Abstract
Data on 35 291 individuals with cancer, aged 13–24 years, in England from 1979 to 2001 were analysed by region and socio-economic deprivation of census ward of residence, as measured by the Townsend deprivation index. The incidence of leukaemia, lymphoma, central nervous system tumours, soft tissue sarcomas, gonadal germ cell tumours, melanoma and carcinomas varied by region (P<0.01, all groups) but bone tumour incidence did not. Lymphomas, central nervous system tumours and gonadal germ cell tumours all had higher incidence in less deprived census wards (P<0.01), while chronic myeloid leukaemia and carcinoma of the cervix had higher incidence in more deprived wards (P<0.01). In the least deprived wards, melanoma incidence was nearly twice that in the most deprived, but this trend varied between regions (P<0.001). These cancer incidence patterns differ from those seen in both children and older adults and have implications for aetiology and prevention.
Keywords: teenage and young adult cancer, geographical incidence, socioeconomic deprivation, cancer registration
Cancer affects approximately one-third of the United Kingdom (UK) population before their 75th birthday and accounts for about 1 in 4 deaths (Coleman et al, 1999). Although predominantly a disease of old age, cancer in teenagers and young adults (TYAs) is an appreciable health problem and the commonest disease-related cause of death at ages 15–24 years in England (Office for National Statistics, 2002). Cancers in later adulthood are often associated with environmental or lifestyle risk factors and can be ascribed to the cumulative effect of exposures over a long time period. Chronic environmental exposures are thought to be less important in TYA cancers and genetic factors may play a greater role, although little is known about their relative importance (Birch, 2005).
Descriptive epidemiological studies indicate how disease incidence varies by demographic group and over geographic region and time. Such studies can provide clues to aetiology and disease prevention. Geographical variability in the incidence of childhood cancer has been studied extensively (COMARE, 2006), but cancer patterns in TYAs have been relatively neglected. National cancer registration in England provides reliable population-based incidence data over a long period, with substantial numbers even for rare cancers (Quinn et al, 2001). We have used national data to investigate variations in TYA cancer incidence by geographic region and economic deprivation for specific diagnostic groups.
METHODS
Incidence data on all registered neoplasms diagnosed in 13–24 year olds in England from 1979 to 2001 inclusive were supplied by the National Cancer Intelligence Centre, Office for National Statistics, London (ONS). Data items included date of birth, age and year of diagnosis, sex, and coded diagnosis. Cases diagnosed from 1979 to 1994 were allocated International Classification of Diseases (ICD), Ninth Revision, disease codes and International Classification of Diseases for Oncology (ICD-O), First Edition, morphology codes (World Health Organisation, 1975, 1976). Cases from 1995 to 2001 were allocated ICD Tenth Revision disease codes and ICD-O, Second Edition morphology codes (Percy et al, 1990; World Health Organisation, 1992). Cancers were grouped according to the diagnostic scheme described by Birch et al (2002). Non-melanoma skin cancers were excluded but benign intracranial and intraspinal neoplasms were included.
The Government Office Region (GOR) and Townsend deprivation index (TDI) (Townsend et al, 1988) for the census ward of residence of the case at the time of diagnosis were also supplied. Cases from 1979 to 1995 inclusive had 1991 census ward TDIs and the 1996–2001 had 2001 census ward TDIs. The four components of the TDI are the proportion of households in the census ward that: have an unemployed economically active head; have no access to a car or van; are overcrowded are not owner-occupied. These components and resident populations by census ward for the 1991 and 2001 censuses were obtained from the national censuses (Office of Population Censuses and Surveys, 1991; Office for National Statistics, 2001). Annual population estimates for England by sex and single year of age and by sex and five year age groups for each of the nine GORs were obtained from the Population Estimates Unit of ONS.
The underlying population and number of cases in each cancer group were tabulated by time period, age group, sex and GOR. The periods were 1979–84, 1985–89, 1990–95 and 1996–2001 and the age groups were 13–14, 15–19 and 20–24 years. These populations and case counts were used to calculate observed and expected incidence and rates for census wards and GORs. The incidence rates by GOR were standardised to the European standard population using the direct method (Quinn et al, 2005). The relationship with TDI was analysed by grouping census wards into quintiles so the expected incidence for all cancers across England was the same in each quintile. For each cancer group and quintile, the percentage observed over expected (percentage risk ratio) was calculated and the significance of the trend and heterogeneity in risk after taking into account the trend was assessed using Poisson regression (McCullagh and Nelder, 1989). Poisson regression was used to compare the observed and expected incidence by GOR. Additionally, the incidence rates by GOR and TDI quintile were examined for variation by GOR after taking into account the trend by TDI (P-value after TDI) and to see if the trend in TDI varied by GOR.
RESULTS
There were 35 291 cases occurring during 186 million person years at risk (mpyr), yielding an overall incidence rate of 188 cases per mpyr (Table 1). All rates given in the text are cases per mpyr. Incidence was slightly higher in males than females and greater in older individuals, but these patterns were not consistent across cancer groups. Across all cancers there was a statistically significant trend towards lower rates with increasing deprivation (Table 2a). After adjustment for TDI there was statistically significant variation in incidence by GOR, with the highest rate in the South East and lowest in the North East (Table 2b). However, incidence patterns by TDI and GOR varied by diagnostic group.
