Abstract
Angiogenesis is essential for development, growth and advancement of solid tumours. Interferon-γ-inducible protein 10 (IP-10) regulates lymphocyte chemotaxis, mediates vascular pericyte proliferation and acts as an angiostatic agent, thus inhibiting tumour growth. This prompted us to study the clinical implications of IP-10 expression related to angiogenesis in uterine cervical cancers. The levels of IP-10 decreased with advancement, and the prognosis of the 30 patients with low IP-10 expression in uterine cervical cancers was poor (66%), whereas the 24-month survival rate of the other patients with high IP-10 expression was 90%. Furthermore, IP-10 levels significantly reverse-correlated with vascular endothelial growth factor (VEGF) levels in uterine cervical cancers. Interferon-γ-inducible protein 10 might work on suppression of angiogenesis associated with VEGF in advancement, and can be recognised as a prognostic indicator. Furthermore, IP-10 activation might be effective on the suppression of regrowth or recurrence after intensive treatment for advanced cervical cancers.
Keywords: IP-10, VEGF, angiogenesis, angiogenic inhibitor, uterine cervical cancer
Angiogenesis is essential for development, growth and advancement of solid tumours (Folkman, 1985). Angiogenic factors from tumours induce and activate matrix metalloproteinase, plasminogen activator, collagenase and other enzymes in endothelial cells (ECs). The enzymes dissolve basement membrane of ECs, after which the ECs proliferate and migrate under the influence of angiogenic factors. Angiogenic factors induce production of integrins in the ECs. The ECs then form immature capillary tubes. Specific angiogenic factors interacting with specific angiogenic inhibitors produce specific angiogenesis in each tumour. The angiogenic factors vascular endothelial growth factor (VEGF) (Fujimoto et al, 1998d, 1998a, 1999a, 2001, 2004), thymidine phosphorylase identified with platelet-derived EC growth factor (Fujimoto et al, 1998b, 1998c, 1999b, 1999c, 2000a) interleukin (IL)-8 and basic fibroblast growth factor (bFGF) along with the angiogenic transcription factor ETS-1 (Fujimoto et al, 2002b) interacting with angiogenic inhibitors work on angiogenesis.
We have reported the positive correlation between microvessel counts and IL-8, and that IL-8 works as an angiogenic factor. There was a significant difference in IL-8 levels between early-stage and late-stage uterine cervical cancers. The prognosis of the higher group was radically poorer than that of the lower group. This indicates that IL-8 might be a prognostic indicator as an angiogenic factor supplied from macrophages within and around the tumour (Fujimoto et al, 2000b, 2002a). The expression of VEGF was correlated with microvessel density in uterine cervical cancers. The levels of VEGF and VEGF165 and VEGF121 mRNAs were remarkably higher in some stages II and III/IV adenocarcinomas of the cervix than in other cases including normal cervices. Therefore, the elevation of VEGF165 and VEGF121 might contribute to the relatively late advancing via angiogenic activity in some adenocarcinomas of the cervix (Fujimoto et al, 1999a, 2004). The expression of basic FGF was significantly higher in advanced primary uterine cervical cancers, regardless of histological type. This status might contribute to the acceleration of growth, invasion, and metastasis with neovascularization in advanced uterine cervical cancers (Fujimoto et al, 1995, 1997a, 1997b).
Specific angiogenic inhibitors have not been well known in uterine cervical cancers. Among angiogenic inhibitors, interferon-γ-inducible protein (IP-10) is a member of the CXC chemokine family, whose members have four highly conserved cysteine amino-acid residues, with the first two cysteines separated by one non-conserved amino-acid residue, hence the name CXC (Rollins, 1997), that has been shown to induce chemotaxis of activated T cells and to inhibit angiogenesis (Strieter et al, 1995). It is produced by activated monocytes, fibroblasts, ECs, epithelial cells and keratinocytes. Interferon-γ-inducible protein 10 binds to a seven-transmembrane G protein-coupled receptor, CXCR3, expressed on activated T cells, leading to chemotaxis (Loetscher et al, 1996). Downregulation of the angiostatic chemokine IP-10 has been found to be a factor in the development of idiopathic pulmonary fibrosis (Keane et al, 1997) and endometriosis (Yoshino et al, 2003). On the other hand, the overexpression of IP-10 in human lymphoma grown in nude mice leads to spontaneous regression directly related to impaired angiogenesis (Sgadari et al, 1996a). This has been substantiated further in model systems of human non-small cell lung cancer tumourigenesis in severe combined immunodeficiency mice (Arenberg et al, 2001). Retroviral gene transfer of IP-10 inhibited growth of human melanoma xenografts in an angiogenesis-dependent manner (Feldman et al, 2002).
