors, such as menstrual cycle, emotional state, health and perhaps age (Penn & Potts 1998a; Ackerl et al. 2001; Singh & Bronstad 2001), and yet despite these changes, each individual may retain his or her own particular scent (Romanes 1887). People can distinguish the scent of different individuals, especially if they are unrelated or have different diets, and can recognize their own and their mate's scent (Russell 1976; Hold & Schleidt 1977; Wallace 1977; Schleidt 1980; Pause et al. 1998). Mothers can recognize their newborn infants by olfactory cues after a few hours of contact, and infants quickly learn to recognize their mother's scent (Porter 1998). When offered human scent, canines can also discriminate individuals, though identical twins are more difficult (Kalmus 1955), and they can recognize individuals with varying degrees of accuracy (Brisbin et al. 2000; Curran et al. 2005). Mosquitoes are more attracted to some individuals than others depending on variation in chemical cues (Schreck et al. 1990; Qiu et al. 2006). These findings are consistent with the individual odour hypothesis; however, these studies do not provide estimates for inter-individual variability or intra-individual consistency, nor do they shed light on the volatile compounds that comprise individual odour.
Some studies have used analytical chemistry techniques, especially gas chromatograph–mass spectrometry (GC–MS), to describe the volatile organic compounds (VOCs) that comprise human sweat, breath and other emanations, though mostly to characterize malodour rather than individual odour per se. These show that sweat contains a complex mixture of volatiles, including short- and long-chain hydrocarbons, alcohols, carboxylic acids, ketones and aldehydes (Nicolaides 1974; Sastry et al. 1980; Zeng et al. 1991, 1996; Bernier et al. 2000, 2002; Natsch et al. 2006). Several studies report inter-individual variation and sex differences in VOCs (Sommerville et al. 1994; Bernier et al. 1999; Curran et al. 2005; Natsch et al. 2006), but they sampled only a few subjects and did not report the reproducibility of their techniques or the chemical signatures. Two studies surveyed 50 subjects, collecting samples from breath (Phillips et al. 1999) and sweat (Ostrovskaya et al. 2002), but neither determined whether individual profiles were repeatable or consistent over time. One study examined individual constancy in skin compounds, but sampled only 15 subjects and two samples per individual (Zhang et al. 2005). The problem is that studies with few subjects and few or no repeat samples risk finding false GC–MS markers, and false positives provide a serious challenge for GC–MS, much like gene expression (microarray) studies (Tsai et al. 2003). For example, if 1000 GC–MS peaks are detected in 10 subjects, then we expect on average 9.46 peaks to be detected uniquely in one individual, even if there is an underlying random (50%) presence/absence distribution. Therefore, determining whether body odour contains individual fingerprints requires sampling a large number of subjects longitudinally using reproducible analytical techniques. A recent debate addresses additional problems, potential pitfalls and differences in terminology used by researchers studying human odour (Curran et al. 2006; Preti et al. 2006).
We conducted a survey in which we collected axillary sweat samples, saliva and urine from human subjects for GC–MS analyses. The axillary region is of particular interest, as it contains dense aggregations of eccrine, apocrine, apoeccrine and sebaceous glands that nurture diverse communities of microbiota thought to play an important role in generating individual odour (Albone et al. 1977; Leyden et al. 1981; Stoddart 1990; Taylor et al. 2003; James et al. 2004b). To overcome the challenges of statistical inference, we sampled a large number of individuals repeatedly over time. Subjects agreed to follow strict instructions to minimize interference from deodorants and other artificial contaminants, and we used a systematic sampling schedule to control for time effects and a variety of other potential confounding factors. We collected axillary samples using a novel method that eliminates the need for pads or other intermediate media which may not be analytically clean (and may even collect exogenous contaminants) or fail to transfer certain compounds, and we used internal standards for precise, quantitative chemical analyses (Soini et al. 2005, 2006). We used novel chemometric methods for peak detection and alignment (Dixon et al. in press) and pattern recognition techniques to analyse the chromatographic profiles of VOCs (Brereton 2003). Finally, we obtained structural identities of key marker compounds by interpretation of mass spectra, internal retention indices and matching to a mass spectral reference database.
2. Methods
2.1 Subjects
We recruited subjects from large families (89 males, 108 females, ages 18–91, mean 44 years) living in a small village in the Austrian Alps. We interviewed recruits to construct pedigrees, and identified 16 families (from 10 to 31 members). We scheduled appointments for sampling in a systematic, balanced design, i.e. sex, age and families were randomized over week, weekday and time of day (using a computer macro we programmed). To minimize potential confounding factors, especially from cosmetic products that contain contaminants and alter microflora, subjects were given several instructions to follow. (i) To refrain from using deodorants containing aluminium chloride (ACH), and to use the ACH- and perfume-free deodorant we provided at least 7 days before sampling, and the perfume-free wash lotion/soap we provided at least 1 day before sampling. (ii) Not to shave axillae 2 days before sampling. (iii) To refrain from washing axillae 12 h before each sampling and not to use any deodorants at all after their last wash before sampling. (iv) To wear the t-shirt we provided (washed with perfume-free detergent) after their last wash before sampling. We did not restrict medication, drugs, alcohol or tobacco use, or diet. During each sampling, we provided a questionnaire to obtain information about factors known or suspected to influence odour (sex, menstrual cycle, contraceptive use, pregnancy status, exercise, diet, hygiene, pets, medication, alcohol, drug and tobacco use), and to determine whether subjects had followed the instructions. Participation was voluntary, subjects were informed about the goals of the study and were compensated for their participation. The appropriate institutional, ethics and research boards approved this study (Austrian Institutional Research Board: the Ethics Committee of the Medical University of Vienna and the Vienna General Hospital, and by the Human Subjects Research Review Board and the Indiana University Human Subjects Committee, both in USA).
