Abstract
The aim of the study is to determine the role of lymphadenectomy in advanced epithelial ovarian cancer. The data were obtained from the Surveillance, Epidemiology and End Results (SEER) program reported between 1988 and 2001. Kaplan–Meier estimates and Cox proportional hazards regression models were used for analysis. Of 13 918 women with stage III–IV epithelial ovarian cancer (median age: 64 years), 87.9% were Caucasian, 5.6% African Americans, and 4.4% Asians. A total of 4260 (30.6%) underwent lymph node dissections with a median number of six nodes reported. For all patients, a more extensive lymph node dissection (0, 1, 2–5, 6–10, 11–20, and >20 nodes) was associated with an improved 5-year disease-specific survival of 26.1, 35.2, 42.6, 48.4, 47.5, and 47.8%, respectively (P<0.001). Of the stage IIIC patients with nodal metastases, the extent of nodal resection (1, 2–5, 6–10, 11–20, and >20 nodes) was associated with improved survivals of 36.9, 45.0, 47.8, 48.7, and 51.1%, respectively (P=0.023). On multivariate analysis, the extent of lymph node dissection and number of positive nodes were significant independent prognosticators after adjusting for age, year at diagnosis, stage, and grade of disease. The extent of lymphadenectomy is associated with an improved disease-specific survival of women with advanced epithelial ovarian cancer.
Keywords: lymph node resection, ovarian cancer, survival
Ovarian cancer is the most lethal gynaecologic malignancy, and is the fifth-highest cause of cancer deaths in women in the United States. In 2006, it was estimated that 20 180 women will be diagnosed with ovarian cancer, and 15 310 will die of the disease (Jemal et al, 2006). The standard of care in the treatment of patients with advanced-stage epithelial ovarian cancer includes primary cytoreduction surgery, followed by a platinum-based chemotherapy regimen (Marsden et al, 2000; Bristow et al, 2002).
A major controversy in the surgical treatment of advanced-stage ovarian cancer concerns the optimal management of the retroperitoneal lymph nodes. Approaches ranging from biopsy of only grossly enlarged nodes to systematic dissection of bilateral pelvic and paraaortic lymph nodes have been employed. Retroperitoneal lymph node involvement is reported in 50–75% of patients with advanced-stage disease at the time of primary surgery (Chen and Lee, 1983; Burghardt et al, 1989, 1991; Onda et al, 1996). It is unclear whether lymphadenectomy aids in better staging of patients, or whether the procedure itself has therapeutic value by debulking gross and occult disease. Prior retrospective reports support the role of lymphadenectomy in ovarian epithelial cancer (Burghardt et al, 1986; Scarabelli et al, 1995). However, others have not found a benefit associated with systematic lymphadenectomy (Spirtos et al, 1995).
Benedetti Panici et al (2005) reported the results of a prospective trial on 427 patients with optimally debulked stage IIIB–IV epithelial ovarian cancer randomised to systematic pelvic and paraaortic lymphadenectomy vs resection of bulky nodes only. Those who underwent a systematic lymph node dissection had a 7-month improvement in progression-free survival (29.4 vs 22.4 months). However, they were unable to demonstrate a significant overall survival benefit.
Given that most of the prior retrospective studies have been limited by a small sample size, we performed a large population-based study to investigate the role of lymphadenectomy in patients with advanced-stage ovarian cancers. Our study analysed the disease-specific survival outcomes of 13 918 patients diagnosed with advanced-stage epithelial ovarian cancer to determine the potential role of lymph node dissection.
MATERIALS AND METHODS
Demographic, clinicopathologic, surgical, and survival information of women diagnosed with epithelial ovarian cancer during the period from 1 January 1988 to 31 December 2001 were extracted with permission from the Surveillance, Epidemiology and End Results (SEER) program of the United States National Cancer Institute. This data represent approximately 14% of the US population and are reported from 12 population-based registries including San Francisco-Oakland, Connecticut, metropolitan Detroit, Hawaii, Iowa, New Mexico, Seattle (Puget Sound), Utah, metropolitan Atlanta, Alaska, San Jose-Monterey, and Los Angeles (Hankey et al, 1999).
Only patients with advanced (stage III–IV) disease who had undergone a surgical staging procedure were included in the analysis. Patients with borderline tumours of the ovary, as well as those patients with germ cell, sex cord stromal, and sarcoma histologies, were excluded. Patients were assigned to the following categories based on race classifications described by the SEER program: Caucasian, African Americans, Asians, and others. Asians were defined as Chinese, Japanese, Korean, Vietnamese, and Filipino. All other race and ethnicity groups were defined as others.
