Abstract
Aggregation of the microtubule-associated protein tau contributes to the formation of neurofibrillary lesions in Alzheimer’s disease and is a useful marker of disease progression. Although filter trap assays have been employed to assess the extent of tau aggregation in cells, tissues, and in vitro, their performance relative to other assay modalities has not been reported. To clarify this issue, the ability of the filter trap approach to quantify aggregation of purified recombinant full-length tau protein in vitro was examined as a function of membrane chemistry in 96-well format. Results showed that nitrocellulose yielded the greatest assay sensitivity relative to polyvinylidene fluoride or cellulose acetate at equal membrane porosity. However, all combinations of filter chemistries, porosities, and monoclonal detection antibodies yielded non-linear correlations between signal intensity and analyte concentration. When corrected for non-linearity, the filter trap assay determined a value for the critical monomer concentration for tau aggregation that was statistically identical to determinations made by electron microscopy assay. The data suggest conditions under which filter trap assays can be used to estimate tau aggregation kinetics.
Keywords: Tau, aggregation, Alzheimer’s disease, filter assay
Introduction
Abnormal aggregates of the microtubule-associated protein tau are found in several progressive neurodegenerative diseases, including Alzheimer’s disease (AD) 1 and frontotemporal dementia (1). In AD, clinical progression of symptoms correlates well with the temporal and spatial spread of tau aggregates in the brains of affected individuals (2-4). Thus tau aggregation is a useful marker of disease, and characterization of its mechanism of formation can yield information on underlying pathological processes. In addition, in vitro modeling of tau fibrillization can be used to clarify the kinetics of the process as well as identify compounds potentially capable of modulating lesion formation (5).
Tau aggregation has been quantified using various methods, including dye-based fluorescence spectroscopy (6, 7), laser light scattering (8, 9), high-speed centrifugation (10), and electron microscopy (11). Each approach has its own advantages and disadvantages. For example, transmission electron microscopy provides direct visualization of the aggregates, which establishes morphology, as well as the length distribution of filaments, which reflects aggregation mechanism. However, this method is low throughput and may be subject to measurement bias depending on conditions of experimentation (11).
Recently, filtration methods have been used to quantify the products of protein aggregation reactions (12, 13). In this approach, reaction products are filtered through a membrane that traps and retains large protein aggregates while small species including protein monomers pass through. When combined with solid-phase immunodetection, the approach can yield a highly sensitive estimation of protein aggregation. Early versions of the assay used cellulose acetate as the capture membrane (13), which proved capable of trapping tau aggregates in extracts of human and transgenic mouse brain tissue (14). Subsequent assay of tau aggregation in cultured cell extracts and also in vitro using purified protein preparations employed filters with greater protein binding affinity, including nitrocellulose (15) and PVDF (16). However, a full characterization of any filter-based assay for tau, including the effect of membrane composition and porosity, has not been reported. Moreover, the relative sensitivity and linearity of these assays have not been disclosed.
Here we characterize a vacuum-based 96-well format filter assay for assessment of tau fibril formation in vitro. Results indicate that while assay sensitivity is a function of both filter composition and porosity, analyte concentration dependence is non-linear under all conditions tested. However, control for non-linearity through use of calibration standards yields a quantitative assay capable of estimating aggregation parameters with a precision equal to microscopy-based methods.
Materials and Methods
Materials
Recombinant htau40 was prepared as described previously (17). Primary mouse monoclonal antibodies Tau1 (18) and Tau5 (19) were the gifts of L. I. Binder (Northwestern University), whereas Alz50 (20) was the gift of P. Davies (Albert Einstein College of Medicine). HRP-linked goat anti-mouse IgG and goat anti-mouse IgM were from Kirkegaard and Perry (Gaithersburg, MD). Filter membranes used included 0.45 μm cellulose acetate from Sterlitech Corporation (Kent, WA), 0.2 μm and 0.45 μm nitrocellulose from Bio-Rad Laboratories (Hercules, CA), and 0.45 μm PVDF from Millipore (Billerica, MA). Formvar/carbon-coated copper grids (300 mesh), glutaraldehyde, and uranyl acetate were obtained from Electron Microscopy Sciences (Ft. Washington, PA). Thiazine red was from ICN Biomedicals (Aurora, OH). Octadecyl sulfate detergent (Lancaster Synthesis, Pelham, NH) was dissolved in 1:1 isopropanol/H2O before use.
Tau fibrillization
Tau protein was incubated (50 μl final volume) at 37°C for up to 24 h in assembly buffer (10 mM HEPES, pH 7.4, 100 mM NaCl, and 5 mM dithiothreitol) in the presence and absence of either Thiazine red (100 μM) or ODS (50 μM) fibrillization inducers. Reactions were immediately subjected to either filter or electron microscopy assays described below.
