Abstract
LIM and SH3 protein 1 (LASP-1), initially identified from human breast cancer, is a specific focal adhesion protein involved in cell proliferation and migration. In the present work, we analysed the effect of LASP-1 on biology and function of human ovarian cancer cell line SKOV-3 using small interfering RNA technique (siRNA).Transfection with LASP-1-specific siRNA resulted in a reduced protein level of LASP-1 in SKOV-3 cells. The siRNA-treated cells were arrested in G2/M phase of the cell cycle and proliferation of the tumour cells was suppressed by 60–90% corresponding to around 70% of the cells being transfected successfully as seen by immunofluorescence. Moreover, transfected tumour cells showed a 40% reduced migration. LASP-1 silencing is accompanied by a reduced binding of the LASP-1-binding partner zyxin to focal contacts without changes in actin stress fibre and microtubule organisation or focal adhesion morphology as observed by immunofluorescence. In contrast, silencing of zyxin is not influencing cell migration and had neither influence on LASP-1 expression nor actin cytoskeleton and focal contact morphology suggesting that LASP-1 is necessary and sufficient for recruiting zyxin to focal contacts.The data provide evidence for an essential role of LASP-1 in tumour cell growth and migration, possibly through influencing zyxin localization.
Keywords: LASP-1, ovarian cancer, zyxin, 14-3-3, SKOV-3, migration
Ovarian cancer is the sixth most common cancer among women worldwide, with estimated 190 000 new cases and 114 000 deaths caused through this neoplasm each year (Parkin et al, 2001). Epithelial ovarian cancer represents 90–95% of all ovarian tumours (Auersperg et al, 2001; Quirk and Natarajan, 2005), which is detected in 60.6% cases in advanced stages of disease due to unspecific or absent symptoms in early stages. Although tremendous efforts have been undertaken to improve the therapeutic outcome, ovarian cancer still remains the most lethal malignoma among gynaecological tumours of women in the western world, given that only 35% of ovarian cancer patients show a 5-year survival (Legge et al, 2005).
About 90% of all epithelial ovarian tumours are sporadic and are diagnosed in women without germline mutations in known susceptibility loci. However, the remaining cases are heritable. Data from several international studies suggest that patients with a loss of heterozygosity of the BRCA1 and BRCA2 genes, which are located on 17q21 and found in hereditary forms of breast cancer, have a 6–61-fold increased lifetime risk of ovarian cancer compared with general population rates (Antoniou et al, 2003).
The Lim and SH3 domain protein LASP-1 was initially identified from a cDNA library of breast cancer metastases. The gene was also mapped to human chromosome 17q21 in a region that is altered in 20–30% of human breast cancers (Tomasetto et al, 1995a, 1995b), suggesting that it could play a role in tumour development and metastasis of breast and ovarian cancer.
Human LASP-1 encodes a membrane-associated protein of 261 amino acids containing an N-terminal LIM domain, followed by two actin-binding sites and a C-terminal src homology SH3 domain. The actin-binding domains in the core of the LASP-1 protein mediate an interaction between LASP-1 and actin at cell membrane extensions, but not along the actin stress fibres (Schreiber et al, 1998; Chew et al, 2002; Butt et al, 2003; Keicher et al 2004; Nakagawa et al, 2006). The exact cellular function of LASP-1 is still not known, but the protein has previously been reported to localise within multiple sites of dynamic actin assembly such as focal contacts, focal adhesions, lamellipodia, membrane ruffles and pseudopodia (Tomasetto et al, 1995a; Chew et al, 1998, 2000, 2002; Lin et al, 2004).
The SH3 domain at the C-terminus is involved in protein–protein interactions through binding to proline-rich sequences, specifically with zyxin, pallidin, lipoma preferred partner (LPP) and vasodilator-stimulated phosphoprotein (VASP) (Keicher et al, 2004; Li et al, 2004; Rachlin and Otey, 2006).