In the assessment of demographic trends in the patient cohort, and to determine 5-year disease-specific survival, χ2-tests and Kaplan–Meier analyses with log-rank tests were performed. The outcome of specific interest was death due to ovarian cancer (disease-specific survival). The Cox proportional hazards model was used to investigate the significance of extent of lymph node resection (characterised by the number of lymph nodes reported) after adjusting for other patient features, including age, race, year of diagnosis, stage, and grade, and number of positive nodes. For the analysis of the patients who had received a lymph node dissection as part of their primary surgical treatment, the patients were grouped based on the extent of the lymphadenectomy (1, 2–5, 6–10, 11–20, and >20 nodes) to meet proportionality assumptions. All data were analysed using Intercooled STATA (version 8.0; STATA Corporation, College Station, TX, USA) and SAS (version 6.12; SAS Inc., Cary, NC, USA).
RESULTS
Of the 13 918 patients with stage III–IV epithelial ovarian cancer, the median age was 62.7 years (range: 12–101). The median year of diagnosis was 1995. The majority of patients were Caucasian (87.9%), with African Americans, Asians, and others making up 5.6, 4.4, and 1.9% of patients, respectively (Table 1). After undergoing surgical staging, 8062 patients had stage III disease with 448 having stage IIIA, 672 stage IIIB, 4576 stage IIIC, and 5856 patients having stage IV ovarian cancer. Of the entire cohort, 4260 had a lymphadenectomy performed as part of their surgery, with a median number of 6 nodes removed (range: 1–90). For patients with positive nodes, the median number of positive nodes was two (range: 1–54) and the median number of total nodes recovered was seven (range: 1–90). In the study group, 66.8% of tumours were of serous histology, 9.2% endometrioid, 5.6% mucinous, and 2.8% clear cell. A large proportion of patients had grade 3 disease (60%), with 4.2 and 17.6% of patients having grade 1 and 2 disease, respectively (Table 2). The median time of follow-up was 22 months (range: 0–167 months).
Table 1. Patient characteristics.
| Total (n=13 918) No. (%)a | 0 nodes (n=9658) No. (%)a | 1 node (n=824) No. (%)a | 2–5 nodes (n=1202) No. (%)a | 6–10 nodes (n=714) No. (%)a | 11–20 nodes (n=813) No. (%)a | >20 nodes (n=707) No. (%)a | |
|---|---|---|---|---|---|---|---|
| Age at diagnosis (years) | |||||||
| Mean | 62.7 (±0.1) | 64.1 (±0.1) | 60.9 (±0.4) | 59.9 (±0.4) | 59.1 (±0.5) | 58.1 (±0.5) | 57.1 (±0.5) |
| Median (range) | 64.0 (12–101) | 65.0 (13–101) | 61.0 (22–90) | 60.0 (20–94) | 59.5 (20–93) | 58.0 (12–90) | 57.0 (15–91) |
| Median year of diagnosis | 1995 | 1994 | 1995 | 1996 | 1997 | 1997 | 1998 |
| Race | |||||||
| Caucasians | 12240 (87.9%) | 8498 (88.0%) | 709 (86.0%) | 1037 (86.3%) | 629 (88.1%) | 741 (91.1%) | 626 (88.5%) |
| African Americans | 782 (5.6%) | 549 (5.7%) | 57 (6.9%) | 83 (6.9%) | 41 (5.7%) | 32 (3.9%) | 20 (2.8%) |
| Asiansb | 612 (4.4%) | 407 (4.2%) | 36 (4.4%) | 57 (4.7%) | 30 (4.2%) | 31 (3.8%) | 51 (7.2%) |
| Othersc | 267 (1.9%) | 195 (2.0%) | 22 (2.7%) | 24 (2.0%) | 12 (1.7%) | 8 (1.0%) | 6 (0.8%) |
| Unknown | 17 (0.1%) | 9 (0.1%) | 0 (0.0%) | 1 (0.1%) | 2 (0.3%) | 1 (0.1%) | 4 (0.6%) |
| Year of diagnosis | |||||||
| 1988–1992 | 4142 (29.8%) | 3288 (34.0%) | 265 (32.2%) | 267 (22.2%) | 129 (18.1%) | 122 (15.0%) | 71 (10.0%) |
| 1993–1997 | 5327 (38.3%) | 3766 (39.0%) | 283 (34.3%) | 489 (40.7%) | 276 (38.7%) | 296 (36.4%) | 217 (30.7%) |
| 1998–2001 | 4449 (31.9%) | 2604 (27.0%) | 276 (33.5%) | 446 (37.1%) | 309 (43.3%) | 395 (48.6%) | 419 (59.3%) |
Percentage of patients for given parameter.
Asians were defined as Chinese, Japanese, Korean, Vietnamese, and Filipino.
Others were defined as all other race/ethnicity parameters.