This prompted us to study the expression manner of IP-10 interacting with various angiogenic factors, VEGF (Boulday et al, 2006), IL-8 (Elner et al, 2006), IL-12 (Sgadari et al, 1996b), TNF-α (Gottlieb et al, 2005) according to various clinical backgrounds of uterine cervical cancer patients, to know the clinical implications of IP-10 in uterine cervical cancers.
MATERIALS AND METHODS
Patients
Informed consent for the following studies was obtained from all patients and the Research Committee for Human Subjects, Gifu University School of Medicine. Sixty patients ranging from 26 to 78 years of age with uterine cervical cancers (22 stage-I cases, 26 stage-II cases, 12 stage-III cases; and 47 squamous cell carcinomas, 13 adenocarcinomas) underwent curative surgery at the Department of Obstetrics and Gynecology, Gifu University School of Medicine, between December 1998 and January 2004. None of the patients had received any therapy before uterine cervical cancer tissue was taken. A part of each tissue of uterine cervical cancers was snap-frozen in liquid nitrogen to determine IP-10, IL-8, and VEGF and a neighbouring part of the tissues was submitted for histopathological study including immunohistochemical staining for IP-10. The clinical stage of uterine cervical cancers was determined by International Federation of Obstetrics and Gynecology (FIGO) classification (International Federation of Obstetrics and Gynecology (FIGO) News, 1989).
Immunohistochemistry
Sections (4 μm) of formalin-fixed paraffin-embedded tissues of uterine cervical cancers were cut with a microtome and dried overnight at 37°C on a silanised-slide (Dako, Carpinteria, CA, USA). Samples were deparaffinised in xylene at room temperature (RT) for 80 min and washed with a graded ethanol/water mixture and then with distiled water. Immunohistochemical staining for factor VIII-related antigen and CD34, which are synthesised by vascular ECs, are specific for the ECs of blood vessels (Bell and Flotte, 1982) and are useful for detecting tumour angiogenesis (Weidner et al, 1991). The samples were soaked in a citrate buffer, and then microwaved at 100°C for 10 min. The protocol for a DakoCytomation LSAB+ System-HRP (Dako) was followed for each sample. In the described procedures, goat anti-human IP-10 (R&D Systems, Minneapolis, MN, USA), rabbit anti-factor VIII-related antigen (Zymed, San Francisco, CA, USA) and mouse CD34 (Dako) were used at dilutions of 1 : 25, 1 : 2 and 1 : 40, respectively, as the first antibodies. The addition of the first antibody, goat anti-human IP-10, rabbit anti-factor VIII-related antigen or mouse CD34, was omitted in the protocols for negative controls of IP-10, factor VIII-related antigen and CD34, respectively.
Vessels were counted in the five highest density areas at × 200 magnification (using a combination of × 20 objective and × 10 ocular, 0.785 mm2 field−1) by blinded investigators. Microvessel counts were expressed as the mean numbers of vessels in the five highest density areas (Maeda et al, 1996). Microvessel density was evaluated by the counting of microvessels.
Enzyme immunoassay for determination of IP-10, IL-8 and VEGF antigens
All steps were carried out at 4°C. Tissues of uterine cervical cancers (wet weight: 10–20 mg) were homogenised in HG buffer (5 mM Tris–HCl (pH 7.4), 5 mM NaCl, 1 mM CaCl2, 2 mM ethyleneglycol-bis-[β-aminoethylether]-N,N,N′,N′-tetraacetic acid, 1 mM MgCl2, 2 mM dithiothreitol, 25 μg ml−1 aprotinin, and 25 μg ml−1 leupeptin) with a Polytron homogenizer (Kinematics, Luzern, Switzerland). This suspension was centrifuged in a microfuge at 12 000 r.p.m. (10 000 g) for 3 min to obtain the supernatant. The protein concentration of samples was measured by the method of Bradford (Bradford, 1976) to standardise IP-10, IL-8 and VEGF antigen levels.