Moreover, recent data showed that LASP-1 specifically interacts via its nebulin-like repeats with Krp1, a focal adhesion protein involved in cell migration. Mutation analysis of LASP-1 demonstrates that its SH3 domain is necessary for pseudopodial extension and invasion (Spence et al, 2006). Thus the protein–protein interactions mediated by the LIM and SH3 domains can be regarded as scaffolds for the formation of complexes of higher order.
LASP-1 is a cAMP- and cGMP-dependent protein kinase substrate with a specific phosphorylation site on serine 146 (Butt et al, 2003). In rabbit parietal cells, elevation of intracellular cAMP by forskolin induced a partial translocation of LASP-1 to the apically directed F-actin-rich intracellular canaliculus, which is the site of active HCl secretion (Chew et al, 1998, 2000). Beyond this, phosphorylation on serine 146 resulted in translocation of the protein from the membrane to the cytosol and was followed by reduced cell migration (Butt et al, 2003). It has also been shown that the SH3 domain of LASP-1 interacts with the N-terminus of Ableson tyrosine kinase and that phosphorylation of LASP-1 at tyrosine 171 is associated with the loss of LASP-1 from focal adhesions and the initiation of cell death, but without changes in dynamic of migratory processes (Lin et al, 2004).
In addition, LASP-1 expression has been reported to be increased in metastatic breast cancer, suggesting that overexpression of LASP-1 may be involved in the migratory process of these cells (Tomasetto et al, 1995a). Surprisingly, both increase and depletion of LASP-1 in COS-7, HEK293 and MCF-7 cells inhibited basal and growth factor-stimulated cell migration (Lin et al, 2004).
Interestingly, recent work has shown, that knock-down of LASP-1 in metastatic breast cancer cell lines BT-20 and MCF-7 results in a strong inhibition of proliferation and migration and leads to a reduction of zyxin at focal contacts through absence of LASP-1 (Grunewald et al, 2006).
In this study we demonstrate that LASP-1 is highly overexpressed in ovarian cancer tissue and metastatic ovarian cancer cell lines. Silencing of the LASP-1 gene by RNA interference in the ovarian cancer cell line SKOV-3 reduced cell proliferation and cell migration in vitro without influencing the actin cytoskeleton, microtubule polymerisation and focal adhesion morphology. Furthermore, the knock-down of LASP-1 severely affected zyxin localisation.
MATERIALS AND METHODS
Tissue samples
The studies were performed with approval of the Ethics Committee of the University of Wurzburg. Tissue samples of 26 archival cases each of serous epithelial ovarian carcinomas with and without invasive components (obtained from the Department of Pathology of the University of Wurzburg and reviewed by a pathologist to confirm the diagnosis), as well as two samples of ascitic fluid containing ovarian cancer cells of women with metastatic ovarian cancer were analysed.
Immunohistochemistry
For immunohistochemical staining procedures, endogenous peroxidase was blocked by incubation in 0.1% hydrogen peroxide in PBS for 5 min. The slides were then incubated with the polyclonal anti-LASP-1 antibody (Butt et al, 2003) diluted 1 : 1000 in ‘antibody diluent’ (DAKO, Hamburg, Germany), followed by the EnVision/rabbit detection system (DAKO). Histogreen (Linaris, Wertheim, Germany) was used as the chromogen and cells were counterstained with haematoxylin (Sigma, Deisenhofen, Germany).
Cell culture conditions
Cell lines (SKOV-3, OAW-42 and PA-1) were obtained from the Cell Line Services (Heidelberg, Germany) and grown at 1 × 105 cells ml−1in a plastic cell culture flask in a humidified incubator at 37°C under 5% CO2 atmosphere in RPMI 1640 medium (PAA, Linz, Austria) containing 10% heat-inactivated foetal bovine serum (PAA) and 1% streptomycin/ampicillin (Invitrogen, Karlsruhe, Germany). For primary tumour cell culture, effusions (20–500 ml) were centrifuged, cell pellets washed twice in PBS (Biochrom, Berlin, Germany),resuspended in RPMI 1640 medium supplemented as described and then seeded on the bottom of a cell culture flask. After 1 h, all nonadherent (mostly leucocytes) cells were washed away and adherent tumour cells cultured in RPMI 1640/10%FCS/streptomycin/ampillicin. Contaminating fibroblasts were deleted by trypsin treatment every other day and the remaining tumour cell monolayer was cultured until homogeneous morphology of the cells (passage 3–4) was reached. Cells were checked routinely and found to be free of contamination by bacteria or fungi.