2.2 Sampling emanations
We collected samples from 197 individuals, five times each (once every fortnight) over a 10-week period (from 18 June to 26 August 2005). To sample axillary sweat, we devised a novel sampling technique using Twister polydimethylsiloxane (PDMS)-coated stir bars (10 mm, 0.5 mm in film thickness, 24 μl PDMS volume; Gerstel GmbH) for stir bar sorptive extraction (SBSE; Baltussen et al. 2002; Soini et al. 2005). The stir bars were held by a special roller device and then placed directly on skin (Soini et al. 2006). Each stir bar was first conditioned at 280°C in helium flow and embedded with two internal standards (8 ng of 7-tridecanone from Aldrich and 50 ng of C-13-labelled benzyl alcohol from Cambridge Isotope Laboratories), and then shipped cooled in special clean, airtight vials from the USA to Austria. Sampled stir bars were stored refrigerated in glass vials at approximately 4°C and shipped cooled (Chillers) each week from Austria to the USA. For comparison, we also collected saliva and urine samples (four repeats each); we asked subjects to spit into a plastic container from which we collected 3 ml samples, and subjects collected their urine (midstream) in the morning and before eating on each day of axillary sampling. All samples were stored at −70°C and shipped as frozen on dry ice. We sampled all subjects in the same room to control for environmental variation.
2.3 Gas chromatograph–mass spectrometry analyses
Samples were analysed using SBSE in connection with thermal desorption GC–MS (Baltussen et al. 2002; Soini et al. 2005, 2006). The GC–MS used for the compound analyses was an Agilent 6890N GC connected to 5973i MSD MS and equipped with a thermal desorption autosampler (TDSA, Gerstel). The capillary column was a DB-5MS (20 m×0.18 mm, i.d., and 0.18 μm in film thickness) from Agilent Technologies (Wilmington, DE). Electron ionization mode at 70 eV was used with a scanning rate of 4.51 scans s−1 over the mass range of m/z 35–350 amu. The MSD transfer line temperature was set at 280°C. The ion source and quadrupole temperatures were set at 230 and 150°C, respectively. We monitored instrumental performance using results from the repeated quality control samples and considered the peak area of the internal standard, 7-tridecanone as performance criteria. Relative standard deviation for the peak area of 7-tridecanone was 14.30% (n=958). Absolute variation for the peak area was 2.14±0.31 (peak area×106, mean±s.d.). Examples of chromatograms are shown in the electronic supplementary material. The structures for compounds were obtained by interpretation of mass spectra, by matching retention times (RTs) and spectra with our internal retention index/reference spectrum database, and by confirmation with commercial synthetic standards (Aldrich Chemical Company, Milwaukee, WI).
2.4 Chemometric analyses
We sampled all 197 subjects five times and, before performing any statistical analyses, we removed 20 GC–MS sweat profiles with analytical problems (samples showing contamination from the laboratory, a very large baseline or problems with the instrument during analysis), resulting in 965 chromatograms. To analyse this large number of GC–MS (with 241 peaks per chromatogram on average), we developed semi-automated methods for data processing, including alignment and peak picking (with such a large number of samples, it is impractical to detect and integrate each peak manually, as this would require approximately 19 years, working at the rate of 50 h week−1 and 45 week yr−1; Dixon et al. in press). To identify peaks in the chromatograms, peaks with similar mass spectra and elution times were aligned across the chromatograms, and all peaks identified in less than five chromatograms were removed to provide a data table that consisted of 965 samples×4941 peaks. Of these 4941 unique peaks detected in at least five out of the 965 samples (minimum threshold), 373 peaks (8%) were detected in at least one individual in four out of their five samples after removing known background peaks (i.e. obvious contaminants). Our subsequent analyses were based on these 373 consistent peaks. The square root of the candidate marker peak areas was calculated in each chromatogram (to reduce the influence of large peaks) and summed to constant total (normalized). Logarithmic scaling was not suitable in this study due to the problem of undetected peaks. We examined similarities within and between individuals and sexes using the proportion of GC–MS peaks in common to two chromatograms (qualitative model) and quantitative similarities between GC–MS profiles, and employed pattern recognition techniques to determine trends. To compare the sexes, we used two statistical indicators, including a univariate t-statistic and multivariate discriminatory partial least-squares weights (Brereton 2003). These statistics were calculated on the column standardized row normalized square-root intensity data for the 373 potential markers for each of the five fortnights, and the peaks ranked on each fortnight from 1 (most significant) to 373 (least significant). Peaks that had a high rank in four out of five fortnights were retained as potential markers for gender. To identify peaks characteristic of an individual, the dataset was divided into family groups. In each family, the normalized square-root intensity data was standardized, so the mean area of each potential marker was 0 and the standard deviation was 1. A principal component analysis (PCA) was performed on each family, and the first three components were analysed by visual examination of three-dimensional scores and loadings plots.
3. Results
We found that axillary sweat is richer in volatiles and semi-volatiles than saliva or urine. For example, using SBSE-based sampling methods and identical GC–MS analytical conditions, we found on average 241 peaks in the GC–MS of sweat, 179 in saliva and 163 in urine per individual. Although most compounds in sweat did not show within-subject consistency, we found 373 peaks that were consistent, and we therefore conducted our statistical analyses on these compounds. Of these 373 consistent peaks in sweat, 166 were also found in saliva and 78 in urine, indicating similarities and differences among these emanations for potential signature compounds. Nearly all of these consistent axillary markers were uncommon and were detected in a minority of individuals, and although a few were common and occurred in at least one sample of most subjects (see table 1 of electronic supplementary material), we found no markers universally associated with all individuals. This does not rule out the possibilities that there are universal compounds that fluctuate over time or were below our detection limits. We found very few peaks common to all samples (e.g. only two peaks were detected in at least 900 samples and 38 in at least half the (965) samples, suggesting that most markers detected are quite specific to small numbers or groups of individuals). Thus, we found a substantial number of marker compounds that can potentially differentiate individuals or groups.
