Abstract
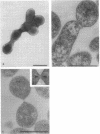
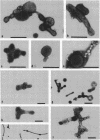
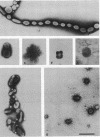
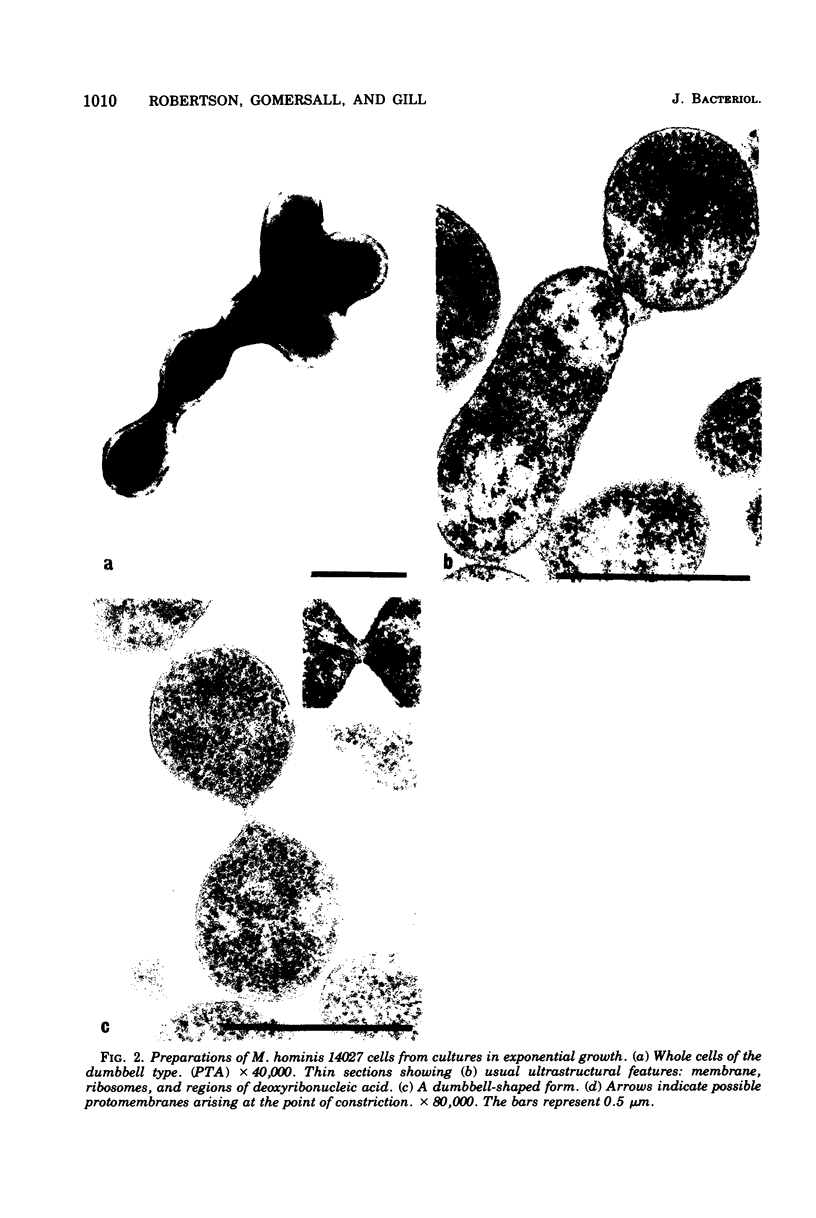
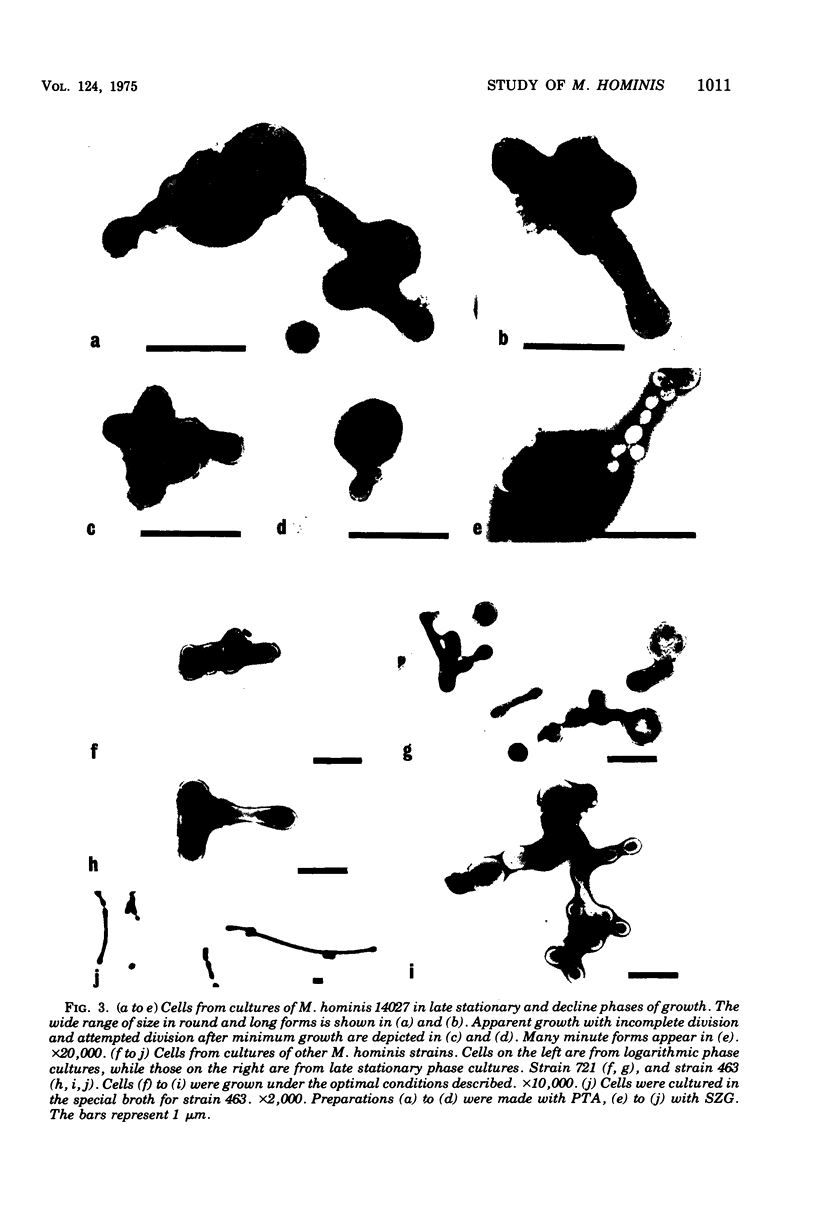
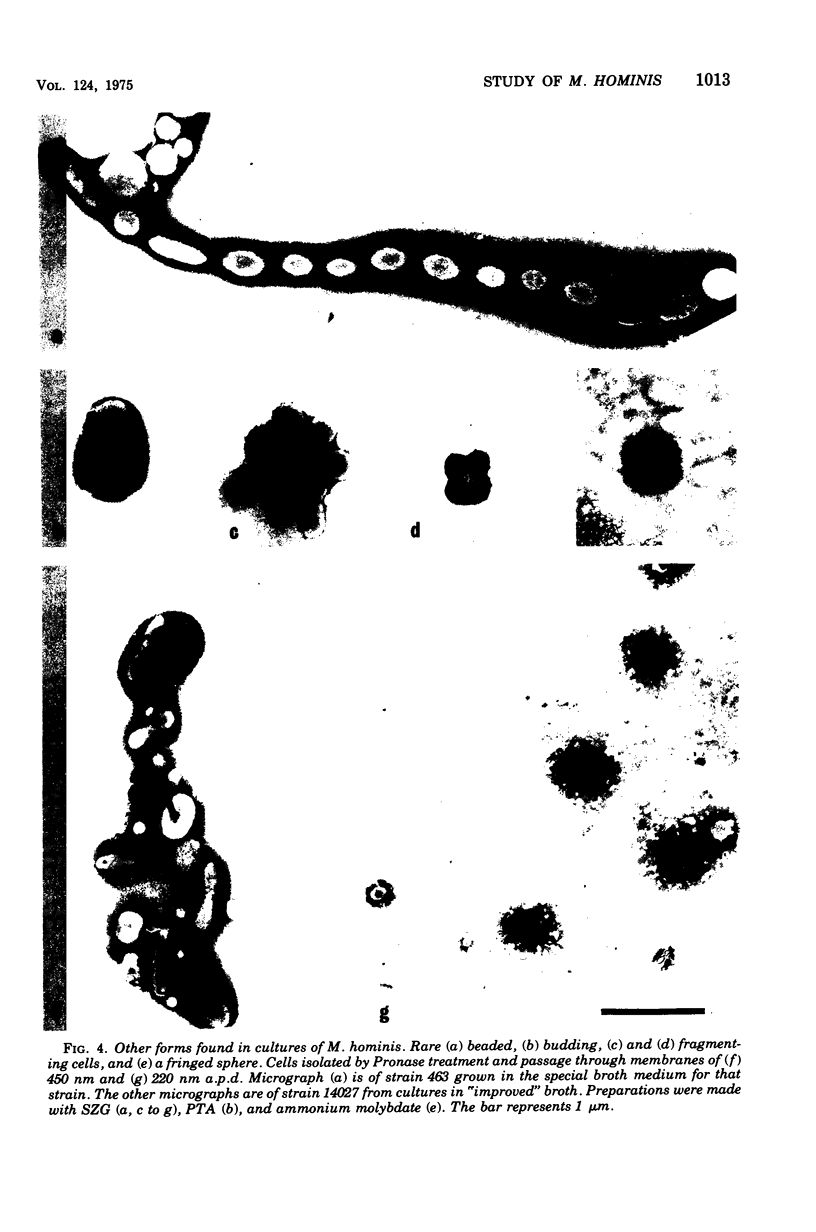
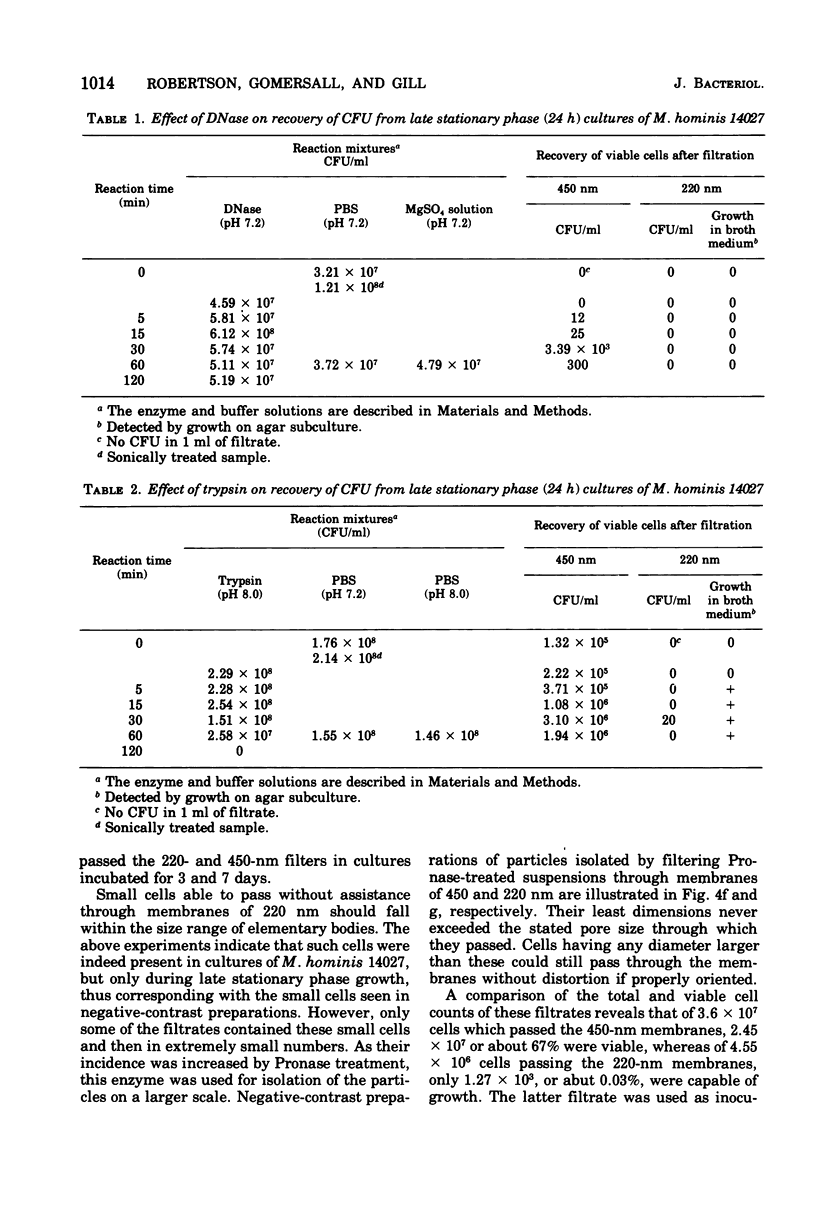
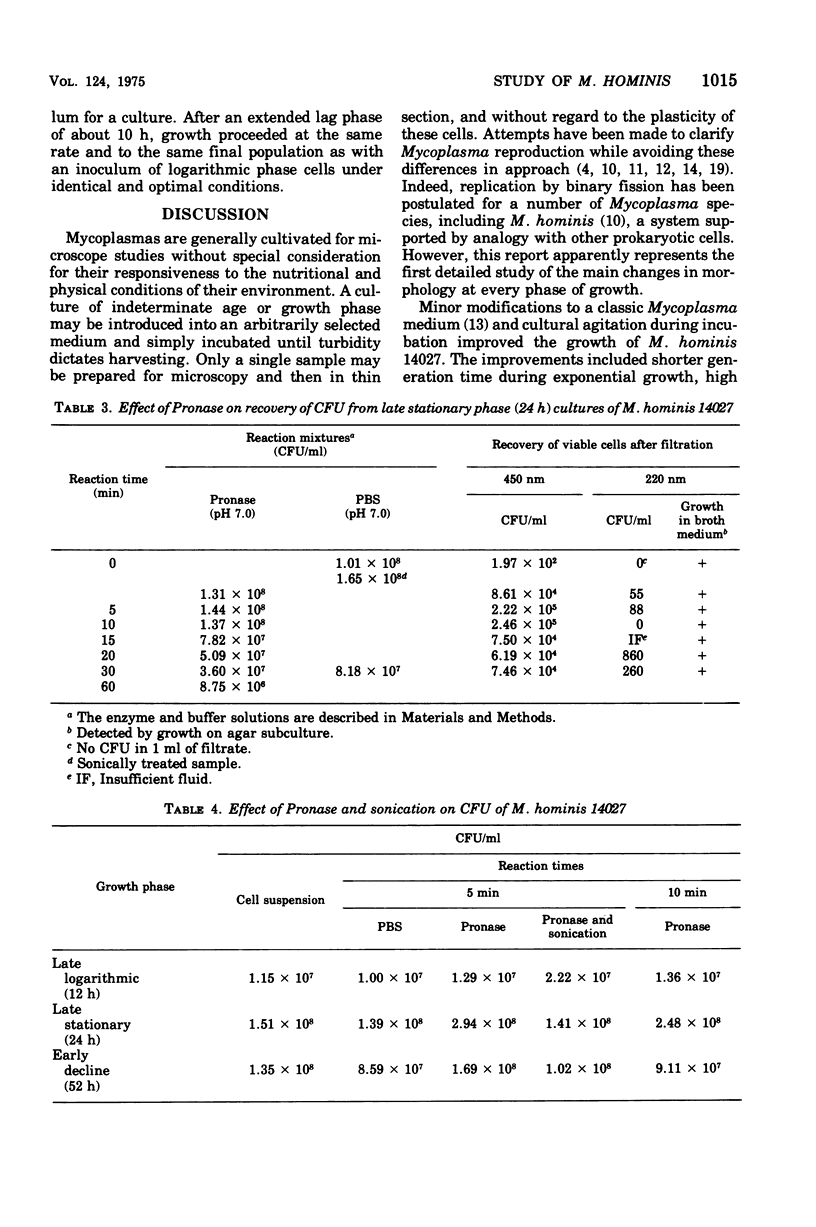
Improved methods for studying the growth of Mycoplasma hominis (ATCC 14027) have been developed, involving modified growth conditions and preparation of the organisms under minimally distorting conditions. Cells so prepared from batch cultures show relatively uniform exponential growth and appear to be dividing by binary fission; but pleomorphic forms appear upon further incubation. Similar behavior was demonstrated by another laboratory-adapted strain and by three clinical isolates, and therefore seems characteristic of the species. The pleomorphic populations contain small forms having diameters within the 100- to 250-nm size range reported for "elementary bodies." Such forms were isolated from this strain of M. hominis by sequential filtration