Table 1. Incidence of cancers (rate per million person years at risk) in England, 1979–2001, by age group, cancer group and sex.
|
Age group
|
Sex
|
All
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
13–14 years
|
15–19 years
|
20–24 years
|
Male
|
Female
|
||||||||
| N | Rate | N | Rate | N | Rate | N | Rate | N | Rate | N | Rate | |
| Leukaemia | 713 | 24.2 | 1739 | 22.8 | 1483 | 18.5 | 2375 | 25.2 | 1560 | 17.1 | 3935 | 21.2 |
| Lymphoma | 674 | 22.9 | 3183 | 41.7 | 4641 | 58.0 | 4794 | 50.5 | 3704 | 40.0 | 8498 | 45.3 |
| CNS neoplasms | 771 | 26.2 | 1842 | 24.1 | 2431 | 30.4 | 2671 | 28.2 | 2373 | 25.9 | 5044 | 27.1 |
| Bone tumours | 434 | 14.8 | 1114 | 14.6 | 644 | 8.0 | 1308 | 13.9 | 884 | 9.8 | 2192 | 11.9 |
| Soft tissue sarcoma | 191 | 6.5 | 703 | 9.2 | 810 | 10.1 | 930 | 9.8 | 774 | 8.4 | 1704 | 9.1 |
| Germ cell tumour | 155 | 5.3 | 1171 | 15.3 | 3370 | 42.1 | 4152 | 43.3 | 544 | 6.0 | 4696 | 24.8 |
| Melanoma | 82 | 2.8 | 703 | 9.2 | 1995 | 24.9 | 967 | 10.1 | 1813 | 19.4 | 2780 | 14.7 |
| Carcinoma | 264 | 9.0 | 1354 | 17.7 | 4166 | 52.0 | 1658 | 17.4 | 4126 | 44.0 | 5784 | 30.6 |
| Misc. NEC | 72 | 2.4 | 168 | 2.2 | 195 | 2.4 | 200 | 2.1 | 235 | 2.6 | 435 | 2.3 |
| Unclassified | 14 | 0.5 | 74 | 1.0 | 135 | 1.7 | 95 | 1.0 | 128 | 1.4 | 223 | 1.2 |
| Total | 3370 | 113.6 | 12051 | 157.7 | 19870 | 248.1 | 19150 | 201.5 | 16141 | 174.5 | 35291 | 188.7 |
Abbreviation: CNS, central nervous system.
Table 2. Incidence of all cancers, leukaemias and lymphomas in teenagers and young adults (13–24 years) in England, 1979–2001, by TDI and GOR.
|
Percentage risk ratio by TDI quintile
|
P-values
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total cases | 1 (least) | 2 | 3 | 4 | 5 (most) | Trend | Heterogeneity | |||||
| (a) Case distribution by Townsend deprivation index | ||||||||||||
| All cancers | 35291 | 102 | 102 | 101 | 98 | 96 | <0.0001 | 0.44 | ||||
| Leukaemia | 3935 | 93 | 101 | 96 | 103 | 108 | 0.007 | 0.43 | ||||
| ALL | 1970 | 93 | 105 | 91 | 104 | 107 | 0.08 | 0.10 | ||||
| AML | 1371 | 101 | 100 | 103 | 103 | 93 | 0.55 | 0.69 | ||||
| CML | 325 | 79 | 91 | 86 | 103 | 141 | 0.0005 | 0.45 | ||||
| Other leukaemia | 269 | 79 | 84 | 102 | 97 | 140 | 0.002 | 0.58 | ||||
| Lymphomas | 8498 | 106 | 101 | 103 | 95 | 95 | 0.0002 | 0.36 | ||||
| NHL | 2489 | 97 | 98 | 98 | 97 | 110 | 0.09 | 0.33 | ||||
| HL | 6009 | 109 | 103 | 106 | 94 | 89 | <0.0001 | 0.15 | ||||
|
Government Office Region
|
||||||||||||
| Cancer group | North East | North West | Yorkshire and Humber | East Midlands | West Midlands | Easta | London | South East | South West | All | P-value | P after TDI |
| (b) Rate per million person years at risk by Government Office Region | ||||||||||||
| All cancers | 173.1 | 185.0 | 185.4 | 181.2 | 181.1 | 177.7 | 187.1 | 208.1 | 208.0 | 188.7 | <0.0001 | <0.0001 |
| Leukaemia | 21.1 | 20.7 | 19.6 | 20.5 | 19.6 | 18.2 | 24.1 | 23.3 | 22.6 | 21.2 | 0.0002 | <0.0001 |
| ALL | 11.1 | 10.5 | 9.9 | 9.9 | 10.6 | 9.8 | 11.3 | 11.7 | 11.3 | 10.7 | 0.48 | 0.30 |
| AML | 7.4 | 7.5 | 6.7 | 7.3 | 6.7 | 5.6 | 8.2 | 8.6 | 7.4 | 7.4 | 0.02 | 0.003 |
| CML | 1.9 | 1.5 | 1.5 | 1.7 | 1.0 | 1.9 | 2.4 | 1.6 | 2.3 | 1.7 | 0.03 | 0.03 |
| Other leukaemia | 0.8 | 1.2 | 1.5 | 1.7 | 1.3 | 0.9 | 2.2 | 1.4 | 1.6 | 1.4 | 0.010 | 0.03 |
| Lymphoma | 39.9 | 42.2 | 42.5 | 40.7 | 44.1 | 46.6 | 47.6 | 51.0 | 48.3 | 45.3 | <0.0001 | <0.0001 |
| NHL | 12.2 | 12.1 | 12.3 | 11.1 | 12.9 | 12.0 | 15.6 | 14.7 | 14.8 | 13.3 | 0.0001 | 0.0001 |
| HL | 27.7 | 30.1 | 30.2 | 29.6 | 31.2 | 34.5 | 32.0 | 36.3 | 33.5 | 32.0 | <0.0001 | 0.005 |
Abbreviations: ALL, acute lymphoblastic leukaemia; AML, acute myeloid leukaemia; CML, chronic myeloid leukaemia; GOR, Government Office Region; HL, Hodgkin lymphoma; NHL, non-Hodgkin lymphoma; TDI, Townsend deprivation index.