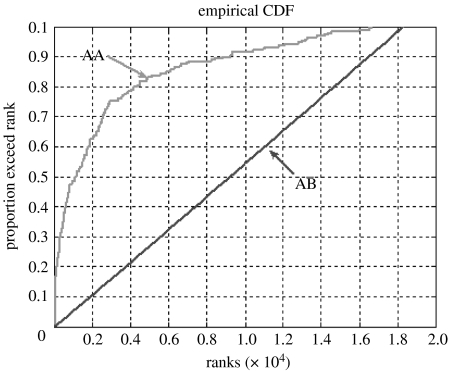
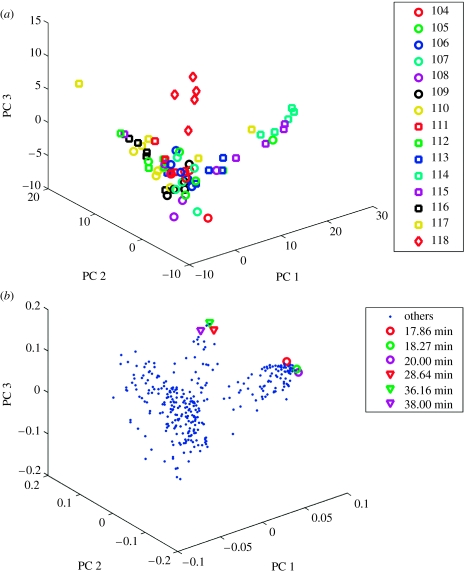
Indeed, when we analysed the 373 candidate compounds in axillary sweat using a variety of pattern recognition techniques, we found strong evidence for individual fingerprints. We calculated the pairwise similarities between GC–MS fingerprints of all 965 samples, using a qualitative presence/absence criterion, and found that repeat samples from the same individual are significantly more similar than samples from different individuals (Kolmorogov–Smirnoff rank test, KS statistic=0.588, p=1.59×10−60; figure 1). For illustration, we show a PCA of one family consisting of 15 individuals (figure 2). The scores plot (figure 2a) shows that repeat samples of individuals cluster closely, indicating a reproducible individual fingerprint. Expansion of the centre of the scores plot in figure 2a shows a similar though weaker pattern (data not shown), and a similar result is found for all other families. It also shows that certain subjects are reproducible outliers, suggesting that some individuals have a more characteristic odour than others. Subjects 114 and 118 (a non-cohabitating aunt and niece), for example, show very characteristic fingerprints and cluster at a distance outside of the rest of the family, which may be a result of diet or other personal habits (e.g. subject 114 lived with eight pets). Furthermore, GC–MS peaks characteristic of an individual's sweat can be determined by comparing the loadings to the scores plot. For example, peaks with RTs 17.86, 18.27 and 20.00 min are highly characteristic of subject 114, whereas peaks with RTs 28.64, 36.16 and 38.00 min are characteristic of subject 118 (table 1). These peaks are coloured in figure 2b and correspond most closely to the individuals in figure 2a. These are detected much more frequently in the particular individual as compared to the population as a whole. Hence, we are able to detect diagnostic compounds that are consistent with individual fingerprints, and we identified the chemical structures of 44 out of these 66 marker compounds characteristic of individuals (table 2).
Figure 1.
Intra- versus inter-individual variation. This empirical cumulative distribution function shows the result of a test using qualitative distance to compare the similarity of GC–MS spectra. AA represents the average distance of the repeats of the same subject (intra-individual) and AB shows the average distance between different subjects (inter-individual). AA is significantly different from AB, and AA is always above AB. The significant Kolmorogov–Smirnoff rank test means that the samples in AA comparisons generally receive a lower rank than those in AB comparisons. This indicates that the repeats of GC–MS spectra of the same subject are more similar to each other than samples from different subjects. A quantitative model shows the same result, and though the difference is also significant (Kolmorogov–Smirnoff rank test, KS statistic=0.321, p=3.74×10−18), it is not as dramatic. This suggests that the intra-individual variation is much smaller using a qualitative distance metric than using a quantitative one.
Figure 2.
(a) Scores and (b) loadings plots showing markers that distinguish individuals. This is an example of one family with individuals numbered and coded with coloured symbols in the scores plots. Key peaks are indicated and their retention times listed in the loadings plot. The scores and loadings for principal component analysis on these data are presented: scores provide information about samples (or subjects), whereas loadings provide complementary information about the compounds that characterize these samples.
Table 1.
Marker compounds in axillary sweat characteristic of two individuals (114 and 118). Two markers (RTs 17.86 and 18.27) are possibly exogenous compounds.
| RT (min) | subject | identification | times detected in 965 samples | times detected in L114 | times detected in L118 |
|---|---|---|---|---|---|
| 17.86 | 114 | methyl-N-methylanthranilate | 43 (4.46%) | 5 (100%) | 0 |
| 18.27 | 114 | α-ionone | 94 (9.74%) | 5 (100%) | 0 |
| 20.00 | 114 | an unknown bicyclic compound | 92 (9.53%) | 5 (100%) | 0 |
| 28.64 | 118 | 4-phenyltridecane | 36 (3.73%) | 2 | 4 (80%) |
| 36.16 | 118 | unknown | 16 (1.66%) | 2 | 4 (80%) |
| 38.00 | 118 | dodecyl octanoate | 144 (14.92%) | 0 | 5 (100%) |
Table 2.
Marker compounds in axillary sweat characteristic of individuals. These ‘individual markers’ were detected in either quantitative or qualitative models. Among these compounds, the following have been previously found on human skin: 2-phenylethanol (Bernier et al. 2000; Zhang et al. 2005), 1-tridecanol (Bernier et al. 2000), undecanal (Curran et al. 2005), lilial and diphenyl ether (Zhang et al. 2005). RT (min) of additional individual marker compounds of unknown identity: 18.42, 22.52, 23.65, 23.74, 23.90, 24.43, 24.50, 26.79, 26.86, 28.41, 28.48, 30.01, 31.41, 32.06, 33.53, 33.87, 34.22, 34.56, 36.16, 36.66, 40.33, 40.77.