using gravity alone, after cell aggregates were dispersed by Pronase treatment. Of the small bodies which traversed membranes of 220-nm pore size, a negligible number grew in liquid or on solid media, suggesting that these were not essential reproductive units in a life cycle, but involution forms due to growth in an altered environment.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bredt W. Experimentelle Untersuchungen über Morphologie und Vermehrung der beim Menschen vorkommenden Mycoplasmen unter besonderer Berücksichtigung von Mycoplasma hominis. Z Med Mikrobiol Immunol. 1970;155(3):248–274. [PubMed] [Google Scholar]
- Bredt W., Heunert H. H., Höfling K. H., Milthaler B. Microcinematographic studies of Mycoplasma hominis cells. J Bacteriol. 1973 Mar;113(3):1223–1227. doi: 10.1128/jb.113.3.1223-1227.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eng J., Froholm O. Immune electron microscopy of not cell-bound antigen of Mycoplasma pneumoniae. Acta Pathol Microbiol Scand B Microbiol Immunol. 1971;79(6):759–763. doi: 10.1111/j.1699-0463.1971.tb00108.x. [DOI] [PubMed] [Google Scholar]
- Engelman D. M., Morowitz H. J. Characterization of the plasma membrane of Mycoplasma laidlawii. 3. The formation and aggregation of small lipoprotein structures derived from sodium dodecyl sulfate-solubilized membrane components. Biochim Biophys Acta. 1968 Apr 29;150(3):376–384. doi: 10.1016/0005-2736(68)90136-3. [DOI] [PubMed] [Google Scholar]
- Engelman D. M., Morowitz H. J. Characterization of the plasma membrane of Mycoplasma laidlawii. IV. Structure and composition of membrane and aggregated components. Biochim Biophys Acta. 1968 Apr 29;150(3):385–396. doi: 10.1016/0005-2736(68)90137-5. [DOI] [PubMed] [Google Scholar]
- Furness G., De Maggio M. D. Binucleate classical mycoplasmas pathogenic for goats. Infect Immun. 1972 Apr;5(4):433–441. doi: 10.1128/iai.5.4.433-441.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furness G. The growth and morphology of Mycoplasmas replicating in synchrony. J Infect Dis. 1970 Sep;122(3):146–158. doi: 10.1093/infdis/122.3.146. [DOI] [PubMed] [Google Scholar]