small interfering RNA preparation and transfection
Expression of human LASP-1 was knocked down with siRNA duplexes targeting the sequence 5′-AAG GTG AAC TGT CTG GAT AAG-3′ (bases 49–69), 5′-CUUAUCCAGACAGUUCACCdTdT-3′. The control siRNA 5′-AGAGAUGUAGUCGCUCGCUdTdT-3′ targeting no known mRNA sequence was used as a control. Both siRNAs were obtained from Dharmacon RNA Technologies (Lafayette, CO, USA). The siControl nontargeting siRNA from Dharmacon could not be used in our cell system owing to toxic effects. A BLAST search against the complete human and murine genome verified that the selected sequences were specific for the respective target gene.
Cells in the exponential phase of growth were plated in six-well plates at a density of 0.5 × 105 cells/well, grown for 24 h and transfected with 1 μg (60 nM) siRNA in reduced serum medium OPTI-MEM-I (Gibco, Paisley, UK) at 30–50% confluence. For the formation of the siRNA–lipid complexes, 3 μl siRNA stock solution (20 μM) was diluted in 100 μl OPTI-MEM, mixed with 3 μl Metafectene (Biontex, Munich, Germany) in 100 μl OPTI-MEM and incubated at room temperature for 17 min. Subsequently, the culture medium was removed and replaced by 1 ml OPTI-MEM-I and the siRNA–lipid complexes (1.2 ml total volume). After 4 h incubation at 37°C, 1.2 ml of cell culture medium with 20% foetal bovine serum was added, and incubation was continued for 36–56 h. For control cells, Metafectene alone (MOCK-transfection) and/or 1 μg scrambled control-siRNA were used.
Silencing of zyxin was achieved with Hs_ZYX_1_HP validated siRNA at a final concentration of 10 nM using HiPerfect transfection reagent (Qiagen, Hilden, Germany) according to the manufacturer's directions. For zyxin control experiments, nonsilencing control siRNA, Alexa Fluor 488 labelled, provided by Quiagen, was used.
At least three independent experiments were performed for each cell line, and representative results are shown.
Cell proliferation assay
For proliferation assay, cells were transfected as described. At the time points indicated, cells were trypsinised and cell numbers were determined by a Coulter counter (Beckman, Fullerton, CA, USA). Experiments were performed in triplicate for each time point. Cell viability was evaluated by counting trypan blue-positive and -negative cells under a phase-contrast microscope (Zeiss Axiovert, Aalen, Germany).
Western blot analysis
For Western blotting, cells were prepared by lysing in Laemmli sample buffer and equal amounts of protein, according to the cell count, were resolved by 12% SDS–PAGE. After blotting on nitrocellulose membrane and blocking with 3% nonfat dry milk in 10 mM Tris, pH 7.5, 100 mM NaCl and 0.1% (w/v) Tween 20, the membrane was first incubated with the antibodies raised against LASP-1 (1 : 10 000) (Butt et al, 2003), caspase-3 (1 : 1000) (New England Biolabs, Frankfurt, Germany) or mouse zyxin hybridoma supernatant (1 : 100; kind gift from Dr J Wehland, GBF Braunschweig, Germany; Rottner et al, 2000), followed by incubation with horseradish peroxidase-coupled goat anti-rabbit IgG or goat anti mouse IgG (Biorad, Munich, Germany), diluted 1 : 5000 and detection by ECL or ECL plus (Amersham Biosciences, Freiburg, Germany). Protein bands were visualised by autoradiography. Quantification of the signals was carried out by densitometry using the Odyssey system (Li-Cor, Bad Homburg, Germany).