Table 2. Clinicopathologic data.
| Total (n=13 918) No. (%)a | 0 nodes (n=9658) No. (%)a | 1 node (n=824) No. (%)a | 2–5 nodes (n=1,202) No. (%)a | 6–10 nodes (n=714) No. (%)a | 11–20 nodes (n=813) No. (%)a | >20 Nodes (n=707) No. (%)a | Chi-square Test P-value | |
|---|---|---|---|---|---|---|---|---|
| Stage of disease | ||||||||
| Stage IIIA | 448 (3.2) | 281 (2.9) | 25 (3.0) | 56 (4.7) | 36 (5.0) | 33 (4.1) | 17 (2.4) | P<0.001 |
| Stage IIIB | 672 (4.8) | 477 (4.9) | 26 (3.2) | 58 (4.8) | 42 (5.9) | 46 (5.7) | 23 (3.3) | |
| Stage IIIC | 4576 (32.9) | 2384 (24.7) | 415 (50.4) | 592 (49.3) | 350 (49.0) | 429 (52.8) | 406 (57.4) | |
| Stage III, NOS | 2366 (17.0) | 2010 (20.8) | 62 (7.5) | 107 (8.9) | 58 (8.1) | 73 (9.0) | 56 (7.9) | |
| Stage IV | 5856 (42.1) | 4506 (46.7) | 296 (35.9) | 389 (32.4) | 228 (31.9) | 232 (28.5) | 205 (29.0) | |
| Grade of disease | ||||||||
| Grade 1 | 580 (4.2) | 366 (3.8) | 30 (3.6) | 47 (3.9) | 43 (6.0) | 47 (5.8) | 47 (6.6) | P=0.005 |
| Grade 2 | 2443 (17.6) | 1684 (17.4) | 135 (16.4) | 211 (17.6) | 123 (17.2) | 164 (20.2) | 126 (17.8) | |
| Grade 3 | 8349 (60.0) | 5673 (58.7) | 531 (64.4) | 765 (63.6) | 437 (61.2) | 490 (60.3) | 453 (64.1) | |
| Unknown | 2546 (18.3) | 1935 (20.0) | 128 (15.5) | 179 (14.9) | 111 (15.5) | 112 (13.8) | 81 (11.5) | |
| Histology | ||||||||
| Serous | 9294 (66.8) | 6474 (67.0) | 553 (67.1) | 805 (67.0) | 457 (64.0) | 532 (65.4) | 473 (66.9) | P<0.001 |
| Endometrioid | 1275 (9.2) | 778 (8.1) | 78 (9.5) | 137 (11.4) | 91 (12.7) | 100 (12.3) | 91 (12.9) | |
| Mucinous | 775 (5.6) | 554 (5.7) | 46 (5.6) | 61 (5.1) | 36 (5.0) | 39 (4.8) | 39 (5.5) | |
| Clear cell | 394 (2.8) | 206 (2.1) | 23 (2.8) | 62 (5.2) | 35 (4.9) | 39 (4.8) | 29 (4.1) | |
| Others or NOS | 2180 (15.7) | 1646 (17.0) | 124 (15.0) | 137 (11.4) | 95 (13.3) | 103 (12.7) | 75 (10.6) | |
Percentage of patients for given parameter.
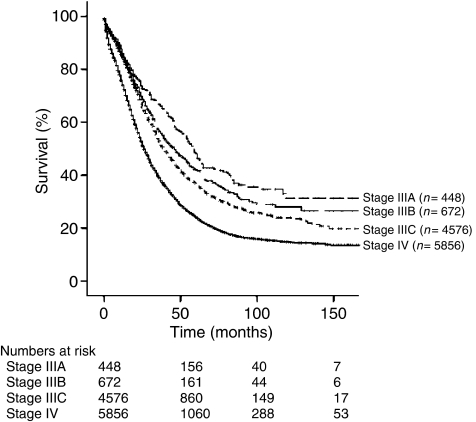
The 5-year disease-specific survival for those ⩽64 years was 37.1 vs 24.4% for those >64 years (P<0.001). The survival of patients with stage IIIA was 48.0%, IIIB 42.1%, and IIIC 36.7%. Stage IV patients had a survival of 24.1% (Figure 1). Women with grade 1, 2, and 3 tumours had survivals of 56.9, 33.4, and 29.2%, respectively (P<0.001). The survival estimates based on histologies were serous 30.6%, endometrioid 43.6%, mucinous 33.3%, and clear cell 25.5% (P<0.001).
Figure 1.
Kaplan–Meier analysis based on stage of disease (n=13 918; P<0.001).