| RT (min) | identification | commentsa | |
|---|---|---|---|
| alcohols and phenols | |||
| 9.98 | 2-phenylethanolb | en | |
| 12.30 | α-terpineolb | ex | |
| 13.82 | geraniolb | s | en |
| 16.48 | eugenolb | s, u | en |
| 18.93 | isoeugenolb | s | |
| 21.93 | 1-tridecanolb | en | |
| aldehydes | |||
| 14.38 | geranialb | s | en |
| 15.46 | undecanalb | s | en |
| 20.61 | tridecanalb | s | en |
| 20.83 | lilial | s | ex |
| ketones | |||
| 17.47 | jasmone | s | ex |
| 18.27 | α-iononeb | s | ex |
| 23.09 | benzophenoneb | s | |
| 28.70 | 2-acetyl-3,5,5,6,8,8-hexa-methyl-5,6,7,8-tetrahydronaphthalene | s | ex |
| 29.22 | 7-acetyl-6-ethyl-1,1,4,4-tetramethyltetralin (Musk 36A) | ex | |
| esters | |||
| 16.37 | α-terpinyl acetateb | s | ex |
| 17.86 | methyl-N-methylanthranilate | ex | |
| 18.28 | 2-hexyl-2-pentenoate | ||
| 18.79 | E-cinnamyl acetate | ||
| 21.24 | α-trichloromethylbenzyl acetate | s | ex |
| 22.69 | isoeugenol acetate | ||
| 23.73 | methyl-cis-dihydrojasmonate | s, u | ex |
| 24.15 | 3Z-1-hexenyl salicylate | s | |
| 27.38 | ethyl tetradecanoateb | ||
| 27.53 | 2-ethylhexyl salicylate | s | |
| 30.06 | ethyl pentadecanoate | ||
| 30.32 | 2-phenylethyl phenylacetate | ||
| 32.29 | decyl octanoate | ||
| 32.36 | dodecyl hexanoate | ||
| 35.64 | ethyl heptadecanoate | ||
| 36.47 | a branched dodecyl benzoate | ||
| 38.00 | dodecyl octanoate | ||
| 38.72 | dodecyl benzoate | ||
| 43.19 | tridecyl benzoate | ||
| 47.56 | tetradecyl octanoate | ||
| 49.15 | tetradecyl benzoate | ||
| hydrocarbons | |||
| 26.96 | a propyl-substituted dodecane | ||
| 28.64 | 4-phenyltridecane | ||
| 29.54 | 3-methyloctadecane | s | en |
| 32.19 | 3-methylnonadecane | u | en |
| others | |||
| 16.81 | 4-sec-butylaniline | ||
| 17.65 | diphenyl ether | s | |
| 20.00 | an unknown bicyclic compound | s | |
| 26.55 | a diethyl acetal | s | |
Compounds also found in saliva (s) or urine (u), and their likely origin (en, probably endogenous; ex, possibly exogenous).
Compounds confirmed using known standards.
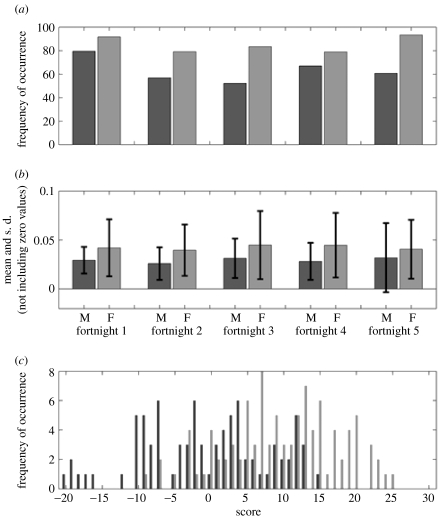
In addition to individual fingerprints, we also found characteristic peaks that distinguish the sexes, and we identified the most significant gender-specific compounds. We determined relative abundance (using square-root normalized data) in each sample, and then calculated the average detection frequency in the GC–MS and the average root-mean-square normalized intensity in the GC–MS for each fortnight and each sex. Figure 3a,b illustrates this result for the compound isopropyl hexadecanoate (RT 33.70 min). We detected no marker compounds that were universally present in one sex and rarely or never in the other, which indicates that the distribution of marker compounds for sex is multivariate, meaning that no single compound provides a marker on its own. Using the presence or absence of the 14 most significant markers, we were able to make a simple model to predict whether a person is male or female from their GC–MS fingerprint (figure 3c). We identified the chemical structures of 12 out of these 14 marker compounds characteristic of gender (table 3). We identified an additional 118 axillary compounds that were not markers characteristic of individuals or sexes (table 2 of electronic supplementary material), a number of which have been found in previous studies (Nicolaides 1974; Zeng et al. 1996; Curran et al. 2005).
Figure 3.
Distributions of markers that distinguish the sexes. (a) The distribution of the marker compound isopropyl hexadecanoate (RT 33.70 min) as the percentage of samples it was detected in and (b) mean and s.d. of the normalized square-root intensity when detected in males and females, over all five fortnights. (c) Distribution of males and females is based on a model using the scoring system (black, male; grey, female). For each fortnight, if the male marker is detected in a specific individual, it is scored as −1, for a female marker it is scored as +1, so an individual scoring +35 contains the strongest possible female fingerprint, whereas an individual scoring −35 the strongest possible male fingerprint. Using a score of five as a divider between the classes, 75% can be correctly classified into their respective genders based on the presence and absence of 14 key markers.
Table 3.
Marker compounds in axillary sweat characteristic of gender. These ‘gender markers’ were detected in either quantitative or qualitative models. Among these compounds, the following have been previously found on human skin: pentadecanoic acid, hexadecanoic acid and heptadecanoic acid (Nicolaides 1974; Bernier et al. 2000), a methylhexadecanoic acid (Nicolaides 1974; Bernier et al. 2000; Curran et al. 2005) and docosane (C-22 linear hydrocarbon; Bernier et al. 2000).
| RT (min) | identification | commentsa | |
|---|---|---|---|
| male | |||
| 13.71 | a ketone | en | |
| 23.18 | 6-phenylundecane | ||
| 28.12 | unknown | u | |
| 29.31 | pentadecanoic acid | s | en |
| 31.96 | hexadecanoic acid | s, u | en |
| 33.48 | a methylhexadecanoic acid | u | en |
| 34.94 | heptadecanoic acid | s, u | en |
| female | |||
| 24.17 | a dialkyl ether | ||
| 30.22 | nonadecane | s, u | en |
| 33.70 | isopropyl hexadecanoate | s | |
| 35.80 | unknown | ||
| 37.35 | 2-ethyl-hexyl-4-methoxycinnamate | s, u | |
| 38.79 | docosane (C-22 linear hydrocarbon) | s | en |
| 43.35 | 1-octyl-4-methoxycinnamate | s | |
Compounds also found in saliva (s) or urine (u), and their likely origin (en, probably endogenous).