East is the label for the East of England GOR.
Leukaemia and lymphoma
Leukaemia incidence overall was highest in the most deprived quintile, particularly for chronic myeloid leukaemia (CML), but there were no significant trends for acute lymphoblastic leukaemia (ALL) and myeloid (AML). Highest rates overall were seen in London and the South East, but rates for ALL did not vary significantly by GOR (Table 2b).
Lymphoma incidence showed a marked trend towards lower rates with increasing deprivation, entirely due to Hodgkin lymphoma (HL). However, both HL and non-Hodgkin lymphoma (NHL) incidence varied by GOR, with higher rates in regions in the South than in the Midlands and North (Table 2a and b).
CNS tumours
Overall incidence of CNS tumours varied by TD1 (Table 3a), with higher rates in less deprived census wards, but the difference was only significant for unspecified CNS tumours. Rates varied significantly by GOR for astrocytomas, other gliomas, other specified tumours and unspecified tumours (Table 3b). A greater proportion of registrations from the West Midlands had unspecified morphology, 26% compared with 5% in other regions, and also had the lowest incidence among specified tumour groups except medulloblastoma. However, the inter-region variability in incidence of CNS tumours was still significant when the West Midlands was omitted from the analyses (P=<0.0001).
Table 3. Incidence of CNS neoplasms in teenagers and young adults (13–24 years) in England, 1979–2001, by TDI and GOR.
|
Percentage risk ratio by TDI quintile
|
P-values
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total cases | 1 (least) | 2 | 3 | 4 | 5 (most) | Trend | Heterogeneity | |||||
| (a) Case distribution by Townsend deprivation index | ||||||||||||
| All CNS neoplasms | 5044 | 104 | 103 | 104 | 94 | 94 | 0.003 | 0.39 | ||||
| Astrocytoma | 1725 | 104 | 100 | 101 | 96 | 99 | 0.45 | 0.91 | ||||
| Other gliomas | 683 | 106 | 96 | 114 | 100 | 83 | 0.11 | 0.17 | ||||
| Ependymoma | 264 | 104 | 109 | 97 | 89 | 100 | 0.54 | 0.84 | ||||
| PNET | 331 | 95 | 119 | 106 | 80 | 99 | 0.43 | 0.18 | ||||
| Other specified | 1679 | 105 | 102 | 102 | 97 | 94 | 0.12 | 0.97 | ||||
| Unspecified | 362 | 113 | 112 | 112 | 78 | 83 | 0.01 | 0.41 | ||||
| Government Office Region | ||||||||||||
| Cancer group | North East | North West | Yorkshire and Humber | East Midland | West Midlands | East | London | South East | South West | All | P-value | P after TDI |
| (b) Rate per million person years at risk by Government Office Region | ||||||||||||
| All CNS neoplasms | 26.0 | 27.5 | 28.6 | 28.4 | 23.2 | 23.4 | 25.2 | 30.2 | 30.6 | 27.1 | <0.0001 | <0.0001 |
| Astrocytoma | 8.8 | 10.0 | 10.7 | 9.2 | 7.4 | 7.6 | 8.4 | 10.3 | 10.8 | 9.3 | 0.0002 | 0.0001 |
| Other Gliomas | 4.0 | 3.3 | 3.5 | 3.3 | 2.0 | 4.1 | 3.8 | 4.7 | 4.0 | 3.7 | 0.0003 | 0.0004 |
| Ependymoma | 1.4 | 1.5 | 1.2 | 1.4 | 1.0 | 1.2 | 1.8 | 1.6 | 1.2 | 1.4 | 0.29 | 0.19 |
| PNET | 1.1 | 1.9 | 2.0 | 2.0 | 1.3 | 1.8 | 1.9 | 2.1 | 1.5 | 1.8 | 0.24 | 0.26 |
| Other specified | 10.1 | 9.6 | 10.2 | 11.8 | 5.6 | 7.5 | 8.3 | 9.3 | 9.5 | 9.0 | <0.0001 | <0.0001 |
| Unspecified | 0.7 | 1.1 | 0.9 | 0.8 | 6.0 | 1.2 | 0.9 | 2.2 | 3.5 | 1.9 | <0.0001 | <0.0001 |
Abbreviations: CNS, central nervous system; GOR, Government Office Region; TDI, Townsend deprivation index.
Bone and soft tissue sarcoma
Incidence of bone tumours was unrelated to TDI (Table 4a) and GOR (Table 4b), overall and for osteosarcoma, chondrosarcoma and Ewings tumours. The ‘other bone tumours’ group showed considerable variability, incidence being highest in the West Midlands, again, due to excess cases with unspecified morphology.