FACS
For cell cycle analysis, SKOV-3 cells were harvested 48 h after LASP-1 siRNA transfection. The cells were pelleted and stained with DAPI (Sigma) at a final concentration of 2 μg ml−1 in permeabilisation buffer containing 0.1 M Tris—HCl, pH 7.4, 0.154 M NaCl, 0.5 mM MgCl2, 1 mM CaCl2, 0.1% NP-40 and 0.2% BSA in ddH2O for 30 min at 4°C in the dark. Bivariate flow histograms were recorded on an analytical, dual-laser-equipped cytometer (LSR1, Becton Dickinson Biosciences, Heidelberg, Germany) using UV excitation. Resulting cell cycle distributions were quantified with the MPLUS AV software (Phoenix Flow Systems, San Diego, CA, USA). For technical details, see Schindler and Hoehn (1999).
Two-dimensional-gelelectrophoreses and mass spectrometry
Isoelectric focusing for two-dimensional (2D) gel electrophoresis was performed using the Protean IEF cell from Biorad (Munich, Germany) according to the instructions of the manufacturer. The SKOV-3 homogenate (about 200 μg protein) was solubilised for 15 min by sonication in 320 μl lysis buffer containing 7 M urea, 2 M thiourea, 4% (w/v) CHAPS, 15 mM DTT (electrophoresis grade), 0.5% carrier ampholytes, pH 3–10. Pellet homogenate was loaded on a 17-cm immobilised IPG strip, pH 3–10 and resolved overnight at 50 V. Focussing was carried out for 1 h at 250 V, 1 h at 500 V and 15 h at 7000 V. After equilibration in 50 mM Tris, pH 8.9, 6 M urea, 30% (w/v) glycerol and 2% (w/v) SDS, gels were immediately applied to a vertical 10% SDS gel without a stacking gel. Electrophoresis was carried out at 8°C with a constant current of 40 mA per gel. Proteins were visualised by Coomassie Brilliant Blue R-250 (Sigma) staining.
Gel pieces were washed two times alternating with 50 mM ammonium hydrogen carbonate buffer and 25 mM ammonium hydrogen carbonate buffer with 50% acetonitrile. Proteins were reduced with 10 mM DTT for 30 min at 56°C and subsequently alkylated by incubation with 20 mM iodoacetamide at room temperature for 30 min. Again samples were washed as described before. Gel pieces were shrunken in a SpeedVac (Thermo Electron, Dreieich, Germany) and rehydrated with 12.5 ng of trypsin in 50 mM ammonium hydrogen carbonate buffer. Digestion was performed by incubation at 37°C overnight. The resulting peptides were extracted by application of 15 μl of 5% formic acid for 10 min.
Separation of complex peptide mixtures was achieved by using reversed-phase chromatography. For nano-LC-ESI-MS/MS experiments, a setup consisting of an autosampler (Famos, Dionex, Idstein, Germany) and precolumn concentration (Switchos, Dionex) before nano-LC separation (Ultimate, Dionex) was used. Precolumns (300-μm inner diameter × 1-mm length) and separation columns (75 μm inner diameter × 150-mm length, C18 PepMapTM) were purchased from Dionex. Gradient elution was performed using a linear gradient from 5 to 50% solvent B (84% acetonitrile, 0.1% formic acid) during a period of 2 h. Solvent A was 0.1% formic acid in water. Separation was followed by rinsing the column with 95% B for 5 min before equilibration to 5% solvent B before the next separation cycle.
Peptides were directly eluted into an ESI mass spectrometer. For mass spectrometric analysis, an ESI linear ion trap LTQ (Thermo Electron, Dreieich, Germany), using distal-coated fused silica tips (New Objective, Woburn, MA, USA), spray voltage was set around 1800 V. A survey scan (m/z 350–2000) was followed by five MS/MS scans fragmenting the five most intensive peptide signals.