Interferon-γ-inducible protein 10, IL-8 and VEGF antigen levels in the samples were determined by a sandwich enzyme immunoassay using a Human IP-10 Quantikine (R&D Systems), a human IL-8 Quantikine (R&D System) and a human VEGF Assay Kit-IBL (Immuno Biological Laboratories, Gunma, Japan), respectively. The levels of IP-10, IL-8 and VEGF were standardised with the corresponding cellular protein concentrations.
Western blot analysis for IP-10
Tissues (wet weight: 10–20 mg) were homogenised in a WB–HB buffer (10 mM Tris–HCl (pH 7.4), 150 mM NaCl, 0.5% Triton X-100 and 0.2 mM phenylmethyl sulphonyl fluoride) with a Polytron homogenizer (Kinematics, Luzern, Switzerland). The protein concentrations of samples were measured by protein assay (Bio-Rad, Hercules, CA, USA). Each sample (25 μl) containing 10 μg of protein was added to 25 μl of a sample buffer (12.5 mM Tris–HCl (pH 6.8), 2% glycerol, 0.4% SDS and 1.25% 2-mercaptoethanol) and analysed by 20% SDS–PAGE under nonreducing condition. The gel was transferred to a nitrocellulose membrane (Hybond ECL Western; Amersham, Arlington Heights, IL, USA). The membrane was blocked with 5% skim milk in a blocking buffer (20 mM Tris–HCl (pH 7.6), 137 mM NaCl and 0.1% Tween-20), incubated with rabbit anti-human IP-10 antibody (1 : 2000) (R&D Systems) for 60 min at RT, washed and then incubated with peroxidase-linked species-specific whole antibody, anti-rabbit immunoglobulin from a goat (1 : 5000; Santa Cruz Biotechnology, Santa Cruz, CA, USA) for 60 min at RT. After sequential washing with blocking buffer, PBS with 0.1% Tween 20 and PBS, signals were developed using ECL plus (GE Healthcare Bio-Sciences AB, Uppsala, Sweden) and X-ray film was exposed on the membrane at RT for 10 min. Bands on the exposed film were captured with a Fuji Image Analyzer LAS-1000 and the intensity was measured with NIH-image (National Institutes of Health, Bethesda, MA USA). The IP-10 levels as the arbitrary unit (AU) determined by Western blot analysis for IP-10 were calculated as follows: IP-10 levels by Western blotting=the intensity of IP-10 × 100/the intensity of β-actin.
Statistics
Interferon-γ-inducible protein 10, IL-8 and VEGF were measured from three parts of the same tissue in triplicate. Statistical analysis was performed with Student's t-test. Differences were considered significant when P<0.05. Correlation evaluations between VEGF and IP-10 levels were analysed by Pearson's product–moment corelational coefficient. Positive correlation was considered significant when P<0.05.
RESULTS
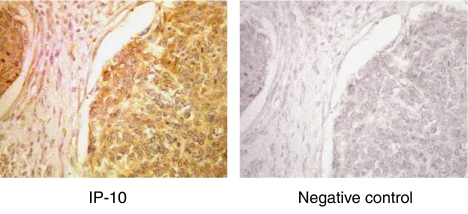
Interferon-γ-inducible protein 10 was diffusely located in the cancer cells, but not in the stromal cells of uterine cervical cancer tissues in all cases given, as shown in Figure 1 for a representative case (71-year-old patient, stage IIb, squamous cell carcinoma).
Figure 1.
Immunohistochemical staining for IP-10 in uterine cervical cancers (original magnification × 100). A representative case of squamous cell carcinoma of the uterine cervix. Goat anti-human IP-10 (R&D Systems, Minneapolis, MN, USA) was used at a dilution of 1 : 50 as the first antibody. Dark brown staining represents positive for IP-10 antigen.
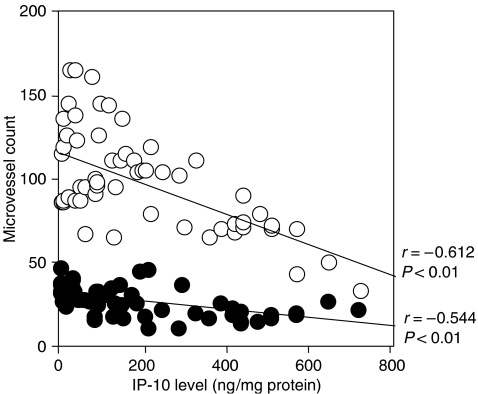
Interferon-γ-inducible protein 10 levels significantly reversely correlated with microvessel counts by immunohistochemical staining for factor VIII-related antigen (MVC-F8) and by staining for CD34 (MVC-CD34) (r=−0.544, P<0.001 and r=−0.612, P<0.001, respectively) as shown in Figure 2.