- Green F., 3rd, Hanson R. P. Ultrastructure and capsule of Mycoplasma meleagridis. J Bacteriol. 1973 Nov;116(2):1011–1018. doi: 10.1128/jb.116.2.1011-1018.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayflick L. Tissue cultures and mycoplasmas. Tex Rep Biol Med. 1965 Jun;23(Suppl):285+–285+. [PubMed] [Google Scholar]
- KELTON W. H. Synchronized division of avian pleuropneumonialike organisms. J Bacteriol. 1962 May;83:948–955. doi: 10.1128/jb.83.5.948-955.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemcke R. M. Osmolar concentration and fixation of mycoplasmas. J Bacteriol. 1972 Jun;110(3):1154–1162. doi: 10.1128/jb.110.3.1154-1162.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemcke R. M. Sizing small organisms. Nature. 1971 Feb 12;229(5285):492–493. doi: 10.1038/229492a0. [DOI] [PubMed] [Google Scholar]
- Metz J., Bredt W. Elektronenmikroskopische Untersuchungen an Mycoplasma hominis (Stamm W 463-69. Z Med Mikrobiol Immunol. 1971;156(4):368–378. [PubMed] [Google Scholar]
- Morowitz H. J., Maniloff J. Analysis of the life cycle of Mycoplasma gallisepticum. J Bacteriol. 1966 Apr;91(4):1638–1644. doi: 10.1128/jb.91.4.1638-1644.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morowitz H. J., Tourtellotte M. E., Pollack M. E. USE OF POROUS CELLULOSE ESTER MEMBRANES IN THE PRIMARY ISOLATION AND SIZE DETERMINATION OF PLEUROPNEUMONIA-LIKE ORGANISMS. J Bacteriol. 1963 Jan;85(1):134–136. doi: 10.1128/jb.85.1.134-136.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razin S., Morowitz H. J., Terry T. M. Membrane subunits of Mycoplasma laidlawii and their assembly to membranelike structures. Proc Natl Acad Sci U S A. 1965 Jul;54(1):219–225. doi: 10.1073/pnas.54.1.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson J., Gomersall M., Gill P. Effect of preparatory techniques on the gross morphology of Mycoplasma hominis. J Bacteriol. 1975 Nov;124(2):1019–1022. doi: 10.1128/jb.124.2.1019-1022.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WATSON D. H., RUSSELL W. C., WILDY P. Electron microscopic particle counts on herpes virus using the phosphotungstate negative staining technique. Virology. 1963 Mar;19:250–260. doi: 10.1016/0042-6822(63)90062-x. [DOI] [PubMed] [Google Scholar]