Mass spectra were transformed into peak lists in dta or mgf format using the wo in-house software solution raw2dta (Boehm et al, 2004). Generated data were processed in parallel with the search algorithms SequestTM, Version 27 (Yates et al, 1995) and MascotTM, Version 2.1.6 (Perkins et al, 1999). For sequence alignment, the swissprot database from October 2005 was used. As fixed modification, carbamidomethylation of cysteine residues was used, and as variable modification oxidation of methionine residues was selected. As filter criteria for Sequest we accepted in the first instance only positive peptide hits with a minimum cross-correlation factor of 2.5, a CN value of 0.25, and a preliminary ranking of one. For the Mascot algorithm the minimum score was set to 40 for each peptide. Only protein hits that were identified with these parameters by both algorithms and had at minimum two identified peptides were accepted. Additionally, all significant hits were revised manually.
Immunfluorescence
For immunfluorescence microscopy, cells were grown on glass chamber slides, fixed in 4% (w/v) paraformaldehyde in PBS, permeabilised with 0.1% (w/v) Triton X-100 in PBS and then stained with affinity-purified LASP-1 antibody (1 : 2000, 1 h), followed by secondary Cy3-labelled anti-rabbit antibody (1 : 500, 30 min) (Dianova, Hamburg, Germany) or mouse zyxin hybridoma supernatant (1 : 10, 2 h), followed by secondary Cy2 labelled goat-anti mouse antibody (1 : 500, 1 h) (Dianova). Oregon green phalloidin (Molecular Probes, Leiden, The Netherlands) was used for actin staining. Tubulin was stained with an anti-α-tubulin antibody (3 μg ml−1) (Calbiochem, Darmstadt, Germany). DNA was counterstained with DAPI (1 : 2500) (Calbiochem) for 2 min.
Migration experiments
Cells were cultured in medium in 25 cm2 flasks to approximately 30–40% confluence and transfected with 10 nM zyxin siRNA or 30 nM LASP-1 siRNA (Keicher et al, 2004) using 10 μl Metafectene. After 48 h incubation and overnight starving, 1 × 105 cells in 100 μl incubation medium (with 1 mM MgCl2) were seeded in the upper chamber of BSA-coated 8 μM pore size transwell Boyden chambers (Corning star, Cambridge, MA, USA). Cells were allowed to migrate through the porous membrane for 4 h at 37°C. Cells remaining at the upper surface were completely removed using a cotton carrier. The lower surfaces of the membranes were then stained in a solution of 1% (w/v) crystal violet in 2% ethanol for 30 s and rinsed afterwards in distilled water. Cell-associated crystal violet was extracted by incubation in 10% acetic acid for 20 min and measured at 595 nm absorbance.
RESULTS
LASP-1 is overexpressed in ovarian cancer tissue
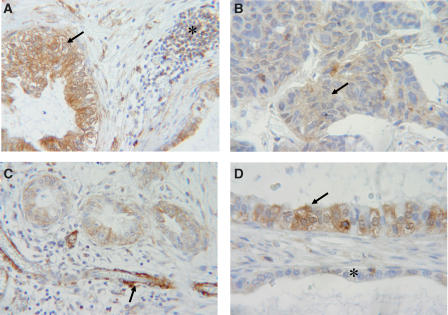
To assess the role of LASP-1 in ovarian cancer, we examined its expression in 26 ovarian cancer samples from different patients with or without invasive components. Immunohistochemistry clearly allowed to localise LASP-1 expression in 14 out of 26 malignant ovarian tissues (53.8%). Strong immunoreactivity was observed in nine cases (Figure 1A), whereas five probes showed a medium to low LASP-1 expression (Figure 1B) and 12 specimens (46.2%) were considered to be LASP-1 negative.
Figure 1.