Figure 2.
Correlation between microvessel counts and IP-10 in uterine cervical cancers. Closed circles, microvessel counts by immunohistochemical staining for factor VIII-related antigen (MVC-F8); open circles, microvessel counts by immunohistochemical staining for CD34 (MVC-CD34).
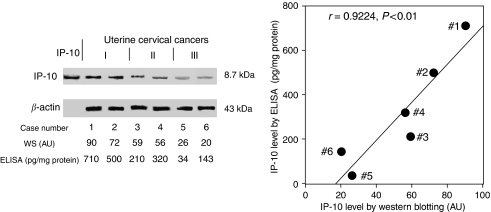
The IP-10 levels determined by Western blotting analysis and ELISA of six representative cases are shown in the left panel of Figure 3. There was a significant positive correlation between the IP-10 levels determined by Western blotting and ELISA as shown in the right panel of Figure 3. In place of the Western blotting, ELISA can be useful to determine the IP-10 levels reliably.
Figure 3.
Correlation between IP-10 levels determined by Western blotting and ELISA. The bands of Western blots of the representative six cases are shown. Representative cases are shown in Figure 4 using n. AU, arbitrary units of IP=10.
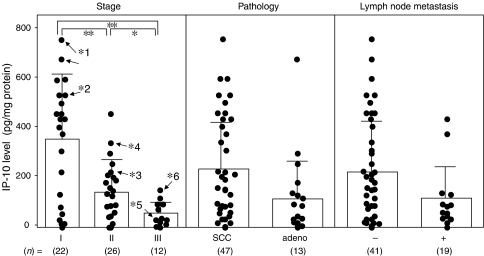
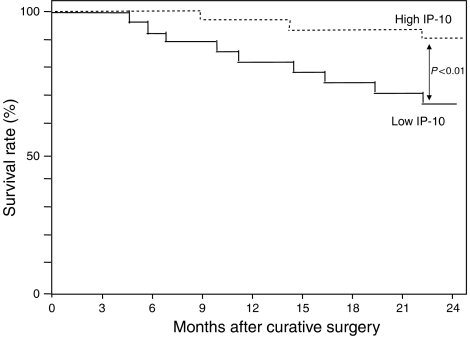
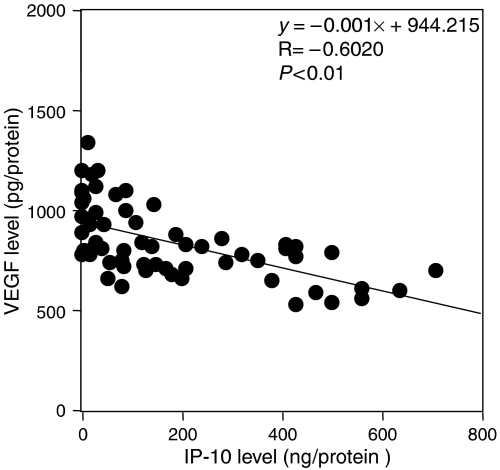
Interferon-γ-inducible protein 10 expression did not demonstrate any significant difference according to histopathological type or lymph node metastasis, as shown in Figure 4. Interferon-γ-inducible protein 10 levels significantly decreased with advancement (between stages I and II, P<0.001; between stages I and III, P<0.001; and between stages II and III, P<0.05) as shown in Figure 4. The 60 patients were divided equally in two, at the median value of 140 pg mg−1 protein, to form the low IP-10 and high IP-10 groups. The prognosis of the 30 patients with high IP-10 expression in uterine cervical cancers was good, whereas the 24-month survival rate of the other 30 patients with low IP-10 expression was significantly poorer, as shown in Figure 5. Interferon-γ-inducible protein 10 levels reversely (r=−0.564, P<0.01) correlated with VEGF levels, as shown in Figure 6, but not with IL-8 levels in uterine cervical cancers (data not shown).
Figure 4.
Levels of IP-10 in uterine cervical cancers classified according to clinical stage, histopathological type and lymph node metastasis. Clinical staging of uterine cervical cancer was done according to FIGO. Each level is the mean of nine determinations. *P<0.05, **P<0.001, n representative cases from Figure 3.