Table 4. Incidence of bone tumours and STS in teenagers and young adults (13–24 years) in England, 1979–2001, by TDI and GOR.
|
Percentage risk ratio by TDI quintile
|
P-values
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total cases | 1 (least) | 2 | 3 | 4 | 5 (most) | Trend | Heterogeneity | |||||
| (a) Case distribution by Townsend deprivation index | ||||||||||||
| Bone tumours | 2192 | 97 | 105 | 100 | 101 | 98 | 0.96 | 0.62 | ||||
| Osteosarcoma | 1105 | 93 | 101 | 101 | 102 | 105 | 0.21 | 0.93 | ||||
| Chondrosarcoma | 155 | 117 | 96 | 74 | 91 | 122 | 0.95 | 0.19 | ||||
| Ewings tumour | 744 | 96 | 112 | 103 | 102 | 86 | 0.30 | 0.24 | ||||
| Other bone tumours | 188 | 109 | 105 | 107 | 97 | 82 | 0.24 | 0.93 | ||||
| Soft tissue sarcomas | 1704 | 97 | 93 | 98 | 108 | 104 | 0.09 | 0.63 | ||||
| Fibromatous neoplasms | 232 | 84 | 97 | 106 | 114 | 99 | 0.29 | 0.73 | ||||
| Rhabdomyosarcoma | 427 | 106 | 90 | 95 | 98 | 111 | 0.67 | 0.53 | ||||
| Other specified STS | 704 | 98 | 93 | 92 | 109 | 107 | 0.21 | 0.61 | ||||
| STS, NOS | 341 | 91 | 96 | 107 | 111 | 95 | 0.51 | 0.66 | ||||
|
Government Office Region
|
||||||||||||
| Cancer group | North East | North West | Yorkshire and Humber | East Midlands | West Midlands | East | London | South East | South West | All | P-value | P after TDI |
| (b) Rate per million person years at risk by Government Office Region | ||||||||||||
| Bone tumours | 11.8 | 11.3 | 11.8 | 13.4 | 11.8 | 12.0 | 12.0 | 12.2 | 10.9 | 11.9 | 0.72 | 0.71 |
| Osteosarcoma | 5.9 | 6.4 | 6.3 | 6.4 | 4.9 | 6.1 | 6.3 | 6.0 | 5.4 | 6.0 | 0.49 | 0.46 |
| Chondrosarcoma | 1.2 | 0.6 | 0.8 | 1.2 | 1.0 | 0.6 | 0.7 | 0.7 | 0.9 | 0.8 | 0.42 | 0.41 |
| Ewings tumour | 4.1 | 3.8 | 4.1 | 4.9 | 2.9 | 4.6 | 4.3 | 4.3 | 3.5 | 4.0 | 0.08 | 0.08 |
| Other bone tumours | 0.6 | 0.4 | 0.5 | 0.8 | 3.1 | 0.7 | 0.7 | 1.2 | 1.0 | 1.0 | <0.0001 | <0.0001 |
| Soft tissue sarcomas | 7.6 | 7.3 | 9.5 | 8.9 | 8.6 | 8.8 | 10.7 | 10.0 | 9.5 | 9.1 | 0.001 | 0.0008 |
| Fibromatous neoplasms | 1.4 | 1.0 | 1.9 | 1.2 | 0.7 | 1.4 | 1.3 | 1.1 | 1.3 | 1.2 | 0.05 | 0.05 |
| Rhabdomyosarcoma | 2.0 | 2.0 | 2.0 | 2.5 | 2.1 | 2.0 | 2.1 | 3.0 | 2.8 | 2.3 | 0.18 | 0.09 |
| Other specified STS | 3.0 | 2.9 | 3.8 | 3.2 | 3.3 | 3.8 | 5.2 | 3.9 | 3.6 | 3.8 | 0.004 | 0.004 |
| STS, NOS | 1.2 | 1.3 | 1.8 | 2.0 | 2.5 | 1.6 | 2.0 | 2.0 | 1.7 | 1.8 | 0.09 | 0.10 |
Abbreviations: GOR, Government Office Region; STS, soft tissue sarcomas; TDI, Townsend deprivation index.
There was no evidence of variability in incidence of soft tissue sarcomas (STS) by TDI (Table 4a). Incidence varied significantly by GOR (Table 4b), with the highest rate in London and the lowest rate in the North West, due mainly to ‘other specified STS’. This group includes synovial sarcoma, liposarcoma, leiomyosarcoma and other rare types, but case numbers were too small for firm inference about which types were causing the effect.
Germ cell tumours and melanoma
There was a significant trend in the incidence of germ cell tumours (GCT) by TDI (Table 5a), with highest rates in the least deprived quintile. This was entirely due to testicular tumours, which formed 85% of GCTs. Incidence varied by GOR with highest rates in the South West.
Table 5. Incidence of germ cell tumours and melanoma in teenagers and young adults (13–24 years) in England, 1979–2001, by TDI and GOR.
|
Percentage risk ratio by TDI quintile
|
P-values
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total cases | 1 (least) | 2 | 3 | 4 | 5 (most) | Trend | Heterogeneity | |||||
| (a) Case distribution by Townsend deprivation index | ||||||||||||
| Germ cell tumours | 4696 | 105 | 105 | 100 | 96 | 93 | 0.001 | 0.91 | ||||
| Gonadal GCT | 4314 | 107 | 106 | 100 | 96 | 92 | 0.0002 | 0.94 | ||||
| Non-gonadal GCT | 382 | 91 | 99 | 105 | 93 | 112 | 0.30 | 0.75 | ||||
| Melanoma | 2780 | 123 | 116 | 106 | 91 | 68 | <0.0001 | 0.01 | ||||
|
Government Office Region
|
||||||||||||
| Cancer group | North East | North West | Yorkshire and Humber | East Midlands | West Midlands | East | London | South East | South West | All | P-value | P after TDI |
| (b) Rate per million person years at risk by Government Office Region | ||||||||||||
| Germ cell tumours | 21.5 | 24.7 | 24.1 | 25.4 | 23.6 | 25.1 | 22.4 | 27.0 | 29.3 | 24.8 | 0.0007 | 0.05 |
| Gonadal GCT | 19.1 | 22.3 | 21.9 | 23.7 | 21.5 | 23.0 | 20.1 | 25.2 | 27.5 | 22.8 | <0.0001 | 0.01 |
| Non-gonadal GCT | 2.4 | 2.3 | 2.2 | 1.7 | 2.1 | 2.0 | 2.3 | 1.8 | 1.8 | 2.0 | 0.74 | 0.76 |
| Melanoma | 13.3 | 15.0 | 15.6 | 12.2 | 12.7 | 13.9 | 11.6 | 17.8 | 19.5 | 14.7 | <0.0001 | <0.0001 |
Abbreviations: GCT, germ cell tumours; GOR, Government Office Region; TDI, Townsend deprivation index.