Filter assay
Tau fibrillization reaction products were diluted up to 10-fold in 2% SDS to prepare a series of descending tau filament concentrations. All samples underwent a further 1:3 dilution in 2% SDS before vacuum filtration through a 96-well dot blot apparatus (Bio-Rad Laboratories, Hercules, CA) containing nitrocellulose, PVDF, or cellulose acetate membranes. The resultant membranes were washed twice with 2% SDS, blocked in 4% nonfat dry milk dissolved in blocking buffer (100 mM Tris-HCl, pH 7.4, 150 mM NaCl) for 2 h, and then incubated with primary antibody at 1:1000 dilution for 1.5 h. Membranes were washed twice in blocking buffer, then incubated with HRP-linked secondary antibody for 1.5 h. The membranes were washed again twice in blocking buffer, then developed with the ECL Western Blotting Analysis System (GE Healthcare, Buckinghamshire, UK). Chemiluminescence was recorded on an Omega 12iC Molecular Imaging System and quantified using UltraQuant software (UltraLum, Claremont, CA).
Transmission electron microscopy
Tau fibrillization reactions were terminated with the addition of 2% glutaraldehyde and then adsorbed onto 300-mesh Formvar/carbon-coated copper grids for 1 min. The grids were rinsed with water, negatively stained for 1 min with 2% uranyl acetate, and washed again with water. Images were captured on a Tecnai G2 Spirit BioTWIN transmission electron microscope (FEI, Hillsboro, OR) operated at 80kV and 23,000× magnification, then analyzed with ImageJ software (National Institutes of Health). Average total filament length was determined as described previously (11).
Analytical methods
Regression analysis was performed with Sigmaplot software (Systat Software Inc., San Jose, CA). Analyte concentration dependence of the filter assay was fit to the power function
| (1) |
where y is the signal intensity produced in the presence of aggregation inducer at tau concentration x, y0 is the background signal produced in the absence of tau aggregation inducer at tau concentration x,, and a and b are constants.
The critical concentration for fibrillization was estimated from the abscissa intercept after least-squares linear regression and is reported ± SEE (9).
Z’-factor for evaluating assay performance was calculated as described previously (21).
Results
To generate a population of tau filaments for testing in filter assays, 1 μM full-length, four-repeat tau protein (htau40; (22)) was incubated (16 h at 37°C) in the presence and absence of Thiazine red inducer. Stable, plateau levels of fibrillization are induced under these conditions (23). In contrast, tau protein incubated in assembly buffer without inducer does not produce detectable aggregates (24). In a preliminary test of the filter trap assay, tau samples prepared as described above were diluted in 2% SDS to form a descending concentration series, then equal volumes of each dilution were vacuum-filtered though a 0.2 μm nitrocellulose membrane in 96-well format. SDS was used as diluent because authentic tau filaments are relatively stable in detergents (25), including SDS (14). Trapped tau protein was then labeled with Tau1 monoclonal antibody in conjunction with an HRP-linked secondary antibody and chemiluminescent substrate. Chemiluminescence was captured using an Omega 12iC Molecular Imaging System. Tau1 was used as the labeling antibody because it binds to a well-characterized linear epitope in non-phosphorylated tau protein with high affinity (17, 26). Results showed that the nitrocellulose filter detected much stronger chemiluminescent signals from the tau sample treated with Thiazine red compared to the non-treated control reaction (Fig. 1), indicating that retention of unaggregated monomer on the membrane was minor relative to trapping of filaments under these conditions. The mean signal to background ratio (i.e, signal from sample with Thizine red inducer compared to sample without inducer) was 29.7 ± 6.9 (n = 3 replicates) for neat sample, and decreased in parallel with the amount of tau aggregate subjected to filtration (Fig. 1). The Z’-factor for assays spanning the 5-fold relative concentration range 0.2 – 1 (Fig. 1) was 0.66 ± 0.15 (n = 9 concentrations). These data indicate that the tau filter trap assay is adequate for high-throughput screening applications under these experimental conditions (21).
Fig. 1. Results from the filter trap assay.
Purified recombinant htau40 (1 μM) was incubated (16 h at 37°C) in the presence and absence of 100 μM Thiazine red inducer, then diluted in 2% SDS to create a descending concentration series of reaction products. These dilutions were vacuum-filtered through a 0.2 μm porosity nitrocellulose membrane, and then stained with Tau1 primary and HRP-conjugated secondary antibodies. Tau immunostaining (chemiluminescence) was then visualized on an Omega 12iC Molecular Imaging System. Under these conditions, substantially more tau is trapped in the presence of aggregation inducer than in its absence.