Figure 5.
Survival rates after curative resection for uterine cervical cancers. Patient prognosis was analysed with a 24-month survival rate. High IP-10, cases with high IP-10 levels (>140 pg mg−1 protein), n=30; low IP-10, cases with low IP-10 levels (<140 pg mg−1 protein), n=30.
Figure 6.
Correlation between IP-10 and VEGF in uterine cervical cancers.
DISCUSSION
In the present study, IP-10 reversely correlated with microvessel counts and might act as an angiostatic agent in uterine cervical cancers. Interferon-γ-inducible protein 10 expressed in the cancer cells decreased with tumour advancement, and downregulated IP-10 correlated with poor patient prognosis in uterine cervical cancers. This indicates that IP-10 works on angiogenesis as an angiogenic inhibitor in uterine cervical cancers. Furthermore, we were interested in how IP-10 interacts with angiogenic factors specific to uterine cervical cancers.
Interferon-γ-inducible protein 10 is identified as one of the TNF-regulated products in keratinocytes (Sgadari et al, 1996b) and it contributes to the antitumoural effects of IL-12 through the recruitment of activated T cells, NK cells, monocytes and macrophages as well as through its inhibitory effects on tumour vasculature (Elner et al, 2006). Human retinal pigment epithelial cells produced very high levels of IP-10 in response to either IL-1α or TNF-α, in the presence of IFN-γ (Boulday et al, 2006). Interferon-γ-inducible protein 10 potently inhibits angiogenesis, working in a dose-dependent manner to inhibit both IL-8 and bFGF-induced endothelial chemotaxis (Strieter et al, 1995). Interferon-γ-inducible protein 10 is expressed in some diseases in association with VEGF. It is reported that VEGF may also play a major role to augment the expression of IP-10 in T cells (Feldman et al, 2002). Interferon-γ-inducible protein 10 also inhibits the proliferation of vascular ECs via IP-10 receptor CXCR3 expressed in ECs (Lasagni et al, 2003). Interferon-γ-inducible protein 10 inhibits VEGF-induced EC tube formation concomitant with blocking motility (Bodnar et al, 2006). Vascular endothelial growth factor augments the effect of IFN-γ on the induction of IP-10 mRNA and protein expression in ECs, and Chinese hamster ovary cells designed to secrete VEGF were injected subcutaneously into the skin of nude mice and found to mediate a time-dependent increase in IP-10 mRNA (Feldman et al, 2002). Therefore, angiogenic factors specific to IP-10 might be IL-8, bFGF and VEGF.
Among angiogenic factors specific to IP-10, angiogenic factors specific to uterine cervical cancers seem to be IL-8 and VEGF. In the present study, IP-10 levels reversely correlated with VEGF levels, but not with IL-8, in uterine cervical cancers. The biological pathway of IP-10 induction by VEGF as a homostatic regulation might be lost with tumour advancement, resulting in drastic tumour angiogenesis that accelerates tumour advancement.
Acknowledgments
We thank Mr John Cole for proofreading the English of this manuscript.