Immunohistochemical staining of cancerous ovarian tissue. LASP-1 was detected using anti-LASP-1 rabbit polyclonal antibody in paraffin-embedded tissue samples. (A) Ovarian cancer tissue with two cystic structures containing malignant cells, which are strongly LASP-1 positive (arrow), and an LASP-1-negative lymphocytic inflammation (star). (B) Infiltrating tumour cells displaying medium LASP-1 expression (arrow). (C) Three ductus with malignant epithelial cells and medium LASP-1 expression and a blood vessel in longitudinal cut with vascular smooth muscle cells showing strong LASP-1 positivity (arrow). (D) Two epithelial strata separated through connective tissue. The arrow indicates malignant LASP-1-positive and the star benign LASP-1-negative epithelial cells. LASP-1+ cells are stained in brown (DAB). All sections were counterstained with haematoxylin.
Normal benign epithelial cells were LASP-1-negative in all ovarian tissues even when malignant epithelial cancer cells close to these normal epithelial cells displayed a strong positivity for LASP-1 (Figure 1D).
In analogy to previous findings in myoepithelial cells of human breast tissue (Grunewald et al, 2006), a massive overexpression in vascular smooth muscle cells could be observed (Figure 1C).
LASP-1 is strongly expressed in ovarian cancer cell lines
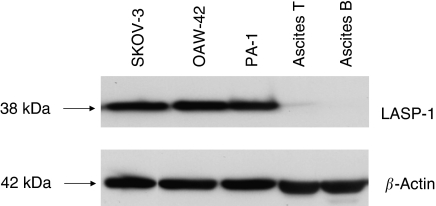
In order to study the significance of LASP-1 overexpression in ovarian cancer, we tested three ovarian cancer cell lines (SKOV-3, OAV-42 and PA-1) as well as two primary cell cultures derived from ascitic fluid of patients with peritoneal metastatic ovarian cancer for LASP-1 expression. Loading was standardised to 3 × 105 cells per slot and controlled by β-actin loading control signal intensity.
Only the three ovarian cancer cell lines showed a high LASP-1 signal, whereas the two primary cell lines were LASP-1 negative (Figure 2). Interestingly, the solid primary ovarian cancer tissue preparations of these two patients showed intensive LASP-1 staining (data not shown).
Figure 2.
Western blot of different ovarian cancer cell lines. A total of 200 000 cells from established ovarian cancer cell lines (SKOV-3, OAW-42 and PA1), as well as primary cells derived from ascitic fluid of two patients (ascitis T and B) with peritoneal metastatic ovarian cancer, were analysed for LASP-1 expression by Western blot. Loading was controlled by β-actin Western blot.
We chose SKOV-3 cells as a cellular model for ovarian cancer because in these cells the BRCA1 and BRCA2 genes, which are located next to the LASP-1 gene on chromosome 17q21, are upregulated (Rauh-Adelmann et al, 2000).
Silencing of LASP-1 in SKOV-3 cells inhibits proliferation in vitro
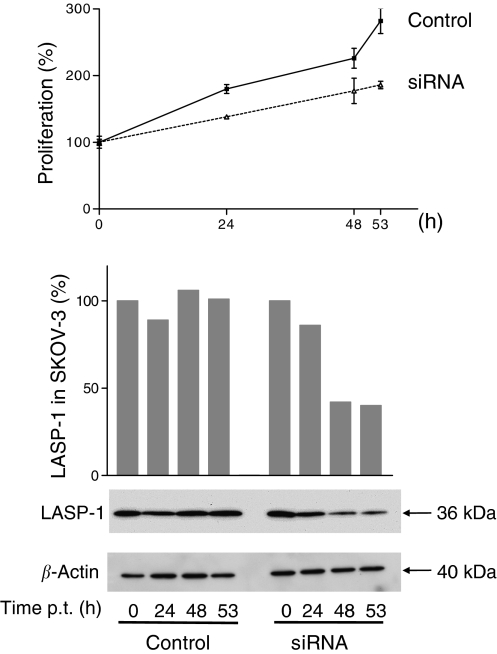
To investigate the function of LASP-1 in the ovarian cancer cell line SKOV-3, we performed a knock-down of the gene using the powerful RNAi technique. The effect of siRNA transfection on the expression of LASP-1 was followed by Western blot analysis 0, 24, 48 and 53 h after transfection. The amount of LASP-1 protein, standardised to β-actin, was reduced up to 58% after 48 h (Figure 3, lower panel) compared with the control siRNA-transfected control cells. This corresponds to around 70% of the cells being transfected successfully as seen by immunofluorescence. Parallel to the protein knock-down, we observed a slower proliferation rate in LASP-1 siRNA-transfected cells compared with control cells (Figure 3, upper panel). The viability of cells was similar in both cultures as trypan blue staining detected no more than 5–8% dead cells in all experiments. To test whether the decreased proliferation could be due to apoptosis, we carried out a Western blot analysis with an anti-caspase-3 antibody. The antibody recognises both the non-active pro-caspase-3 (38 kDa) and the active cleaved caspase-3 protein (17 kDa). As shown in Figure 4B, treatment of SKOV-3 cells with the LASP-1 siRNA duplex produced no active caspase-3, indicating that apoptosis might not explain the reduced proliferation.