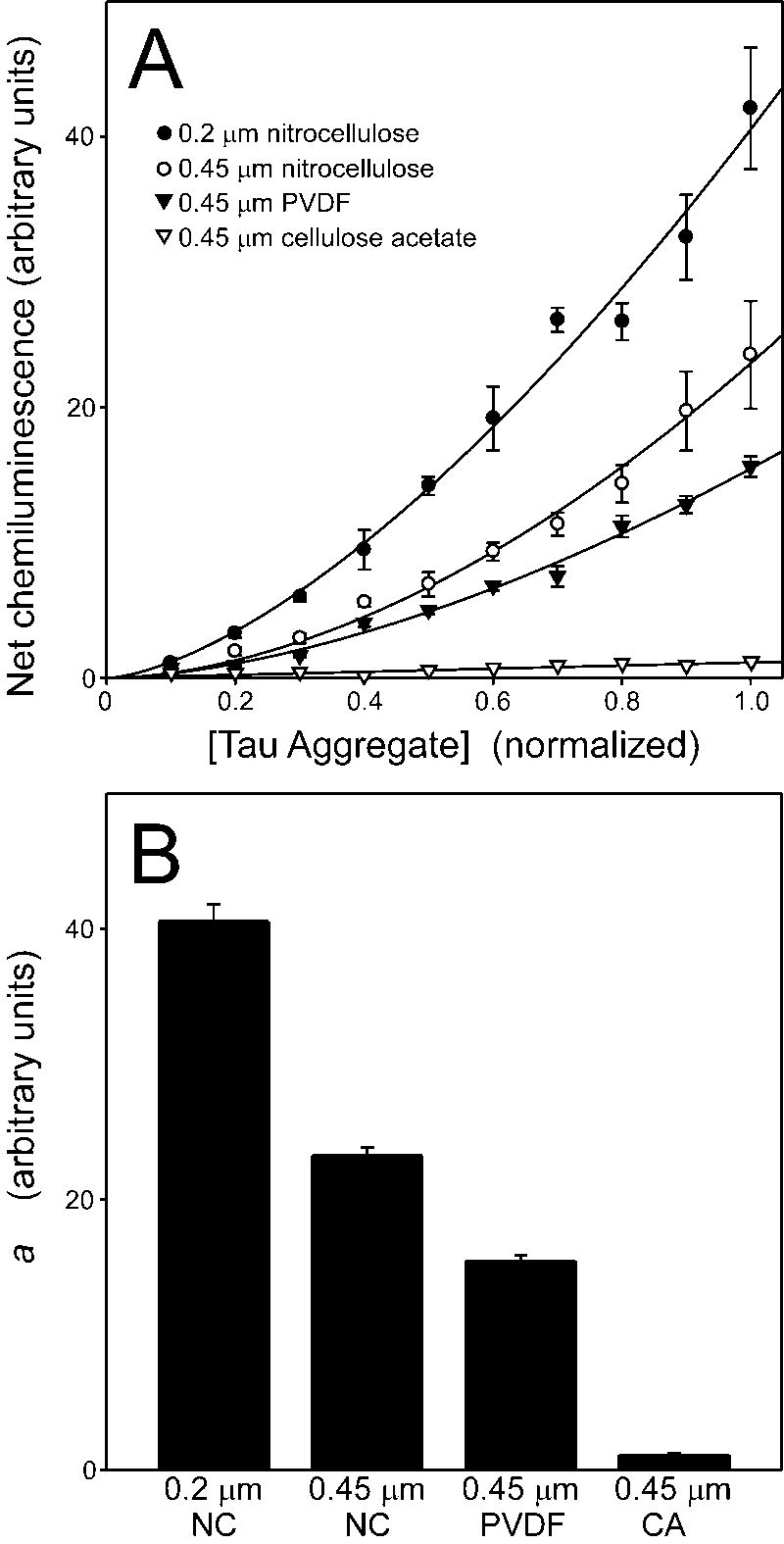
To assess assay linearity, net chemiluminescence (i.e., the difference between Thiazine red-induced and non-induced tau samples) was determined by densitometry and then plotted as a function of relative tau concentration. The resultant curves were nonlinear (Fig. 2A), but could be fit to a simple power function (eq. 1). Nonlinearity did not result from choice of inducer, because similar data was obtained for tau filaments prepared in the presence of anionic surfactant octadecyl sulfate (data not shown). These data suggest that tau aggregates can be selectively trapped and detected by nitrocellulose filters, but that chemiluminescent signal is not linearly related to tau filament concentration under these experimental conditions.