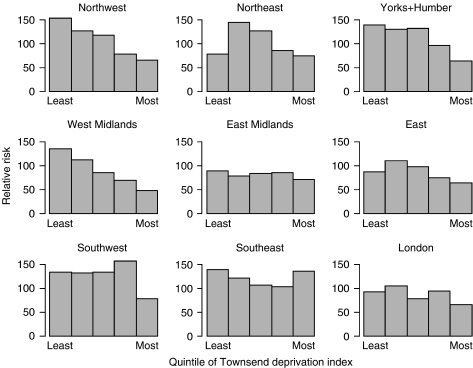
Melanoma incidence varied markedly by TDI (Table 5a). Highest rates were in the least deprived quintile and were almost double those in the most deprived. Rates varied significantly by GOR (Table 5b). Further examination of the data demonstrated that the relationship with TDI differed by GOR (Figure 1), with a uniformly high rate in the South West (P=0.85 for trend), uniformly low rates in the East Midlands (P=0.67) and East of England (P=0.31) and a striking trend in incidence with TDI in the North West, West Midlands and Yorkshire and the Humber (all P⩽0.0001). For these three regions, incidence rates in wards in the least deprived quintile were more than twice those in the most deprived and comparable with the overall rate in the South West.
Figure 1.
Percentage relative risk of melanoma in 13–24 year olds, 1979–2001, by Government Office Region and TDI using common quintile boundaries across England.
Carcinomas
Overall, there was no variation in incidence of carcinomas by TDI, but the incidence of carcinoma of cervix showed significant variability, with higher rates in the most deprived quintiles (Table 6a). There was a less marked trend in the opposite direction for breast cancer. Rates for lung, breast, cervix and colorectal carcinomas varied by GOR particularly for the latter two sites (Table 6b). Highest rates for lung cancer were seen in the South West, where the incidence was five times that in the East of England, although overall rates were low.
Table 6. Incidence of carcinomas in teenagers and young adults (13–24 years) in England, 1979–2001, by TDI and GOR.
|
Percentage risk ratio by TDI quintile
|
P-values
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total cases | 1 (least) | 2 | 3 | 4 | 5 (most) | Trend | Heterogeneity | |||||
| (a) Case distribution by Townsend deprivation index | ||||||||||||
| Carcinomas | 5784 | 94 | 98 | 101 | 100 | 105 | 0.72 | 0.78 | ||||
| Thyroid | 1131 | 95 | 102 | 102 | 103 | 99 | 0.08 | 0.85 | ||||
| Other head and neck | 603 | 88 | 101 | 95 | 103 | 112 | 0.09 | 0.86 | ||||
| Lung | 155 | 87 | 89 | 90 | 108 | 125 | 0.11 | 0.93 | ||||
| Breast | 489 | 103 | 118 | 93 | 103 | 86 | 0.02 | 0.33 | ||||
| Cervix | 929 | 74 | 92 | 111 | 105 | 111 | 0.0004 | 0.16 | ||||
| Other GU tract | 1130 | 104 | 96 | 102 | 95 | 103 | 0.91 | 0.68 | ||||
| Colon and rectum | 576 | 107 | 96 | 99 | 87 | 111 | 0.98 | 0.25 | ||||
| Other GI tract | 387 | 87 | 89 | 97 | 115 | 112 | 0.04 | 0.87 | ||||
| Other specific and unspecific | 384 | 98 | 98 | 105 | 84 | 114 | 0.72 | 0.30 | ||||
|
Government Office Region
|
||||||||||||
| Cancer group | North East | North West | Yorkshire and Humber | East Midlands | West Midlands | East | London | South East | South West | All | P-value | P after TDI |
| (b) Rate per million person years at risk by Government Office Region | ||||||||||||
| Carcinomas | 29.3 | 32.2 | 30.8 | 29.1 | 31.4 | 26.4 | 30.1 | 32.2 | 31.7 | 30.6 | 0.008 | 0.002 |
| Thyroid | 5.5 | 6.1 | 6.4 | 5.6 | 6.1 | 6.6 | 6.3 | 5.5 | 5.6 | 6.0 | 0.84 | 0.86 |
| Other head and neck | 3.6 | 3.4 | 2.3 | 3.2 | 3.0 | 3.0 | 3.6 | 3.7 | 2.8 | 3.2 | 0.20 | 0.17 |
| Lung | 0.7 | 1.0 | 0.6 | 0.5 | 0.8 | 0.3 | 0.8 | 1.0 | 1.5 | 0.8 | 0.002 | 0.001 |
| Breast | 2.6 | 2.4 | 2.5 | 2.4 | 2.5 | 1.7 | 3.0 | 3.1 | 2.3 | 2.6 | 0.10 | 0.02 |
| Cervix | 10.3 | 12.6 | 12.0 | 8.1 | 14.0 | 6.8 | 6.0 | 8.6 | 11.3 | 9.8 | <0.0001 | <0.0001 |
| Other GU tract | 5.1 | 5.9 | 5.3 | 6.6 | 5.6 | 5.5 | 6.1 | 6.4 | 6.6 | 6.0 | 0.56 | 0.62 |
| Colon and rectum | 3.1 | 3.1 | 2.9 | 2.9 | 2.6 | 1.9 | 2.6 | 4.4 | 3.5 | 3.1 | 0.0003 | 0.0002 |
| Other GI tract | 0.9 | 1.8 | 2.5 | 1.7 | 2.0 | 2.1 | 2.3 | 2.2 | 2.3 | 2.1 | 0.10 | 0.08 |
| Other specific and unspecific | 2.7 | 2.1 | 2.2 | 2.2 | 1.8 | 2.2 | 2.2 | 1.8 | 1.7 | 2.1 | 0.76 | 0.59 |
Abbreviations: GI, gastrointestinal; GOR, Government Office Region; GU, genitourinary; TDI, Townsend deprivation index.