4. Conclusions
We found more volatile compounds in axillary sweat than saliva or urine, suggesting that this emanation provides a particularly important source of individual markers. This large diversity of axillary compounds may be generated by microbiota, or perhaps skin simply contains more exogenous contaminants than saliva or urine (Labows et al. 1979). Of the 4941 peaks in the GC–MS profiles from sweat, we found 373 markers that showed consistency over the 10-week sampling period. We limited our search for maker compounds to these consistent ones, and this also helped make our analyses more computationally efficient (as it reduced our dataset from 4941 to 373 peaks×965 samples). We may have subsequently omitted important marker compounds, but our results should be conservative. Using these 373 candidate compounds, we found evidence for both individual and gender-specific GC–MS signatures.
We found significant evidence for individual chemical signatures in the GC–MS profiles from axillary sweat, and we identified the chemical structures of 44 of these compounds (table 2). We found both qualitative (presence/absence) and quantitative differences (variation in the relative ratios of compounds), as previously suggested (Sastry et al. 1980), but we found that qualitative indicators of similarity were more effective than quantitative ones. This may be due to the inherent difficulty of quantifying sweat (i.e. the analytical instrumental methods were quantitative, but the amount of sweat was not controlled). Other studies have suggested that individual differences are mainly quantitative (Bernier et al. 1999; Curran et al. 2005), though this may be due to differences in sample sizes or methodology. Nevertheless, our results suggest that identifying individuals with these ‘individual markers’ would require using pattern recognition of the entire profile pattern rather than particular compounds. Interestingly, we found that many subjects had very distinctive GC–MS signatures, even among subjects that cohabitate or were closely related. The reason for the variation in distinctiveness is unclear. Not all subjects had consistent marker compounds over time, which might be due to physiological, dietary, or other changes, or simply how consistently subjects adhered to the rules we provided (including contacting exogenous compounds beyond our control). Future studies might examine whether humans or other species use these marker compounds for olfactory discrimination of individuals (e.g. these compounds could be manipulated to test whether they affect olfactory discrimination of individuals).
We found that although the axillary sweat of men and women had remarkably similar GC–MS profiles, we could statistically discriminate the sexes, and we identified the chemical structures of 12 of these marker compounds characteristic of gender (table 3). A previous analytical study comparing axillary odour compounds of men versus women (six of each sex) found three compounds present only in women and 34 quantitative differences between the sexes (although they did not report any statistical analyses; Zeng et al. 1996), whereas another study (10 of each sex) found no differences between the sexes (Asano et al. 2002). We found no unique and exclusive markers to discriminate the sexes, and instead we found compounds that were more common in male subjects than females and vice versa. No marker was uniquely indicative of gender, and the difference between the sexes is characterized by a multivariate distribution of marker compounds (i.e. a multivariate fingerprint). Thus, odour may be analogous to facial features, in that no single measurement on a face can easily be used to recognize an individual; it requires a combination of features, and recognition is improved by including other traits (Chang et al. 2003). Some previous studies suggest that people can discriminate the sexes by olfactory cues (Doty et al. 1982), and so future work could examine whether these compounds play a role in this task (again, these compounds could be manipulated to test whether they affect the ability to discriminate the sexes), or other aspects of chemosensory communication.
The origins of these individual and sex-specific volatile compounds are unclear. Individual odour is a phenotype or ‘extended phenotype’, and like other phenotypes variation may be due to genetic factors, environmental factors, or both. There is evidence from twin studies that odour is influenced by genetics (Roberts et al. 2005), and that odour plays a role in people's ability to recognize kin (Weisfeld et al. 2003). There is evidence that odour in humans and other species is influenced by the highly polymorphic major histocompatibility complex (MHC) loci (Wedekind et al. 1995; Penn & Potts 1998b; Yamazaki & Beauchamp 2005). Individual and genetically determined odours are sometimes referred to as ‘odourtypes’ (Yamazaki & Beauchamp 2005), which imply discontinuous variation; however, we found no evidence for this in our GC–MS profiles. Continuous phenotypic variation would suggest that individual odour is a quantitative trait, influenced by many loci, and if so, terms such as ‘chemical signatures’, ‘chemical fingerprints’ or ‘odourprints’ would provide more accurate descriptions. Individual odour is also influenced by environmental factors, such as commensal microflora (though work on this topic mainly examines malodour rather than individual odour per se; Leyden et al. 1981; Taylor et al. 2003; James et al. 2004a), and there is evidence for interactions in which MHC genes influence microflora composition (Toivanen et al. 2001). The individual marker compounds we found provide candidates for MHC and other genetically determined odours, and we are currently trying to determine whether they are influenced by genetics, microflora or both.
Some of the individual marker compounds we found might be artificial contaminants since some also occur in soaps, cosmetics, fragrances, shampoos, detergents and tobacco (we suspect that 10 out of 44 are exogenous; table 2). Such compounds are not necessarily contaminants, however, because some that are used in fragrances are also found in body fluids, arising through metabolic origins (e.g. eugenol and undecanal). Many of the individual compounds we found in axillary samples were also detected in the urine and saliva samples of our subjects (21 out of 44; table 2), suggesting metabolic origins. On the other hand, skin care products (synthetic and natural) can be absorbed into the body (Jimbo 1983) (which is a disturbing news, given the pathological effects they may have on physiology and behaviour; Zala & Penn 2004). Determining the origins of individual and sex-specific odours—and controlling exogenous chemical contaminants—may provide the most important challenge for future GC–MS studies (Labows et al. 1979).