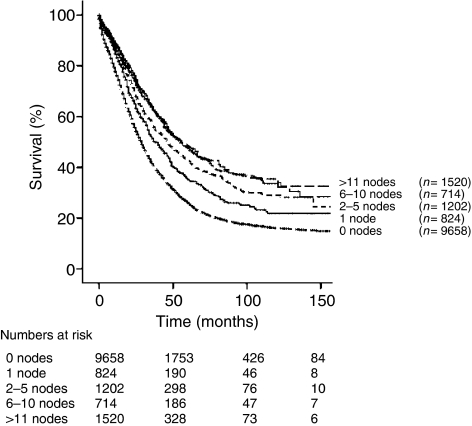
To evaluate the effect of the extent of lymph node dissection, our study cohort was divided into six groups: patients who had 0, 1, 2–5, 6–10, 11–20, and >20 nodes reported. For all stages, we found that the removal of increasing numbers of lymph nodes was associated with a significant increase in 5-year disease-specific survival (Table 3). The findings of 0, 1, 2–5, 6–10, 11–20, and >20 lymph nodes were associated with survivals of 26.1, 35.2, 42.6, 48.4, 47.5, and 47.8%, respectively (P<0.001; Figure 2 also). The effect of extent of lymphadenectomy by histological subtype and by grade is shown in Table 3. The 5-year disease-specific survival rates were found to significantly increase when more nodes were resected, within all grades and histologic types. In an analysis of those patients (n=2563) who were found to have nodal metastases, the removal of a total of 1, 2–5, 6–10, 11–20, and >20 lymph nodes was associated with survival rates of 32.8, 36.8, 38.7, 42.0, and 41.7%, respectively (P=0.002; Table 4). For 2188 patients with stage IIIC disease and positive lymph nodes, survival was noted to be significantly associated with a more extensive lymphadenectomy. Additionally, the effect of the number of positive nodes was investigated in subgroups of patients with IIIC-IV node-positive disease, dividing patients into those with 1, 2–5, and >5 positive lymph nodes. When 1, 2–5, and >5 positive lymph nodes were found, the removal of increasing numbers of negative lymph nodes was associated with improved survival (Table 4).
Table 3. Five-year disease-specific survival based on the extent of lymphadenectomy and clinicopathologic characteristics.
| No. | Total % (s.e.) | 0 nodes % (s.e.) | 1 node % (s.e.) | 2–5 nodes % (s.e.) | 6–10 nodes % (s.e.) | 11–20 nodes % (s.e.) | >20 nodes % (s.e.) | Log-rank | |
|---|---|---|---|---|---|---|---|---|---|
| Stage of disease | P<0.001 | ||||||||
| Stage III–IV | 13 918 | 31.1 (0.5) | 26.1 (0.5) | 35.2 (2.0) | 42.6 (1.8) | 48.4 (2.4) | 47.5 (2.3) | 47.8 (2.8) | P<0.001 |
| Stage III | 8062 | 36.7 (0.7) | 30.5 (0.8) | 37.4 (2.7) | 47.7 (2.2) | 55.2 (2.9) | 51.6 (2.8) | 54.5 (3.2) | P<0.001 |
| Stage IIIA | 448 | 48.0 (2.8) | 40.4 (3.3) | 33.9 (12.6) | 66.8 (8.4) | 61.5 (10.5) | 71.4 (9.6) | 74.7 (17.5) | P<0.001 |
| Stage IIIB | 672 | 42.1 (2.4) | 35.1 (2.7) | 41.0 (12.9) | 55.9 (8.0) | 74.0 (7.6) | 61.0 (8.8) | 81.1 (10.1) | P=0.001 |
| Stage IIIC | 4576 | 36.7 (0.9) | 29.0 (1.2) | 36.9 (3.1) | 45.0 (2.6) | 47.8 (3.6) | 48.7 (3.3) | 51.1 (3.5) | P<0.001 |
| Stage IV | 5856 | 24.1 (0.7) | 21.4 (0.7) | 31.3 (3.1) | 33.1 (2.9) | 34.6 (4.0) | 38.3 (4.1) | 32.2 (5.0) | P<0.001 |
| Grade of disease | P<0.001 | ||||||||
| Grade 1 | 580 | 56.9 (2.4) | 49.3 (3.0) | 44.5 (11.2) | 67.3 (7.7) | 71.9 (7.8) | 75.2 (7.8) | 77.3 (7.6) | P<0.001 |
| Grade 2 | 2443 | 33.4 (1.2) | 28.0 (1.3) | 37.3 (5.6) | 45.0 (4.3) | 59.1 (5.6) | 49.4 (5.1) | 48.3 (6.1) | P<0.001 |
| Grade 3 | 8349 | 29.2 (0.6) | 24.2 (0.7) | 32.6 (2.5) | 40.4 (2.3) | 44.6 (3.1) | 43.5 (2.9) | 46.4 (3.6) | P<0.001 |
| Histology | P<0.001 | ||||||||
| Serous | 9294 | 30.6 (0.6) | 26.1 (0.7) | 32.2 (2.5) | 41.6 (2.2) | 49.7 (3.0) | 45.6 (2.9) | 44.0 (3.5) | P<0.001 |
| Endometrioid | 1275 | 43.6 (1.6) | 35.5 (2.0) | 53.1 (6.4) | 49.8 (5.0) | 50.7 (6.7) | 63.0 (5.9) | 75.4 (5.6) | P<0.001 |
| Mucinous | 775 | 33.3 (2.0) | 28.1 (2.2) | 41.6 (8.5) | 48.7 (8.1) | 46.1 (10.1) | 51.3 (9.6) | 47.0 (9.7) | P<0.001 |
| Clear cell | 394 | 25.5 (2.9) | 18.3 (3.6) | 10.1 (9.1) | 38.0 (7.0) | 39.9 (10.4) | 34.4 (9.4) | 37.6 (10.3) | P=0.007 |
s.e.=standard error.