Fig. 2. Influence of membrane composition and porosity on detection of tau aggregates.
The dilution series described in Fig. 1 were vacuum-filtered through 0.2 μm nitrocellulose, 0.45 μm nitrocellulose (NC), 0.45 μm PVDF, or 0.45 μm cellulose acetate (CA) membranes. Membrane-bound tau was then detected with Tau1 monoclonal antibody followed by densitometric quantification of chemiluminescence. A, Net chemiluminescence, corresponding to the difference between signals generated in the presence and absence of Thiazine red inducer, were plotted versus relative tau filament concentration (where undiluted analyte has a concentration of 1). Each data point represents the mean ± SD of triplicate densitometric measurements whereas the solid curves represent best fits of the data points to a power function (eq. 1). All membranes yielded non-linear concentration dependence curves. B, parameter a estimated from fits to eq. 1 were replotted ± SEE as a measure of assay sensitivity. Assay sensitivity depended on membrane composition and porosity.
Membrane chemistry
Published filter trap assays have employed cellulose acetate (13, 14), nitrocellulose (15), and PVDF (16) membranes. To compare the performance of these membranes at constant porosity, each was subjected in parallel to the assay described above using Tau1 monoclonal antibody in conjunction with an HRP-linked secondary antibody and chemiluminescent substrate. Net chemiluminescence was then plotted as a function of relative tau concentration (Fig. 2A). Because dependence of chemiluminescence on analyte concentration was non-linear under all conditions, data were fit to eq. 1, with parameter a taken as a measure of relative sensitivity (Fig. 2B). Results showed that 0.45 μm cellulose acetate had by far the weakest ability to trap and retain tau aggregates. In contrast, 0.45 μm PVDF improved retention by more than an order of magnitude (Fig. 2B). Filters of the same porosity but composed of nitrocellulose improved sensitivity by a further 1.5 ± 0.1 fold. Finally, narrowing nitrocellulose porosity to 0.2 μm increased sensitivity 1.7 ± 0.1 fold more. These data suggest that filaments prepared in vitro from purified full-length tau preparations are best captured and retained by membranes with high protein binding activity such as nitrocellulose and PVDF. In addition, while smaller pore sizes are more efficient at trapping tau aggregates in the context of nitrocellulose, they do not improve assay linearity.
Primary antibodies
Many monoclonal antibodies are available for detection of tau protein, including those that bind epitopes dependent on conformation. To assess relative performance of the filter trap assay, two additional antibodies, Tau5 and Alz50, were tested as detection reagents. Tau5 is an IgG that binds a linear tau epitope independent of phosphorylation state (17, 27), whereas Alz50 is an IgM with anti-tau binding affinity selective for filamentous tau (17). A comparison of the performance of Tau1, Tau5, and Alz50 is shown in Fig. 3. All three antibodies displayed parabolic concentration dependence curves, with only minor differences in sensitivity. These data suggest that selection of primary antibody has only minor effects on the performance of the filter trap assay.
Fig. 3. Comparison of anti-tau monoclonal antibodies for detection of tau aggregates.
The dilution series described in Fig. 1 was vacuum-filtered through a 0.2 μm nitrocellulose membrane. Net chemiluminescence was quantified using Tau1 (A), Tau5 (B), or Alz50 (C) primary antibodies and appropriate HRP-conjugated secondary antibodies (goat anti-mouse IgG for Tau1 and Tau5, goat anti-mouse IgM for Alz50) and plotted versus relative tau filament concentration (undiluted analyte = 1). Each data point represents the mean ± SD of triplicate measurements, whereas the solid curves represent best fits of the data points to a power function (eq. 1). All primary antibodies yielded non-linear concentration curves.