References
- Arenberg DA, Zlotnick A, Strom SR, Burdick MD, Strieter RM (2001) The murine CC chemokine, 6C-kine, inhibits tumor growth and angiogenesis in a human lung cancer SCID mouse model. Cancer Immunol Immunother 49: 587–592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell DA, Flotte TJ (1982) Factor VIII related antigen in adenomatoid tumors. Cancer 50: 932–938 [DOI] [PubMed] [Google Scholar]
- Bodnar RJ, Yates CC, Wells A (2006) IP-10 blocks vascular endothelial growth factor-induced endothelial cell motility and tube formation via inhibition of calpain. Circ Res 98: 617–625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulday G, Haskova Z, Reinders ME, Pal S, Briscoe DM (2006) Vascular endothelial growth factor-induced signaling pathways in endothelial cells that mediate overexpression of the chemokine IFN-gamma-inducible protein of 10 kDa in vitro and in vivo. J Immunol 176: 3098–3107 [DOI] [PubMed] [Google Scholar]
- Bradford MM (1976) Rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72: 248–254 [DOI] [PubMed] [Google Scholar]
- Elner SG, Delmonte D, Bian ZM, Lukacs NW, Elner VM (2006) Differential expression of retinal pigment epithelium (RPE) IP-10 and interleukin-8. Exp Eye Res 83: 374–379 [DOI] [PubMed] [Google Scholar]
- Feldman AL, Friedl J, Lans TE, Libutti SK, Lorang D, Miller MS, Turner EM, Hewitt SM, Alexander HR (2002) Retroviral gene transfer of interferon-inducible protein 10 inhibits growth of human melanoma xenografts. Int J Cancer 99: 149–155 [DOI] [PubMed] [Google Scholar]
- Folkman J (1985) Tumor angiogenesis. Adv Cancer Res 43: 175–203 [DOI] [PubMed] [Google Scholar]
- Fujimoto J, Aoki I, Khatun S, Toyoki H, Tamaya T (2002a) Clinical implications of expression of interleukin-8 related to myometrial invasion with angiogenesis in uterine endometrial cancers. Ann Oncol 13: 430–434 [DOI] [PubMed] [Google Scholar]
- Fujimoto J, Aoki I, Toyoki H, Khatun S, Tamaya T (2002b) Clinical implications of expression of ETS-1 related to angiogenesis in uterine cervical cancers. Ann Oncol 13: 1598–1604 [DOI] [PubMed] [Google Scholar]
- Fujimoto J, Hori M, Ichigo S, Tamaya T (1995) Expression of basic fibroblast growth factor and its mRNA in uterine endometrial cancers. Invas Metast 15: 203–210 [PubMed] [Google Scholar]
- Fujimoto J, Ichigo S, Hirose R, Sakaguchi H, Tamaya T (1998a) Expressions of vascular endothelial growth factor (VEGF) and its mRNA in uterine endometrial cancers. Cancer Lett 134: 15–22 [DOI] [PubMed] [Google Scholar]
- Fujimoto J, Ichigo S, Hori M, Hirose R, Sakaguchi H, Tamaya T (1997a) Expression of basic fibroblast growth factor and its mRNA in advanced uterine cervical cancers. Cancer Lett 111: 21–26 [DOI] [PubMed] [Google Scholar]
- Fujimoto J, Ichigo S, Hori M, Hirose R, Sakaguchi H, Tamaya T (1997b) Expression of basic fibroblast growth factor and its mRNA in advanced ovarian cancers. Eur J Gynaecol Oncol 18: 349–352 [PubMed] [Google Scholar]
- Fujimoto J, Ichigo S, Sakaguchi H, Hirose R, Tamaya T (1998b) Expression of platelet-derived endothelial cell growth factor (PD-ECGF) and its mRNA in uterine endometrial cancers. Cancer Lett 130: 115–120 [DOI] [PubMed] [Google Scholar]
- Fujimoto J, Ichigo S, Sakaguchi H, Hirose R, Tamaya T (1998c) Expression of platelet-derived endothelial cell growth factor (PD-ECGF) and its mRNA in ovarian cancers. Cancer Lett 126: 83–88 [DOI] [PubMed] [Google Scholar]
- Fujimoto J, Sakaguchi H, Aoki I, Khatun S, Tamaya T (2001) Clinical implications of the expression of vascular endothelial growth factor in metastatic lesions of ovarian cancers. Br J Cancer 85: 313–316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimoto J, Sakaguchi H, Aoki I, Tamaya T (2000a) The value of platelet-derived endothelial cell growth factor as a novel predictor of advancement of uterine cervical cancers. Cancer Res 60: 3662–3665 [PubMed] [Google Scholar]
- Fujimoto J, Sakaguchi H, Aoki I, Tamaya T (2000b) Clinical implications of expression of interleukin 8 related to angiogenesis in uterine cervical cancers. Cancer Res 60: 2632–2635 [PubMed] [Google Scholar]