Our findings shed light on the chemical basis of individual and sex-specific odours, which has implications for understanding how people (and canines) discriminate individuals and the sexes by their odour, and for determining how MHC or other genes influence odour (Penn & Potts 1998b; Penn 2002; Yamazaki & Beauchamp 2005). It has been suggested that individual identification is one of the most important messages used in vertebrate chemical communication (Wilson 1970), and our results are relevant to efforts to understand chemosensory individuality in humans and other species (Penn 2006). Our results also offer practical implications for efforts to design electronic sensor (e-nose) technologies for biometric fingerprinting (Jain 2005), forensic research and disease diagnoses. An individual's odour can change due to a variety of diseases (Penn & Potts 1998a), including cancer (Willis et al. 2004), and variation in odour affects attractiveness to mosquitoes (Schreck et al. 1990; Qiu et al. 2006). Thus, there is increasing interest in determining whether body odour can be used to diagnose disease (Turner & Magan 2004; Phillips et al. 2006) or altered to reduce the risk of attracting mosquitoes and other disease vectors.
Acknowledgments
We thank A. Katzer for her outstanding organizational support at every stage of this work, I. Blantar, K. Zimmer and M. Oberzaucher for their assistance in recruiting subjects and collecting samples, and R. Hengsberger for additional assistance. We are grateful for comments from G. Preti, A. Willse, T. Wyatt, B. Pause and two anonymous reviewers. This work was supported by ARO grant DAAD19-03-1-0215. Opinions, interpretations, conclusions and recommendations are those of the authors and are not necessarily endorsed by the United States Government.
Supplementary Material
Table 1 showing distribution of marker compounds characteristic of individuals, Table 2 showing 118 additional compounds identified from axillary samples, and GC-MS chromatographic profiles of skin volatile compounds
References
- Ackerl K, Atzmüller A, Grammer K. The scent of fear. Neuroendocrinol. Lett. 2001;23:79–84. [PubMed] [Google Scholar]
- Albone E.S, Gosden P.E, Ware G.C. Bacteria as a source of chemical signals in mammals. In: Müller-Schwarze D, Mozell M.M, editors. Chemical signals in vertebrates. Plenum Press; New York, NY: 1977. p. 35. [Google Scholar]
- Asano K.G, Bayne C.K, Horsman K.M, Buchanan M.V. Chemical composition of fingerprints for gender determination. J. Forensic Sci. 2002;47:805–807. [PubMed] [Google Scholar]
- Baltussen E, Cramers C.A, Sandra P.J.F. Sorptive sample preparation—a review. Anal. Bioanal. Chem. 2002;373:3–22. doi: 10.1007/s00216-002-1266-2. [DOI] [PubMed] [Google Scholar]
- Bernier U.R, Booth M.M, Yost R.A. Analysis of human skin emanations by gas chromatography/mass spectrometry. 1. Thermal desorption of attractants for the yellow fever mosquito (Aedes aegypti) from handled glass beads. Anal. Chem. 1999;71:1–7. doi: 10.1021/ac980990v. [DOI] [PubMed] [Google Scholar]
- Bernier U.R, Kline D.L, Barnard D.R, Schreck C.E, Yost R.A. Analysis of human skin emanations by gas chromatography/mass spectrometry. 2. Identification of volatile compounds that are candidate attractants for the yellow fever mosquito (Aedes aegypti) Anal. Chem. 2000;72:747–756. doi: 10.1021/ac990963k. [DOI] [PubMed] [Google Scholar]
- Bernier U.R, Kline D.L, Schreck C.E, Yost R.A, Barnard D.R. Chemical analysis of human skin emanations: comparison of volatiles from humans that differ in attraction of Aedes aegypti (Diptera: Culicidae) J. Am. Mosq. Control Assoc. 2002;18:186–195. [PubMed] [Google Scholar]
- Brereton R.G. Wiley; Chichester, UK: 2003. Chemometrics: data analysis for the laboratory and chemical plant. [Google Scholar]
- Brisbin I.L, Austad S, Jacobson S.K. Canine detectives: the nose knows–or does it? Science. 2000;290:1093. doi: 10.1126/science.290.5494.1093b. [DOI] [PubMed] [Google Scholar]
- Chang K, Bowyer K.W, Sarkar S, Victor B. Comparison and combination of ear and face images in appearance-based biometrics. IEEE Trans. Pattern Anal. 2003;25:1160–1165. doi: 10.1109/TPAMI.2003.1227990. [DOI] [Google Scholar]
- Curran A.M, Rabin S.I, Prada P.A, Furton K.G. Comparison of the volatile organic compounds present in human odor using SPME-GC/MS. J. Chem. Ecol. 2005;31:1607–1619. doi: 10.1007/s10886-005-5801-4. [DOI] [PubMed] [Google Scholar]
- Curran A.M, Rabin S.I, Prada P.A, Furton K.G. On the definition and measurement of human scent: response by Curran et al. J. Chem. Ecol. 2006;32:1617–1623. doi: 10.1007/s10886-006-9096-x. [DOI] [PubMed] [Google Scholar]
- Dixon, S. J., Xu, Y., Brereton, R. G., Oberzaucher, E., Grammer, K., Soini, H. A., Novotny, M. V. & Penn, D. J. In press. An automated method for peak detection and matching in large gas chromatography-mass spectrometry data sets. J. Chemometrics.