Comparison with electron microscopy
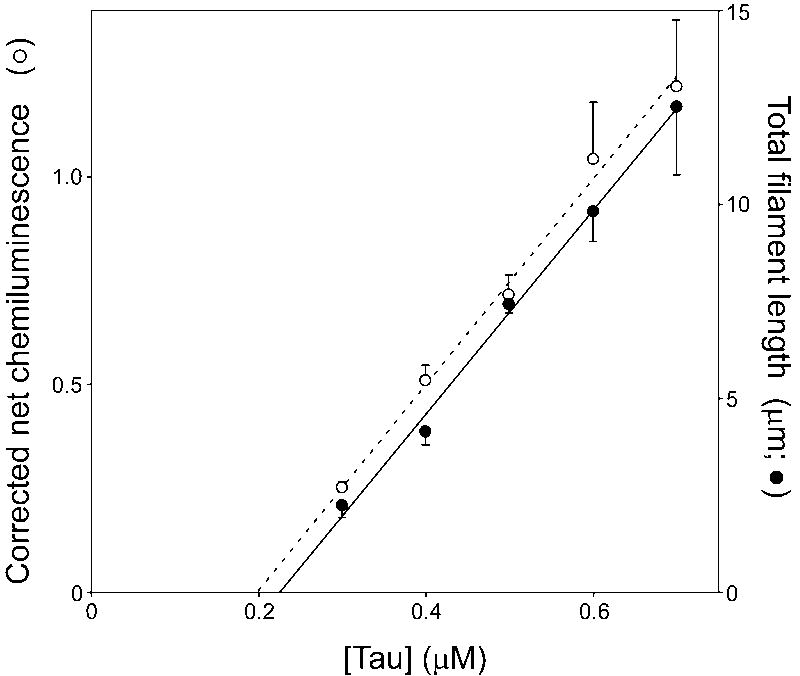
When corrected for non-linearity, the filter trap assay could potentially provide sensitive and high-throughput measurement of tau aggregation parameters. To test this approach, tau critical concentration was estimated by both electron microscopy and corrected filter trap assays. Critical concentration is the highest concentration of tau protein that does not support fibrillization, and therefore corresponds to the abscissa intercept when aggregate mass or total length is plotted as a function of bulk protein concentration (28). To determine tau critical concentration, varying concentrations of tau protein (0.3 – 0.7 μM) were incubated in the presence and absence of Thiazine red inducer, with one aliquot used to determine total filament length by electron microscopy while an identical aliquot was subjected to the filter trap assay (with 0.2 μm nitrocellulose as the filter and Tau1 as the primary detection antibody). For the latter measurements, net chemiluminescence was corrected for non-linearity using eq. 1 fit to a descending concentration series similar to that shown in Figs. 1 and 2. Under these conditions, total filament length as determined by electron microscopy was linearly related to bulk tau concentration (linear regression r2 = 0.995), with an abscissa intercept of 0.23 ± 0.02 μM (Fig. 4). When corrected using eq. 1, net chemiluminescence also was linearly related to bulk tau concentration (linear regression r2 = 0.994), with an abscissa intercept of 0.20 ± 0.02 μM (Fig. 4). The critical concentration values determined by electron microscopy and corrected filter trap assay were in good agreement with previous determinations (23), and were not statistically different from each other (p = 0.37). This result suggests that the precision of the filter assay is comparable to the electron microscopy assay for tau aggregation, and that it may be used to quantify of aggregation kinetics provided a standard curve is used to correct for non-linearity.
Fig. 4. Use of calibrated filter assay to estimate critical concentration.
Varying concentrations of purified recombinant htau40 (0.3 – 0.7 μM) were incubated 16 h at 37°C in the presence and absence of 100 μM Thiazine red inducer. One aliquot of each reaction product was subjected to the filter trap assay (○), while an equal aliquot was subjected to the transmission electron microscopy assay (●). Net chemiluminescence measured in the filter assay was corrected for nonlinearity using a dilution series, whereas total filament length was by electron microscopy measurements. Both were then plotted as a function of bulk tau concentration. Each data point represents the mean ± SD of triplicate measurements whereas the solid lines represent best fits to a linear regression. Critical concentrations, which were determined from the intercepts of these regressions with the abscissa (dotted line), were not statistically different under these conditions (p = 0.37).
Discussion
Filter trap assays can play an important role in the characterization of tau aggregation reactions owing to their sensitivity and high-throughput capability. Results presented here identified areas of concern in employing this method. First, the relationship between amounts of analyte trapped from in vitro aggregation reactions did not correlate linearly with amounts applied to the filter. Nonlinearity was independent of the nature of tau aggregation inducer, membrane composition, membrane porosity, or detection antibody isotype or conformational binding selectivity, suggesting it was intrinsic to the assay methodology. Nonlinear immunoassays can result from concentration-dependent aggregation of analyte as found, for example, with prion protein PrPC (29). However, the tau aggregates employed in the current study were harvested at reaction plateau and therefore were no longer rapidly changing aggregation state (23). In addition, the dissociation rate of tau filaments is slow relative to sample preparation and assay time (30), suggesting that changes in tau aggregation state do not contribute to non-linearity. Alternatively, non-linearity may stem from the use of immunological detection methods. Both the IgGs and the IgM used for detection of bound tau aggregates are multivalent, and therefore subject to avidity effects. Indeed, immobilization of antigen on a solid support is known to enhance avidity effects (31). Finally, it is conceivable that trapped tau aggregates help recruit and retain additional aggregates on the filter resulting in apparent binding cooperativity.