Figure 2.
Kaplan–Meier analysis of patients by extent of lymphadenectomy (n=13 918; P<0.001).
Table 4. Five-year disease-specific survival analysis for node-positive stage IIIC–IV patients based on the extent of lymphadenectomy and number of positive nodes.
|
Total number of nodes removed
|
||||||||
|---|---|---|---|---|---|---|---|---|
| Positive node number | No. | Total % (s.e.) | 1 node % (s.e.) | 2–5 nodes % (s.e.) | 6–10 nodes % (s.e.) | 11–20 nodes % (s.e.) | >20 nodes % (s.e.) | Log-rank |
| All patients | 2563 | 38.0 (1.3) | 32.8 (2.6) | 36.8 (2.4) | 38.7 (3.1) | 42.0 (2.9) | 41.7 (3.3) | P=0.002 |
| 1 positive node | 1067 | 40.1 (1.9) | 32.8 (2.6) | 45.8 (4.1) | 48.1 (6.1) | 43.7 (6.5) | 58.2 (8.1) | P<0.001 |
| 2–5 positive nodes | 972 | 37.0 (2.1) | — | 31.4 (3.0) | 38.9 (4.5) | 44.2 (4.7) | 40.2 (5.7) | P<0.001 |
| >5 positive nodes | 524 | 35.6 (2.8) | — | — | 29.6 (5.5) | 38.7 (4.6) | 36.5 (4.6) | P=0.883 |
s.e.=standard error.
On multivariate analysis, the extent of lymph node dissection, both as a categorical and continuous variable, persisted as an independent prognostic factor. In addition, age at diagnosis, stage, grade, histologic cell type, number of positive nodes, and year of diagnosis were also found to be significant prognosticators (Table 5).
Table 5. Multivariate analysis.
| Prognostic factor | Hazard ratio | 95% confidence interval | P-value |
|---|---|---|---|
| Age at diagnosisa | 1.018 | 1.016–1.019 | P<0.005 |
| Year of diagnosisb | 0.977 | 0.970–0.984 | P<0.005 |
| Stagec | 1.266 | 1.220–1.315 | P<0.005 |
| Graded | 1.933 | 1.684–2.219 | P<0.005 |
| Histologye | 1.994 | 1.716–2.316 | P<0.005 |
| Extent of lymphadenectomyf | 0.911 | 0.861–0.964 | P=0.001 |
| Positive nodesg | 1.338 | 1.215–1.473 | P<0.005 |
Continous.
Continous.
Stage IIIA/B vs IIIC vs IV.
Grade 1 vs 2–3.
Others vs clear cell.
0 vs 1 vs 2–5 vs 6–10 vs ⩾11.
No vs yes.
DISCUSSION
The standard therapy for advanced epithelial ovarian carcinoma includes total hysterectomy, bilateral salpingo-oophorectomy, omentectomy, washings, blind biopsies of diaphragm and peritoneum, and optimal surgical cytoreduction, followed by platinum-based chemotherapy. The prognostic value of complete tumour debulking on the overall survival has been demonstrated in many retrospective analyses (Piver et al, 1988; Covens, 2000; Bristow et al, 2002). On the basis of a recent meta-analysis of 81 cohorts of patients with stage III–IV disease, it was found that for each 10% increase in maximal cytoreduction, there was an associated 5.5% increase in median survival (Bristow et al, 2002). However, the role of retroperitoneal nodal resection remains unclear, particularly for advanced-stage disease.
In a retrospective review of 127 patients, Carnino et al (1997) reported that the probability of finding a lymph node metastasis was significantly higher when more lymph nodes were removed. These authors suggested that systematic lymphadenectomy should be performed, rather than lymph node sampling, to determine the therapeutic impact of lymph node resection in epithelial ovarian cancers. Determining nodal metastases by palpation at the time of surgery has been found to have significant limitations (Petru et al, 1994; Arango et al, 2000; Eisenkop and Spirtos, 2001; Tangjitgamol et al, 2003).
The potential benefit of performing of a systematic lymphadenectomy in the primary surgical evaluation of presumed early-stage ovarian cancer patients has been previously investigated. The value of systematic retroperitoneal node dissection may be associated with the upstaging of patients with clinical stage I cancers, which directs them to further treatment with chemotherapy. Furthermore, when initial surgical staging is adequate, patients with low-risk disease may be spared cytotoxic chemotherapy (Trimbos et al, 2003; Chan et al, 2007).