DISCUSSION
This is the first study of variability in cancer incidence by region and deprivation score for TYA cancer patients in England and makes use of information from all nine regional cancer registries over a 23-year period. Data collection methods differ between the regional registries and some differences in ascertainment may exist. However, a study of registration of childhood cancers (aged 0–14 years) by the regional cancer registries estimated that under-ascertainment was less than 5% (Hawkins and Swerdlow, 1992). Ascertainment of cancers in young people aged 15–24 years is also likely to be high. Overall, cancer registration data from the 1970s onwards are mainly complete and of high quality (Office for National Statistics, 2006). It seems reasonable to suppose, since different cancer groups show different patterns by region and deprivation, that these results cannot be explained by differences in registration practices alone.
Large-scale variability in the incidence of neoplasms at all ages has been studied for the UK and Ireland (Quinn et al, 2005), and in relation to economic deprivation (Quinn et al, 2001, 2005). Since more than 75% of cancers occur over the age of 60 years in the UK, disease patterns in the young are masked by the much greater cancer incidence at older ages. Furthermore, these earlier studies grouped cases by ICD, which uses mainly primary site to classify cancers. As such a classification is inappropriate to cancers in the TYA age group, we developed a more suitable morphology-based classification (Birch et al, 2002) and this has been applied here.
Leukaemia incidence at all ages (Quinn et al, 2005) and also in childhood in Great Britain, 1969–1993 (COMARE, 2006), showed higher rates in areas of higher socioeconomic status, much stronger below age 10 than in 10–14 year olds. Our results show a slight trend in the opposite direction, with lower incidence in TYAs in more prosperous areas. The markedly higher incidence of CML in more deprived areas is a new finding, which should be verified in an independent study. Variability in incidence patterns between age groups may imply differences in aetiology. There is consistent evidence of a role for infections in leukaemia in young children (McNally and Parker, 2006), but other factors such as traffic density (Nordlinger and Järvholm, 1997) and benzene exposure (Schnatter et al, 2005) should be explored in TYAs, especially in densely populated areas with high leukaemia incidence, such as London and the South East (Table 2b).
Alexander et al (1991) found that HL incidence among 0–24 year olds in parts of England and Wales was greater in areas of high socio-economic status, and we confirm this for TYAs over a longer period and larger geographic area. HL in early childhood is related to Epstein Barr virus (EBV) infections, but in TYAs the predominant subtype is nodular sclerosis, which is associated with a much lower incidence of EBV inclusion within the tumour (Jarrett et al, 1996). HL in older childhood has been related to delayed exposure to infection arising from improved socioeconomic conditions (Glaser et al, 1997). Law et al (2003) found marginal evidence for higher NHL incidence in under-15 year olds in less deprived areas in England and Wales, but was not found here. Viruses are implicated in the aetiology of NHL, specifically with HIV l and HTLV l, as well as EBV (Baris and Zahm, 2001). Geographical and socioeconomic variations may reflect differing opportunities for exposure to relevant infectious agents.
McKinney et al (1994) found that incidence of childhood brain tumours in Scotland varied by geographic region, with an excess in more prosperous areas. This pattern is present in our TYA data and is also seen across all ages (Quinn et al, 2005), and in childhood CNS tumours (COMARE, 2006). Regional trends in specific types are difficult to analyse due to the large proportion of unspecified cases from the West Midlands and changes in proportions with specified morphologies over time.
In this study, we found little or no variation in incidence of the majority of bone and soft tissue sarcomas with geographical region and TDI. A comparable lack of variability in incidence was found in the childhood cancer study (COMARE, 2006). These results imply that aetiological agents are acting uniformly across geographical regions and socioeconomic groups, perhaps related to intrinsic factors affecting growth and development and genetic susceptibility (Birch, 2005).
In TYAs, GCTs are dominated by testicular teratomas and seminomas. The geographical pattern of testicular GCTs in the present study is similar to that for testicular cancer of all ages (Quinn et al, 2005), in keeping with most being GCTs, with highest rates below age 50 years. That study, like the present, also found higher rates in the least deprived groups. There is strong evidence that testicular GCTs have their origin during prenatal life and may be associated with maternal exposures during pregnancy (Møller and Evans, 2003), but how such factors relate to observed patterns is unknown.