- Fujimoto J, Sakaguchi H, Hirose R, Ichigo S, Tamaya T (1998d) Biologic implications of the expression of vascular endothelial growth factor subtypes in ovarian carcinoma. Cancer 83: 2528–2533 [DOI] [PubMed] [Google Scholar]
- Fujimoto J, Sakaguchi H, Hirose R, Ichigo S, Tamaya T (1999a) Expression of vascular endothelial growth factor (VEGF) and its mRNA in uterine cervical cancers. Br J Cancer 80: 827–833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimoto J, Sakaguchi H, Hirose R, Ichigo S, Tamaya T (1999b) The expression of platelet-derived endothelial cell growth factor (PD-ECGF) and its mRNA in uterine cervical cancers. Br J Cancer 79: 1249–1254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimoto J, Sakaguchi H, Hirose R, Wen H, Tamaya T (1999c) Clinical implication of expression of platelet-derived endothelial cell growth factor (PD-ECGF) in metastatic lesions of uterine cervical cancers. Cancer Res 59: 3041–3044 [PubMed] [Google Scholar]
- Fujimoto J, Toyoki H, Sato E, Sakaguchi H, Tamaya T (2004) Clinical implication of expression of vascular endothelial growth factor-C in metastatic lymph nodes of uterine cervical cancers. Br J Cancer 91: 466–469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottlieb AB, Chamian F, Masud S, Cardinale I, Abello MV, Lowes MA, Chen F, Magliocco M, Krueger JG (2005) TNF inhibition rapidly down-regulates multiple proinflammatory pathways in psoriasis plaques. J Immunol 175: 2721–2729 [DOI] [PubMed] [Google Scholar]
- International Federation of Obstetrics and Gynecology (FIGO) News (1989) Int J Gynecol Obstet 28: 189–193
- Keane MP, Arenberg DA, Lynch III JP, Whyte RI, Iannettoni MD, Burdick MD, Wilke CA, Morris SB, Glass MC, DiGiovine B, Kunkel SL, Strieter RM (1997) The CXC chemokines, IL-8 and IP-10, regulate angiogenic activity in idiopathic pulmonary fibrosis. J Immunol 159: 1437–1443 [PubMed] [Google Scholar]
- Lasagni L, Francalanci M, Annunziato F, Lazzeri E, Giannini S, Cosmi L, Sagrinati C, Mazzinghi B, Orlando C, Maggi E, Marra F, Romagnani S, Serio M, Romagnani P (2003) An alternatively spliced variant of CXCR3 mediates the inhibition of endothelial cell growth induced by IP-10, Mig, and I-TAC, and acts as functional receptor for platelet factor 4. J Exp Med 197: 1537–1549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loetscher M, Gerber B, Loetscher P, Jones SA, Piali L, Clark-Lewis I, Baggiolini M, Moser B (1996) Chemokine receptor specific for IP10 and mig: structure, function, and expression in activated T-lymphocytes. J Exp Med 184: 799–802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda K, Chung Y, Ogawa Y, Takatsuka S, Kang SM, Ogawa M, Sawada T, Onda N, Kato Y, Sowa M (1996) Thymidine phosphorylase/platelet-derived endothelial cell growth factor expression associated with hepatic metastasis in gastric carcinoma. Br J Cancer 73: 884–888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rollins BJ (1997) Chemokines. Blood 90: 909–928 [PubMed] [Google Scholar]
- Sgadari C, Angiolillo AL, Cherney BW, Pike SE, Farber JM, Koniaris LG, Vanguri P, Burd PR, Sheikh N, Gupta G, Teruya-Feldstein J, Tosato G (1996a) Interferon-inducible protein-10 identified as a mediator of tumor necrosis in vivo. Proc Natl Acad Sci USA 93: 13791–13796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sgadari C, Angiolillo AL, Tosato G (1996b) Inhibition of angiogenesis by interleukin-12 is mediated by the interferon-inducible protein 10. Blood 87: 3877–3882 [PubMed] [Google Scholar]
- Strieter RM, Kunkel SL, Arenberg DA, Burdick MD, Polverini PJ (1995) Interferon gamma-inducible protein 10 (IP-10), a member of the C-X-C chemokine family, is an inhibitor of angiogenesis. Biochem Biophys Res Commun 210: 51–57 [DOI] [PubMed] [Google Scholar]
- Weidner N, Semple JP, Welch WR, Folkman J (1991) Tumor angiogenesis and metastasis – correlation in invasive breast carcinoma. New Engl J Med 324: 1–8 [DOI] [PubMed] [Google Scholar]
- Yoshino O, Osuga Y, Koga K, Hirota Y, Tsutsumi O, Yano T, Morita Y, Momoeda M, Fujiwara T, Kugu K, Taketani Y (2003) Concentrations of interferon-gamma-induced protein-10 (IP-10), an antiangiogenic substance, are decreased in peritoneal fluid of women with advanced endometriosis. Am J Reprod Immunol 50: 60–65 [DOI] [PubMed] [Google Scholar]