The role of systematic lymphadenectomy in advanced stages of ovarian cancer is somewhat unclear. Some prior studies have found an association between systematic node dissection and improved survival. In a retrospective study of 82 patients with stage III disease, Burghardt et al (1986) showed that pelvic lymphadenectomy was associated with an improved survival compared with those patients who did not have a lymphadenectomy. In a retrospective review of 150 epithelial cancer patients based on the Tokai Ovarian Tumor Study Group, Kikkawa et al (1995) found that the performance of a lymphadenectomy was associated with improved survival in a multivariate analysis after controlling for the effects of stage, residual disease, and histological subtype (Hazard Ratio: 0.677; P=0.0497).
In a randomised, controlled multi-institutional study of 427 advanced-staged optimally debulked patients, Benedetti Panici et al (2005) showed a 7-month improvement in disease-free survival in those who underwent a systematic lymphadenectomy compared with patients who had removal of only pathologically enlarged lymph nodes. In another randomised trial of 268 patients with epithelial ovarian cancer macroscopically confined to the pelvis after cytoreductive surgery, Maggioni et al (2006) compared the effects of a systematic lymphadenectomy to random sampling of retroperitoneal lymph nodes. These authors revealed that systematic lymphadenectomy was associated with an improvement in both progression-free and overall survival; however, neither was statistically significant. The investigators stated that this trial lacked the power to detect a significant difference between the two groups. These two studies may also have been limited by the short follow-up duration for assessing long-term survival outcomes (Benedetti Panici et al, 2005). As such, we performed a large population-based study to evaluate the potential role of an extensive lymphadenectomy in women diagnosed with advanced-stage epithelial ovarian cancer.
In this report of 13 918 women with stages III–IV ovarian cancer, 4260 patients had a dissection of at least one lymph node performed as part of their initial surgical evaluation. Our data suggested that a more extensive lymph node dissection was associated with an improved 5-year disease-specific survival. These findings were consistent in patients within substages of stage III disease and those with nodal metastases. Although an increase in the number of positive nodes was associated with a worsened survival, the removal of 1, 2–5, 6–10, and 11–20 nodes improved the outcomes of these patients from 32.8, 36.8, 38.7, 42.0, and 41.7%, respectively. More importantly, multivariate analysis demonstrated that a more extensive node resection, both as a categorical and continuous variable, was associated with an improved survival after adjusting for age, stage, grade, number of positive nodes, and year of diagnosis (Table 5).
This study is one of the largest series to evaluate the role of lymphadenectomy in surgically staged advanced ovarian cancer patients. A large proportion of these patients had an extensive lymph node resection; in fact, 707 patients had >20 lymph nodes removed. Given the large size of this cohort, with 13 918 patients, we were able to perform subset analyses on node-positive stage IIIC and/or IV patients showing consistent results. Similar to the results of a randomised trial, our data also showed that metastatic lymph node involvement is associated with poorer survival. However, in our current separate analysis of over 2563 patients with stage IIIC disease and nodal metastases, we were able to perform a detailed subset analysis showing that increasing numbers of metastatic lymph nodes (1, 2–5, and >5) is associated with a worsened survival (40.1, 37.0, and 35.6%, respectively).
Our analysis was limited by the lack of information on surgeon's subspecialty, volume of residual disease, medical comorbidities, location of nodal resection (pelvic vs paraaortic), adjuvant chemotherapy, and treatment of recurrence. In particular, the extent of extranodal residual disease in stage IIIC and IV patients and its potential impact on the extent of lymphadenectomy were not available in the SEER database. Nevertheless, even among those with stage IIIA disease, defined as microscopic disease in the upper abdomen, the extent of nodal dissection (6–10, 11–20, and >20 nodes) was associated with an improved survival from 61.5, 71.4, and 74.7%, respectively. Moreover, there was no central pathology review. Patients who had a less extensive lymphadenectomy may have had significant medical and/or surgical comorbidities, thus, representing patients with poor prognostic cancers. Furthermore, owing to the retrospective nature of this analysis, there may exist a selection bias where those patients who underwent a more extensive lymphadenectomy may have had less comorbidity, as well as having tumours with more favourable prognostic features. In addition, the extent of a lymphadenectomy may not be truly reflected by the reported number of recovered nodes in our study. Clearly, the extent of the nodal resection by the surgeon as well as comprehensive processing of the specimens by the pathologists influences nodal recovery. In addition, a more thorough lymphadenectomy may be a marker for quality comprehensive medical and surgical care rather than the procedure itself resulting in the improved survival of these patients. Lastly, there are certain patients in whom lymph node sampling or lymphadenectomy may not be feasible owing to comorbidity factors, blood loss, or body habitus. In a prospective randomised trial reported by Benedetti Panici et al (2005) women who underwent a systematic lymphadenectomy were found to have more postoperative complications, mostly consisting of lymphocytes or lymphoedema. Furthermore, the median operating time was 90 min longer and blood loss was 350 ml higher, with 12% more blood transfusions given when a systematic lymphadenectomy was performed.