In the UK, approximately 40% of melanomas occur under 50 years of age. The regional variations among TYAs largely reflect those reported for all ages (Quinn et al, 2005), with high rates in the South West and South East and low rates in London, East Midlands and West Midlands. Both studies also found a marked inverse association with socioeconomic deprivation. However, the restriction in the trend in incidence by TDI at ages 13–24 to certain regions of England is a new and striking finding. It is well documented that the greatest risk factor for melanoma is excessive exposure to ultraviolet radiation, especially during childhood. Light-skinned individuals are more vulnerable (Tucker and Goldstein, 2003), and regional differences in darker skinned ethnic minority populations may contribute to differences in rates, and to regional differences in the incidence gradient with TDI. Differences in reporting practices for melanoma between UK cancer registries may account for some but not all regional differences in rates (Goodwin et al, 2004).
The South West of England has the lowest lung cancer incidence and lower smoking levels than other parts of the UK (Rickards et al, 2004; Quinn et al, 2005). However, it also has high concentrations of residential radon (Green et al, 2002), which has been estimated to increase overall lung cancer risk by 8% per 100 Bq m−3 (Darby et al, 2005). The comparatively high rate of lung cancer and uniformity of incidence by TDI quintile in TYAs is in marked contrast to all ages data (Quinn et al, 2005). This would support the view that tobacco is not an important cause of lung cancer in TYAs, among whom radon exposure may be a factor.
Carcinoma of the cervix is the most common carcinoma in female TYAs in England. In the general adult population, this is related to sexual behaviour and human papilloma virus infection (Wallbloomers et al, 1999), with a much higher incidence in areas of high deprivation and in the North West, Northern and Yorkshire health areas (Quinn et al, 2005). Our similar results imply that aetiology in TYAs may be similar to that in older women.
Although incidence of colorectal carcinoma in most regions was close to the overall rate of 3.1, there was more than a twofold difference between regions with the highest and lowest rates. At older ages, dietary factors affect colorectal cancer risk (Norat et al, 2005), but whether this relates to regional differences in incidence among TYAs is unknown.
Cancer represents a major source of morbidity and mortality in 13–24 year olds. The patterns of incidence observed in the present study merit further investigation. The differences between children, TYAs and older adults may imply differences in aetiology. Information about how current behaviour of children and young people affects their risk of serious illness or death as teenagers and young adults may have a greater influence on personal behaviour than information applicable to diseases that occur in later life.
Acknowledgments
Data used in this study were provided by the nine regional cancer registries in England. Census output is Crown copyright and is reproduced with the permission of the Controller of HMSO and the Queen's Printer for Scotland. Jillian M Birch is a Cancer Research UK Professorial Fellow, University of Manchester and TOB Eden is the Teenage Cancer Trust Professor of Teenage and Young Adult Cancer, University of Manchester.
References
- Alexander FE, McKinney PA, Williams J, Ricketts TJ, Cartwright RA (1991) Community lifestyle characteristics and incidence of Hodgkin's disease in young people. Int J Cancer 48: 10–14 [DOI] [PubMed] [Google Scholar]
- Baris D, Zahm SH (2001) Epidemiology of lymphomas. Curr Opin Oncol 12: 383–394 [DOI] [PubMed] [Google Scholar]
- Birch JM, Alston RD, Kelsey AM, Quinn MJ, Babb P, McNally RJQ (2002) Classification and incidence of cancers in adolescents and young adults in England 1979–1997. Br J Cancer 87: 1267–1274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birch JM (2005) Patterns of incidence of cancer in teenagers and young adults: implications for aetiology. In: Eden, TOB, Barr RD, Bleyer A, Whiteson M (eds) Cancer and the Adolescent, 2nd edn. pp 13–31. Blackwell Publishing: London [Google Scholar]
- Coleman MP, Babb P, Damiecki P, Grosclaude P, Honjo S, Jones J, Knerer G, Pitard A, Quinn MJ, Sloggett A, De Stavola B (1999) Cancer survival Trends in England and Wales 1971–1995: Deprivation and NHS Region. Studies in Medical and Population Subjects 61. The Stationery Office: London [Google Scholar]
- COMARE, Committee on Medical Aspects of Radiation in the Environment (2006) Eleventh Report: the distribution of childhood leukaemia and other childhood cancers in Great Britain 1969–1993. HMSO: London [Google Scholar]
- Darby S, Hill D, Auvinen A, Barros-Dios JM, Baysson H, Bochicchio F, Deo H, Falk R, Forastiere F, Hakama M, Heid I, Kreienbrock L, Kreuzer M, Lagarde F, Mäkeläinen I, Muirhead C, Oberaigner W, Pershagen G, Ruano-Ravina A, Ruosteenoja E, Schaffrath Rosario A, Tirmarche M, Tomášek L, Whitley E, Wichmann HE, Doll R (2005) Radon in homes and risk of lung cancer:collaborative analysis of individual data from 13 European case–control studies. Br Med J 330(7485): 223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaser SL, Lin RJ, Stewart SL, Ambinder RF, Jarrett RF, Brousset P, Pallesen G, Gulley ML, Khan G, O'Grady J, Hummel M, Preciado MV, Knecht H, Chan JKC, Claviez A (1997) Epstein–Barr virus associated Hodgkin's disease: epidemiologic characteristics in international data. Int J Cancer 70: 375–382 [DOI] [PubMed] [Google Scholar]