Regardless of its source, non-linear concentration-dependence of signal intensity was well modeled as a power function. Therefore, it was possible to correct raw signal intensity by including a dilution range of tau fibrils and interpolating the resultant standard curve. When so corrected, the relationship between analyte concentration and filter trapping became linear, and the filter trap assay was capable of determining a value for critical concentration in the presence of Thiazine red inducer that was statistically indistinguishable from the value estimated by transmission electron microscopy. In terms of precision, the ability of the corrected filter assay to quantify the extent of tau fibrillization at reaction plateau was comparable to that of microscopy methods.
Although different membrane chemistries can be used for trapping tau aggregates, the most sensitive detection was found with nitrocellulose followed closely by PVDF when porosity effects were controlled. Cellulose acetate performed poorly with tau filaments prepared from purified recombinant tau, although it efficiently captures tau aggregates from human and animal brain extracts (14). The difference may be attributable to porosity (0.2 μm vs. the 0.45 μm used here) and also the size and complexity of the aggregates. For example, aggregates trapped from brain samples contain β-amyloid as well as tau protein, suggesting that trapped aggregates represent a heterogeneous mixture of protein species. In contrast, tau filaments formed in vitro are free of other proteins and as a result may be retained less efficiently by cellulose acetate.
Of the antibodies tested in this assay, Tau1 was the most sensitive at lower concentrations of aggregates; however all three of the antibodies tested showed similar signal curves with minimal backgrounds. Thus, labeling antibody may be chosen to fit the needs of a particular application (e.g., phosphorylation-or conformation-sensitive antibodies).
When corrected for nonlinearity, and using Tau1 as detection antibody, the performance of the filter trap assay was comparable to electron microscopy (11) and laser light scattering methods (9). However, filtration has special utility for high-throughput quantitation of tau aggregation. Potential applications include determination of the potency and efficacy of aggregation inhibitors (16), the structure-activity relationship of aggregation inducers (32), or as shown here, critical concentration. The commonality in these measurements is constant incubation time. In principle the filter assay could also be used for time-dependent applications (such as estimation of lag time) provided that reactions are stopped at the appropriate time points before they are filtered through the membrane.
Regardless of the application, trapping of aggregates produced in vitro is limited by the porosity of the membrane. As a result, the assay will underestimate the extent of aggregation depending on the length distribution of reaction products. For example, retention of small soluble oligomers is expected to be poor. These species are most abundant at very early time points during filament formation (33), but can appear at any time owing to off-pathway reactions (34). Were the latter large enough to be retained on filters, then it may be possible to selectively detect them using conformation selective antibodies (35). Alternatively, the fibrillized state could be selectively detected in the presence of off-pathway aggregates with fibril-selective antibodies such as Alz50 (17). The difficulty of detecting small aggregates is common to electron microscopy, light scattering, and centrifugation methods as well (28).
In summary, the filter trap assay described above can yield rapid and quantitative assessment of tau aggregation state. The assay is suitable for a number of membranes and antibodies and so can be modified to fit the particular needs of the experimenter. Quantification can be improved by calibrating against dilution standards, which facilitates estimation of aggregation parameters such as critical concentration.
Acknowledgments
We thank Lauren Crissman for assistance with tau expression and purification. This work was supported by grants from the National Institutes of Health (AG14452) and from the Alzheimer’s Association.