There are several possible mechanisms that may explain the improvement in survival that was found to be associated with a more extensive lymphadenectomy in advanced cancers. A more complete lymphadenectomy is likely to remove occult microscopic disease, resulting in a more complete cytoreduction. In the randomised trial reported by Benedetti Panici et al (2005), patients with stage IIIB–C and IV epithelial ovarian cancer randomised to undergo systematic pelvic and paraaortic lymphadenectomy were found to have a statistically significant increase in positive lymph nodes compared to those randomised to resection of bulky nodes only (70 vs 42%; P<0.001). Thus, compared to those who had a limited lymphadenectomy, 28% more patients in the extensive lymphadenectomy arm benefited from cytoreduction of occult nodal metastases. A meta-analysis of the survival effect of maximum cytoreductive surgery in advanced ovarian carcinoma reported that each 10% increase in maximum cytoreduction was associated with a 5.5% increase in median survival time (Bristow et al, 2002). The magnitude of improved survival reported in our current study is consistent with these estimates, suggesting that the improvement in disease-specific survival may be associated with the removal of additional occult disease.
Furthermore, an extensive lymph node resection may lead to an improvement in survival by removing micrometastatic disease within the lymph nodes that may be resistant to chemotherapy. Prior studies on patients who underwent chemotherapy followed by second-look surgery showed that 33.3–65.3% of patients with advanced-stage disease had residual disease in the retroperitoneal lymph nodes (Burghardt and Winter, 1989; Baiocchi et al, 1998). These studies suggested that chemotherapy appears to have minimal effect on tumour deposit in the nodes; thus, retroperitoneal lymphadenectomy should be an integral component of ovarian cancer cytoreductive surgery.
In summary, our retrospective analysis suggests that the extent of lymphadenectomy is associated with an improvement in disease-specific survival in patients with advanced ovarian carcinoma. Furthermore, the extent of nodal disease provides additional prognostic information. Further trials are warranted to investigate the treatment of these high-risk patients with nodal metastases.
References
- Arango HA, Hoffman MS, Roberts WS, DeCesare SL, Fiorica JV, Drake J (2000) Accuracy of lymph node palpation to determine need for lymphadenectomy in gynecologic malignancies. Obstet Gynecol 95: 553–556 [DOI] [PubMed] [Google Scholar]
- Baiocchi G, Grosso G, di Re E, Fontanelli R, Raspagliesi F, di Re F (1998) Systematic pelvic and paraaortic lymphadenectomy at second-look laparotomy for ovarian cancer. Gynecol Oncol 69: 151–156 [DOI] [PubMed] [Google Scholar]
- Benedetti Panici P, Maggioni A, Hacker N, Landoni F, Ackermann S, Campagnutta E, Tamussino K, Winter R, Pellegrino A, Greggi S, Angioli R, Manci N, Scambia G, Dell'Anna T, Fossati R, Floriani I, Rossi RS, Grassi R, Favalli G, Raspagliesi F, Giannarelli D, Martella L, Mangioni C (2005) Systematic aortic and pelvic lymphadenectomy versus resection of bulky nodes only in optimally debulked advanced ovarian cancer: a randomized clinical trial. J Natl Cancer Inst 97: 560–566 [DOI] [PubMed] [Google Scholar]
- Bristow RE, Tomacruz RS, Armstrong DK, Trimble EL, Montz FJ (2002) Survival effect of maximal cytoreductive surgery for advanced ovarian carcinoma during the platinum era: a meta-analysis. J Clin Oncol 20: 1248–1259 [DOI] [PubMed] [Google Scholar]
- Burghardt E, Girardi F, Lahousen M, Tamussino K, Stettner H (1991) Patterns of pelvic and paraaortic lymph node involvement in ovarian cancer. Gynecol Oncol 40: 103–106 [DOI] [PubMed] [Google Scholar]
- Burghardt E, Lahousen M, Stettner H (1989) The significance of pelvic and para-aortic lymphadenectomy in the operative treatment of ovarian cancer. Baillieres Clin Obstet Gynaecol 3: 157–165 [DOI] [PubMed] [Google Scholar]
- Burghardt E, Pickel H, Lahousen M, Stettner H (1986) Pelvic lymphadenectomy in operative treatment of ovarian cancer. Am J Obstet Gynecol 155: 315–319 [DOI] [PubMed] [Google Scholar]
- Burghardt E, Winter R (1989) The effect of chemotherapy on lymph node metastases in ovarian cancer. Baillieres Clin Obstet Gynaecol 3: 167–171 [DOI] [PubMed] [Google Scholar]