- Goodwin RG, Holme SA, Roberts DL (2004) Variations in registration of skin cancer in the United Kingdom. Clin Exp Dermatol 29: 328–330 [DOI] [PubMed] [Google Scholar]
- Green BMR, Miles JCH, Bradley EJ, Rees DM (2002) Radon Atlas of England and Wales. National Radiological Protection Board (NRPB-W26). Chilton: Oxfordshire [Google Scholar]
- Hawkins MM, Swerdlow AJ (1992) Completeness of cancer and death follow-up obtained through the National Health Service Central Register for England and Wales. Br J Cancer 66: 408–413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarrett AF, Armstrong AA, Alexander E (1996) Epidemiology of EBV and Hodgkin's lymphoma. Ann Oncol 7(Suppl 4): 5–10 [DOI] [PubMed] [Google Scholar]
- Law GR, Parslow RC, Roman E, on behalf of the United Kingdom Childhood Cancer Study Investigators (2003) Childhood cancer and population mixing. Am J Epidemiol 158: 328–336 [DOI] [PubMed] [Google Scholar]
- McCullagh P, Nelder JA (1989) Generalized Linear Models, 2nd edn. Chapman and Hall: London [Google Scholar]
- McKinney PA, Ironside JW, Harkness EF, Arango JC, Doyle D, Black RJ (1994) Registration quality and descriptive epidemiology of childhood brain tumours in Scotland 1975–90. Br J Cancer 70: 973–979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNally RJQ, Parker L (2006) Environmental factors and childhood acute leukaemias and lymphomas. Leuk Lymphoma 47(4): 583–598 [DOI] [PubMed] [Google Scholar]
- Møller H, Evans H (2003) Epidemiology of gonadal germ cell cancer in males and females. Acta Pathol Microbiol Immunol Scand Suppl 111: 43–46 [DOI] [PubMed] [Google Scholar]
- Norat T, Bingham S, Ferrari P, Slimani N, Jenab M, Mazuir M, Overvad K, Olsen A, Tjonneland A, Clavel F, Boutron-Ruault M-C, Kesse E, Boeing H, Bergmann MM, Nieters A, Linseisen J, Trichopoulou A, Trichopoulos D, Tountas Y, Berrino F, Palli D, Panico S, Tumino R, Vineis P, Bueno-de-Mesquita B, Peeters PHM, Engeset D, Lund E, Skeie G, Ardanaz E, Gonzalez C, Navarro C, Quiros JR, Sanchez M-J, Berglund G, Mattisson I, Hallmans G, Palmqvist R, Day NE, Khaw K-T, Key TJ, Joaquin MS, Hemon B, Saracci R, Kaaks R, Riboli E (2005) Meat, fish and colorectal cancer risk:the European Prospective Investigation into cancer and nutrition. J Natl Cancer Inst 97: 906–916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordlinger R, Järvholm B (1997) Environmental exposure to gasoline and leukaemia in children and young adults – an ecology study. Int Arch Occup Environ Health 70: 57–60 [DOI] [PubMed] [Google Scholar]
- Office for Population Censuses and Surveys (1991) Census: Small Area Statistics (England and Wales) [computer file]. ESRC/JISC Census Programme, Census Dissemination Unit, MIMAS (University of Manchester)
- Office for National Statistics: Twentieth century mortality (2002) Mortality in England and Wales by Age, Sex, Year and Underlying Cause: Year 2000 Update. Office for National Statistics: London [Google Scholar]
- Office for National Statistics (2001) Census: Standard Area Statistics (England and Wales) [computer file]. ESRC/JISC Census Programme, Census Dissemination Unit, MIMAS (University of Manchester)
- Office of National Statistics (2006) Cancer Statistics Registrations. Registrations of Cancer Diagnosed in 2004, England. Series MB1 no 35. Office for National Statistics: London [Google Scholar]
- Percy C, Van Holten V, Muir C (eds) (1990) International Classification of Diseases for Oncology, 2nd edn. World Health Organisation: Geneva [Google Scholar]
- Quinn MJ, Babb P, Brock A, Kirby L, Jones J (2001) Cancer trends in England and Wales, 1950–1999 Studies on Medical and Population Subjects No. 66. The Stationery Office: London [Google Scholar]
- Quinn MJ, Wood H, Cooper N, Rowan S (eds) (2005) Cancer atlas of the United Kingdom and Ireland 1991–2000. Studies on Medical and Population Subjects 68 Palgrave Macmillan
- Rickards L, Fox K, Roberts C, Fletcher L, Goddard E (2004) Living in Britain: Results from the 2002 General Household Survey. The Stationery Office: London [Google Scholar]
- Schnatter AR, Rosamilia K, Wojcik NC (2005) Review of the literature on benzene exposure and leukemia subtypes. Chem Biol Interact 153–154: 9–21 [DOI] [PubMed] [Google Scholar]
- Townsend P, Phillimore P, Beattie A (1988) Health Deprivation, Inequality and the North. Croom-Helm: London [Google Scholar]
- Tucker MA, Goldstein AM (2003) Melanoma etiology: where are we? Oncogene 22: 3042–3052 [DOI] [PubMed] [Google Scholar]
- Wallbloomers JMM, Jacobs MV, Manos MM, Bosch FX, Kummer JA, Shah KV, Snijders PFJ, Peto J, Meijer CJLM, Muñoz N (1999) Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol 189: 12–19 [DOI] [PubMed] [Google Scholar]
- World Health Organisation (1975) International Classification of Diseases, Injuries and Causes of Death, 9th Revision. World Health Organisation: Geneva [Google Scholar]
- World Health Organisation (1976) International Classification of Diseases for Oncology, 1st edn. World Health Organisation: Geneva [Google Scholar]
- World Health Organisation (1992) International Classification of Diseases and Health Related Problems, 10th Revision. World Health Organisation: Geneva [Google Scholar]