Footnotes
Abbreviations used : AD, Alzheimer’s disease; ECL, enhanced chemiluminescence; HRP, horse radish peroxidase; PVDF, polyvinylidene fluoride; SEE, standard error of the estimate
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Buee L, Bussiere T, Buee-Scherrer V, Delacourte A, Hof PR. Tau protein isoforms, phosphorylation and role in neurodegenerative disorders. Brain Res Brain Res Rev. 2000;33:95–130. doi: 10.1016/s0165-0173(00)00019-9. [DOI] [PubMed] [Google Scholar]
- 2.Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol (Berl) 1991;82:239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- 3.Ghoshal N, Garcia-Sierra F, Wuu J, Leurgans S, Bennett DA, Berry RW, Binder LI. Tau conformational changes correspond to impairments of episodic memory in mild cognitive impairment and Alzheimer’s disease. Exp Neurol. 2002;177:475–493. doi: 10.1006/exnr.2002.8014. [DOI] [PubMed] [Google Scholar]
- 4.Royall DR, Palmer R, Mulroy AR, Polk MJ, Roman GC, David JP, Delacourte A. Pathological determinants of the transition to clinical dementia in Alzheimer’s disease. Exp Aging Res. 2002;28:143–162. doi: 10.1080/03610730252800166. [DOI] [PubMed] [Google Scholar]
- 5.Chirita C, Necula M, Kuret J. Ligand-dependent inhibition and reversal of tau filament formation. Biochemistry. 2004;43:2879–2887. doi: 10.1021/bi036094h. [DOI] [PubMed] [Google Scholar]
- 6.LeVine H., 3rd Thioflavine T interaction with synthetic Alzheimer’s disease beta-amyloid peptides: detection of amyloid aggregation in solution. Protein Sci. 1993;2:404–410. doi: 10.1002/pro.5560020312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Friedhoff P, Schneider A, Mandelkow EM, Mandelkow E. Rapid assembly of Alzheimer-like paired helical filaments from microtubule-associated protein tau monitored by fluorescence in solution. Biochemistry. 1998;37:10223–10230. doi: 10.1021/bi980537d. [DOI] [PubMed] [Google Scholar]
- 8.Gamblin TC, King ME, Dawson H, Vitek MP, Kuret J, Berry RW, Binder LI. In vitro polymerization of tau protein monitored by laser light scattering: method and application to the study of FTDP-17 mutants. Biochemistry. 2000;39:6136–6144. doi: 10.1021/bi000201f. [DOI] [PubMed] [Google Scholar]
- 9.Necula M, Kuret J. A static laser light scattering assay for surfactant-induced tau fibrillization. Anal Biochem. 2004;333:205–215. doi: 10.1016/j.ab.2004.05.044. [DOI] [PubMed] [Google Scholar]
- 10.Bandyopadhyay B, Li G, Yin H, Kuret J. Tau aggregation and toxicity in a cell culture model of tauopathy. J Biol Chem. 2007;282:16454–16464. doi: 10.1074/jbc.M700192200. [DOI] [PubMed] [Google Scholar]
- 11.Necula M, Kuret J. Electron microscopy as a quantitative method for investigating tau fibrillization. Anal Biochem. 2004;329:238–246. doi: 10.1016/j.ab.2004.02.023. [DOI] [PubMed] [Google Scholar]
- 12.Scherzinger E, Lurz R, Turmaine M, Mangiarini L, Hollenbach B, Hasenbank R, Bates GP, Davies SW, Lehrach H, Wanker EE. Huntingtin-encoded polyglutamine expansions form amyloid-like protein aggregates in vitro and in vivo. Cell. 1997;90:549–558. doi: 10.1016/s0092-8674(00)80514-0. [DOI] [PubMed] [Google Scholar]
- 13.Wanker EE, Scherzinger E, Heiser V, Sittler A, Eickhoff H, Lehrach H. Membrane filter assay for detection of amyloid-like polyglutamine-containing protein aggregates. Meth Enzymol. 1999;309:375–386. doi: 10.1016/s0076-6879(99)09026-6. [DOI] [PubMed] [Google Scholar]
- 14.Xu G, Gonzales V, Borchelt DR. Rapid detection of protein aggregates in the brains of Alzheimer patients and transgenic mouse models of amyloidosis. Alzheimer Dis Assoc Disord. 2002;16:191–195. doi: 10.1097/00002093-200207000-00010. [DOI] [PubMed] [Google Scholar]
- 15.Dou F, Netzer WJ, Tanemura K, Li F, Hartl FU, Takashima A, Gouras GK, Greengard P, Xu H. Chaperones increase association of tau protein with microtubules. Proc Natl Acad Sci U S A. 2003;100:721–726. doi: 10.1073/pnas.242720499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pickhardt M, von Bergen M, Gazova Z, Hascher A, Biernat J, Mandelkow EM, Mandelkow E. Screening for inhibitors of tau polymerization. Curr Alzheimer Res. 2005;2:219–226. doi: 10.2174/1567205053585891. [DOI] [PubMed] [Google Scholar]