- Carnino F, Fuda G, Ciccone G, Iskra L, Guercio E, Dadone D, Conte PF (1997) Significance of lymph node sampling in epithelial carcinoma of the ovary. Gynecol Oncol 65: 467–472 [DOI] [PubMed] [Google Scholar]
- Chan JK, Munro EG, Cheung MK, Husain A, Teng NN, Berek JS, Osann K (2007) Association of lymphadenectomy and survival in stage I ovarian cancer patients. Obstet Gynecol 109: 12–19 [DOI] [PubMed] [Google Scholar]
- Chen SS, Lee L (1983) Incidence of para-aortic and pelvic lymph node metastases in epithelial carcinoma of the ovary. Gynecol Oncol 16: 95–100 [DOI] [PubMed] [Google Scholar]
- Covens AL (2000) A critique of surgical cytoreduction in advanced ovarian cancer. Gynecol Oncol 78: 269–274 [DOI] [PubMed] [Google Scholar]
- Eisenkop SM, Spirtos NM (2001) The clinical significance of occult macroscopically positive retroperitoneal nodes in patients with epithelial ovarian cancer. Gynecol Oncol 82: 143–149 [DOI] [PubMed] [Google Scholar]
- Hankey BF, Ries LA, Edwards BK (1999) The surveillance, epidemiology, and end results program: a national resource. Cancer Epidemiol Biomarkers Prev 8: 1117–1121 [PubMed] [Google Scholar]
- Jemal A, Siegel R, Ward E, Murray T, Xu J, Smigal C, Thun MJ (2006) Cancer statistics, 2006. CA Cancer J Clin 56: 106–130 [DOI] [PubMed] [Google Scholar]
- Kikkawa F, Ishikawa H, Tamakoshi K, Suganuma N, Mizuno K, Kawai M, Arii Y, Tamakoshi A, Kuzuya K, Tomoda Y (1995) Prognostic evaluation of lymphadenectomy for epithelial ovarian cancer. J Surg Oncol 60: 227–231 [DOI] [PubMed] [Google Scholar]
- Maggioni A, Benedetti Panici P, Dell'Anna T, Landoni F, Lissoni A, Pellegrino A, Rossi RS, Chiari S, Campagnutta E, Greggi S, Angioli R, Manci N, Calcagno M, Scambia G, Fossati R, Floriani I, Torri V, Grassi R, Mangioni C (2006) Randomised study of systematic lymphadenectomy in patients with epithelial ovarian cancer macroscopically confined to the pelvis. Br J Cancer 95: 699–704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsden DE, Friedlander M, Hacker NF (2000) Current management of epithelial ovarian carcinoma: a review. Semin Surg Oncol 19: 11–19 [DOI] [PubMed] [Google Scholar]
- Onda T, Yoshikawa H, Yokota H, Yasugi T, Taketani Y (1996) Assessment of metastases to aortic and pelvic lymph nodes in epithelial ovarian carcinoma. A proposal for essential sites for lymph node biopsy. Cancer 78: 803–808 [DOI] [PubMed] [Google Scholar]
- Petru E, Lahousen M, Tamussino K, Pickel H, Stranzl H, Stettner H, Winter R (1994) Lymphadenectomy in stage I ovarian cancer. Am J Obstet Gynecol 170: 656–662 [DOI] [PubMed] [Google Scholar]
- Piver MS, Lele SB, Marchetti DL, Baker TR, Tsukada Y, Emrich LJ (1988) The impact of aggressive debulking surgery and cisplatin-based chemotherapy on progression-free survival in stage III and IV ovarian carcinoma. J Clin Oncol 6: 983–989 [DOI] [PubMed] [Google Scholar]
- Scarabelli C, Gallo A, Zarrelli A, Visentin C, Campagnutta E (1995) Systematic pelvic and para-aortic lymphadenectomy during cytoreductive surgery in advanced ovarian cancer: potential benefit on survival. Gynecol Oncol 56: 328–337 [DOI] [PubMed] [Google Scholar]
- Spirtos NM, Gross GM, Freddo JL, Ballon SC (1995) Cytoreductive surgery in advanced epithelial cancer of the ovary: the impact of aortic and pelvic lymphadenectomy. Gynecol Oncol 56: 345–352 [DOI] [PubMed] [Google Scholar]
- Surveillance, Epidemiology and End Results (SEER) program SEER*Stat Database: Incidence – SEER 9 Regs Public-Use, Nov 2004 Sub (1973–2002), National Cancer Institute, DCCPS, Surveillance Research Program, Cancer Statistics Branch http//:www.seer.cancer.gov
- Tangjitgamol S, Manusirivithaya S, Sheanakul C, Leelahakorn S, Sripramote M, Thawaramara T, Kaewpila N (2003) Can we rely on the size of the lymph node in determining nodal metastasis in ovarian carcinoma? Int J Gynecol Cancer 13: 297–302 [DOI] [PubMed] [Google Scholar]
- Trimbos JB, Vergote I, Bolis G, Vermorken JB, Mangioni C, Madronal C, Franchi M, Tateo S, Zanetta G, Scarfone G, Giurgea L, Timmers P, Coens C, Pecorelli S (2003) Impact of adjuvant chemotherapy and surgical staging in early-stage ovarian carcinoma: European Organisation for Research and Treatment of Cancer-Adjuvant ChemoTherapy in Ovarian Neoplasm trial. J Natl Cancer Inst 95: 113–125 [PubMed] [Google Scholar]