- Doty R.L, Green P.A, Ram C, Yankell S.L. Communication of gender from human breath odors: relationship to perceived intensity and pleasantness. Horm. Behav. 1982;16:13–22. doi: 10.1016/0018-506X(82)90002-2. [DOI] [PubMed] [Google Scholar]
- Hold B.C.L, Schleidt M. The importance of human odour in non-verbal communication. Zeitschrift für Tierpsychologie. 1977;43:225. doi: 10.1111/j.1439-0310.1977.tb00072.x. [DOI] [PubMed] [Google Scholar]
- Jain A.K. Biometric recognition: how do I know who you are? Image analysis and processing—Iciap 2005 Proceedings. 2005;3617:19–26. doi: 10.1109/SIU.2004.1338241. [DOI] [Google Scholar]
- James A.G, Casey J, Hyliands D, Mycock G. Fatty acid metabolism by cutaneous bacteria and its role in axillary malodour. World J. Microbiol. Biotechnol. 2004a;20:787–793. doi: 10.1007/s11274-004-5843-8. [DOI] [Google Scholar]
- James A.G, Hyliands D, Johnston H. Generation of volatile fatty acids by axillary bacteria. Int. J. Cosmet. Sci. 2004b;26:149–156. doi: 10.1111/j.1467-2494.2004.00214.x. [DOI] [PubMed] [Google Scholar]
- Jimbo Y. Penetration of fragrance compounds through human epidermis. J. Dermatol. 1983;10:229–239. doi: 10.1111/j.1346-8138.1983.tb01132.x. [DOI] [PubMed] [Google Scholar]
- Kalmus H. The discrimination by the nose of the dog of individual human odours and in particular of the odours of twins. Br. J. Anim. Behav. 1955;3:25–31. doi: 10.1016/S0950-5601(55)80072-X. [DOI] [Google Scholar]
- Labows J, Preti G, Hoelzle E, Leyden J, Kligman A. Analysis of human axillary volatiles: compounds of exogenous origin. J. Chromatogr. 1979;163:294–299. doi: 10.1016/s0378-4347(00)81417-6. [DOI] [PubMed] [Google Scholar]
- Leyden J.J, McGinley K.J, Holzle E, Labows J.N, Kligman A.M. The microbiology of the human axilla and its relationship to axillary odor. J. Invest. Dermatol. 1981;77:413–416. doi: 10.1111/1523-1747.ep12494624. [DOI] [PubMed] [Google Scholar]
- Natsch A, Derrer S, Flachsmann F, Schmid J. A broad diversity of volatile carboxylic acids, released by a bacterial aminoacylase from axilla secretions, as candidate molecules for the determination of human-body odor type. Chem. Biodiv. 2006;3:1–20. doi: 10.1002/cbdv.200690015. [DOI] [PubMed] [Google Scholar]
- Nicolaides N. Skin lipids: their biochemical uniqueness. Science. 1974;186:19–26. doi: 10.1126/science.186.4158.19. [DOI] [PubMed] [Google Scholar]
- Ostrovskaya A, Landa P.A, Sokolinsky M, Rosalia A.D, Maes D. Study and identification of volatile compounds from human skin. J. Cosmet. Sci. 2002;53:147–148. [Google Scholar]
- Pause B.M, Krauel K, Sojka B, Ferstl R. Body odor evoked potentials: a new method to study the chemosensory perception of self and non-self in humans. Genetica. 1998;104:285–294. doi: 10.1023/A:1026462701154. [DOI] [PubMed] [Google Scholar]
- Penn D.J. The scent of genetic compatibility: sexual selection and the major histocompatibility complex. Ethology. 2002;108:1–21. doi: 10.1046/j.1439-0310.2002.00768.x. [DOI] [Google Scholar]
- Penn D.J. Chemical communication: five major challenges in the post-genomics age. In: Dicke M, Takken W, editors. Chemical ecology: from gene to ecosystem. vol. 16. Springer; Dordrecht, The Netherlands: 2006. pp. 9–18. [Google Scholar]
- Penn D, Potts W.K. Chemical signals and parasite-mediated sexual selection. Trends Ecol. Evol. 1998a;13:391–396. doi: 10.1016/S0169-5347(98)01473-6. [DOI] [PubMed] [Google Scholar]
- Penn D, Potts W. How do major histocompatibility complex genes influence odor and mating preferences? Adv. Immunol. 1998b;69:411–435. doi: 10.1016/s0065-2776(08)60612-4. [DOI] [PubMed] [Google Scholar]
- Phillips M, Herrera J, Krishnan S, Zain M, Greenberg J, Cataneo R.N. Variation in volatile organic compounds in the breath of normal humans. J. Chromatogr. B Biomed. Sci. Appl. 1999;729:75–88. doi: 10.1016/S0378-4347(99)00127-9. [DOI] [PubMed] [Google Scholar]
- Phillips M, Cataneo R.N, Ditkoff B.A, Fisher P, Greenberg J, Gunawardena R, Kwon C.S, Tietje O, Wong C. Prediction of breast cancer using volatile biomarkers in the breath. Breast Cancer Res. 2006;99:19–21. doi: 10.1007/s10549-006-9176-1. [DOI] [PubMed] [Google Scholar]
- Porter R.H. Olfaction and human kin recognition. Genetica. 1998;104:259–263. doi: 10.1023/A:1026404319384. [DOI] [PubMed] [Google Scholar]
- Preti G, Willse A, Labows J, Leyden J.J, Wahl J, Kwak J. On the definition and measurement of human scent: comments on Curran et al. J. Chem. Ecol. 2006;32:1613–1616. doi: 10.1007/s10886-006-9095-y. [DOI] [PubMed] [Google Scholar]
- Qiu Y.T, Smallegange R.C, Van Loon J.J.A, Ter Braak C.J.F, Takken W. Interindividual variation in the attractiveness of human odours to the malaria mosquito Anopheles gambiae s.s. Med. Vet. Entomol. 2006;20:280–287. doi: 10.1111/j.1365-2915.2006.00627.x. [DOI] [PubMed] [Google Scholar]
- Roberts S.C, Gosling L.M, Spector T.D, Miller P, Penn D.J, Petrie M. Body odor similarity in noncohabiting twins. Chem. Senses. 2005;30:651–656. doi: 10.1093/chemse/bji058. [DOI] [PubMed] [Google Scholar]
- Romanes G.J. Experiments on the sense of smell in dogs. Nature. 1887;36:273–274. [Google Scholar]