- 17.Carmel G, Mager EM, Binder LI, Kuret J. The structural basis of monoclonal antibody Alz50’s selectivity for Alzheimer’s disease pathology. J Biol Chem. 1996;271:32789–32795. doi: 10.1074/jbc.271.51.32789. [DOI] [PubMed] [Google Scholar]
- 18.Binder LI, Frankfurter A, Rebhun LI. The distribution of tau in the mammalian central nervous system. J Cell Biol. 1985;101:1371–1378. doi: 10.1083/jcb.101.4.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.LoPresti P, Szuchet S, Papasozomenos SC, Zinkowski RP, Binder LI. Functional implications for the microtubule-associated protein tau: localization in oligodendrocytes. Proc Natl Acad Sci U S A. 1995;92:10369–10373. doi: 10.1073/pnas.92.22.10369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wolozin BL, Pruchnicki A, Dickson DW, Davies P. A neuronal antigen in the brains of Alzheimer patients. Science. 1986;232:648–650. doi: 10.1126/science.3083509. [DOI] [PubMed] [Google Scholar]
- 21.Zhang JH, Chung TD, Oldenburg KR. A Simple Statistical Parameter for Use in Evaluation and Validation of High Throughput Screening Assays. J Biomol Screen. 1999;4:67–73. doi: 10.1177/108705719900400206. [DOI] [PubMed] [Google Scholar]
- 22.Goedert M, Spillantini MG, Jakes R, Rutherford D, Crowther RA. Multiple isoforms of human microtubule-associated protein tau: sequences and localization in neurofibrillary tangles of Alzheimer’s disease. Neuron. 1989;3:519–526. doi: 10.1016/0896-6273(89)90210-9. [DOI] [PubMed] [Google Scholar]
- 23.Chirita CN, Congdon EE, Yin H, Kuret J. Triggers of full-length tau aggregation: a role for partially folded intermediates. Biochemistry. 2005;44:5862–5872. doi: 10.1021/bi0500123. [DOI] [PubMed] [Google Scholar]
- 24.King ME, Ahuja V, Binder LI, Kuret J. Ligand-dependent tau filament formation: implications for Alzheimer’s disease progression. Biochemistry. 1999;38:14851–14859. doi: 10.1021/bi9911839. [DOI] [PubMed] [Google Scholar]
- 25.Greenberg SG, Davies P. A preparation of Alzheimer paired helical filaments that displays distinct tau proteins by polyacrylamide gel electrophoresis. Proc Natl Acad Sci U S A. 1990;87:5827–5831. doi: 10.1073/pnas.87.15.5827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Szendrei GI, Lee VM, Otvos L., Jr Recognition of the minimal epitope of monoclonal antibody Tau-1 depends upon the presence of a phosphate group but not its location. J Neurosci Res. 1993;34:243–249. doi: 10.1002/jnr.490340212. [DOI] [PubMed] [Google Scholar]
- 27.Porzig R, Singer D, Hoffmann R. Epitope mapping of mAbs AT8 and Tau5 directed against hyperphosphorylated regions of the human tau protein. Biochem Biophys Res Commun. 2007;358:644–649. doi: 10.1016/j.bbrc.2007.04.187. [DOI] [PubMed] [Google Scholar]
- 28.Kuret J, Congdon EE, Li G, Yin H, Yu X, Zhong Q. Evaluating triggers and enhancers of tau fibrillization. Microsc Res Tech. 2005;67:141–155. doi: 10.1002/jemt.20187. [DOI] [PubMed] [Google Scholar]
- 29.Meyer RK, Lustig A, Oesch B, Fatzer R, Zurbriggen A, Vandevelde M. A monomer-dimer equilibrium of a cellular prion protein (PrPC) not observed with recombinant PrP. J Biol Chem. 2000;275:38081–38087. doi: 10.1074/jbc.M007114200. [DOI] [PubMed] [Google Scholar]
- 30.Necula M, Kuret J. Site-specific pseudophosphorylation modulates the rate of tau filament dissociation. FEBS Lett. 2005;579:1453–1457. doi: 10.1016/j.febslet.2005.01.047. [DOI] [PubMed] [Google Scholar]
- 31.Harlow E, Lane D. Using Antibodies: A Laboratory Manual. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, NY: 1999. [Google Scholar]
- 32.Chirita CN, Necula M, Kuret J. Anionic Micelles and Vesicles Induce tau Fibrillization in vitro. J Biol Chem. 2003;278:25644–25650. doi: 10.1074/jbc.M301663200. [DOI] [PubMed] [Google Scholar]
- 33.Hu JF, Matzavinos A, Othmer HG. A theoretical approach to actin filament dynamics. J Stat Phys. 2007;128:111–138. [Google Scholar]
- 34.Necula M, Kayed R, Milton S, Glabe CG. Small molecule inhibitors of aggregation indicate that amyloid beta oligomerization and fibrillization pathways are independent and distinct. J Biol Chem. 2007;282:10311–10324. doi: 10.1074/jbc.M608207200. [DOI] [PubMed] [Google Scholar]
- 35.Kayed R, Head E, Thompson JL, McIntire TM, Milton SC, Cotman CW, Glabe CG. Common structure of soluble amyloid oligomers implies common mechanism of pathogenesis. Science. 2003;300:486–489. doi: 10.1126/science.1079469. [DOI] [PubMed] [Google Scholar]