- Russell M.J. Human olfactory communication. Nature. 1976;260:520–522. doi: 10.1038/260520a0. [DOI] [PubMed] [Google Scholar]
- Sastry S.D, Buck K.T, Janák J, Dressler M, Preti G. Volatiles emitted by humans. In: Waller G.R, Dermer O.C, editors. Biochemical applications of mass spectrometry: first supplementary volume. Wiley; Chichester, UK: 1980. pp. 1085–1129. [Google Scholar]
- Schleidt M. Personal odor and nonverbal communication. Ethol. Sociobiol. 1980;1:225–231. doi: 10.1016/0162-3095(80)90009-6. [DOI] [Google Scholar]
- Schreck C.E, Kline D.L, Carlson D.A. Mosquito attraction to substances from the skin of different humans. J. Am. Mosq. Control Assoc. 1990;6:406–410. [PubMed] [Google Scholar]
- Singh D, Bronstad P.M. Female body odour is a potential cue to ovulation. Proc. R. Soc. B. 2001;268:797–801. doi: 10.1098/rspb.2001.1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soini H.A, Bruce K.E, Wiesler D, David F, Sandra P, Novotny M.V. Stir bar sorptive extraction: a new quantitative and comprehensive sampling technique for determination of chemical signal profiles from biological media. J. Chem. Ecol. 2005;31:377–392. doi: 10.1007/s10886-005-1347-8. [DOI] [PubMed] [Google Scholar]
- Soini H.A, Bruce K.E, Klouckova I, Brereton R.G, Penn D.J, Novotny M.V. In-situ surface sampling of biological objects and preconcentration of their volatiles for chromatographic analysis. Anal. Chem. 2006;78:7161–7168. doi: 10.1021/ac0606204. [DOI] [PubMed] [Google Scholar]
- Sommerville B.A, McClintock J.P, Broom D.M. Analysis of human sweat volatiles: An example of pattern recognition in the analysis and interpretation of gas chromatograms. Pest. Sci. 1994;41:365–368. doi: 10.1002/ps.2780410413. [DOI] [Google Scholar]
- Stoddart D.M. Cambridge University Press; Cambridge, UK: 1990. The scented ape: the biology and culture of human odor. [Google Scholar]
- Taylor D, Daulby A, Grimshaw S, James G, Mercer J.G, Vaziri S. Characterization of the microflora of the huma axilla. Int. J. Cosmet. Sci. 2003;25:137–145. doi: 10.1046/j.1467-2494.2003.00181.x. [DOI] [PubMed] [Google Scholar]
- Toivanen P, Vaahtovuo J, Eerola E. Influence of major histocompatibility complex on bacterial composition of fecal flora. Infect. Immun. 2001;69:2372–2377. doi: 10.1128/IAI.69.4.2372-2377.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai C.A, Hsueh H.M, Chen J.J. Estimation of false discovery rates in multiple testing: application to gene microarray data. Biometrics. 2003;59:1071–1081. doi: 10.1111/j.0006-341X.2003.00123.x. [DOI] [PubMed] [Google Scholar]
- Turner A.P, Magan N. Electronic noses and disease diagnostics. Nat. Rev. Microbiol. 2004;2:161–166. doi: 10.1038/nrmicro823. [DOI] [PubMed] [Google Scholar]
- Wallace P. Individual discrimination of humans by odors. Physiol. Behav. 1977;19:577–579. doi: 10.1016/0031-9384(77)90238-4. [DOI] [PubMed] [Google Scholar]
- Wedekind C, Seebeck T, Bettens F, Paebke A.J. MHC-dependent mate-preferences in humans. Proc. R. Soc. B. 1995;260:245–249. doi: 10.1098/rspb.1995.0087. [DOI] [PubMed] [Google Scholar]
- Weisfeld G.E, Czilli T, Phillips K.A, Gall J.A, Lichtman C.M. Possible olfaction-based mechanisms in human kin recognition and inbreeding avoidance. J. Exp. Child Psychol. 2003;85:279–295. doi: 10.1016/S0022-0965(03)00061-4. [DOI] [PubMed] [Google Scholar]
- Willis C.M, Church S.M, Guest C.M, Cook W.A, McCarthy N, Bransbury A.J, Church M.R, Church J.C. Olfactory detection of human bladder cancer by dogs: proof of principle study. Br. Med. J. 2004;329:712. doi: 10.1136/bmj.329.7468.712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson E.O. Chemical communication within animal species. In: Sondheimer E, Simeone J.B, editors. Chemical ecology. Academic Press; New York, NY: 1970. pp. 133–155. [Google Scholar]
- Yamazaki K, Beauchamp G.K. Chemosensory recognition of olfactory individuality. Chem. Senses. 2005;30:I142–I143. doi: 10.1093/chemse/bjh154. [DOI] [PubMed] [Google Scholar]
- Zala S.M, Penn D.J. Abnormal behaviours induced by chemical pollution: a review of the evidence and new challenges. Anim. Behav. 2004;68:649–664. doi: 10.1016/j.anbehav.2004.01.005. [DOI] [Google Scholar]
- Zeng X.N, Leyden J.J, Lawley H.J, Sawano K, Nohara I, Preti G. Analysis of characteristic odors from human male axillae. J. Chem. Ecol. 1991;17:1469–1492. doi: 10.1007/BF00983777. [DOI] [PubMed] [Google Scholar]
- Zeng X.N, Leyden J.J, Speilman A.I, Preti G. Analysis of characteristic human female axillary odors: qualitative comparison to males. J. Chem. Ecol. 1996;22:237–257. doi: 10.1007/BF02055096. [DOI] [PubMed] [Google Scholar]
- Zhang Z.M, Cai J.J, Ruan G.H, Li G.K. The study of fingerprint characteristics of the emanations from human arm skin using the original sampling system by SPME-GC/MS. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2005;822:244–252. doi: 10.1016/j.jchromb.2005.06.026. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table 1 showing distribution of marker compounds characteristic of individuals, Table 2 showing 118 additional compounds identified from axillary samples, and GC-MS chromatographic profiles of skin volatile compounds