Figure 3.
Silencing of LASP-1 in SKOV-3 cells inhibits proliferation. A total of 40 000 cells of the SKOV-3 cells were plated and allowed to grow for 24 h (up to 40% confluence). Small interfering RNA LASP-1 was transfected into cells in a concentration of 60 nM. Cells were harvested after 0, 24, 44 and 53 h of siRNA treatment. Control cells were treated with control siRNA. Upper panel: treatment with siRNA LASP-1 impairs SKOV-3 cell proliferation. After the indicated periods of time, the cells were harvested, and their total number was determined using a Coulter counter. Lower panel: Densitometric quantification and Western blot analysis of LASP-1 expression standardised to β-actin at the corresponding time points shows a reduction of LASP-1 expression of about 60% 44 h after transfection with siRNA LASP-1.
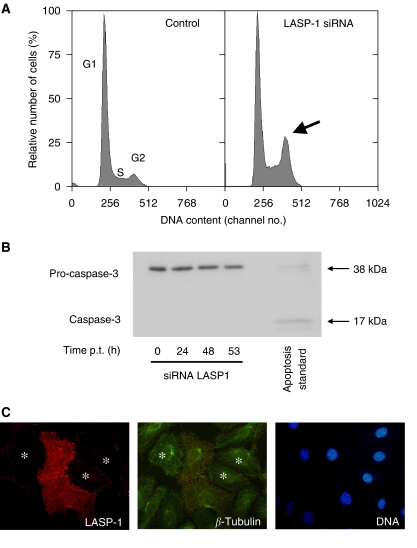
Figure 4.
LASP-1 siRNA treatment of SKOV-3 cells induces G2 phase accumulation without triggering apoptosis. (A) SKOV-3 cells were transfected with siRNA LASP-1 and transfection reagent Metafectene or treated only with Metafectene (MOCK transfection). After 48 h, the cells were harvested and prepared for flow cytometric cell cycle analysis. LIM and SH3 protein 1 (LASP-1) siRNA-treated cells show G2 phase accumulation as opposed to the same cells with MOCK transfection. (B) Several hours after siRNA LASP-1 treatment, cells were harvested and prepared for Western blot analysis using an anti-caspase-3 antibody, the antibody recognises both the nonactive pro-caspase-3 (38 kDa) and the active cleaved caspase-3 protein (17 kDa). No active caspase-3 could be detected. (C) Immunfluorescence of LASP-1 (red), α-tubulin (green) and DNA (blue) of siRNA LASP-1-transfected (*) and nontransfected SKOV-3 cells.
Downregulation of LASP-1 induces G2 phase accumulation in SKOV-3 cells
We next analysed cell cycle distributions of siRNA-treated SKOV-3 cells using flow cytometry. After incubation with LASP-1 siRNA for 48 h, the proportion of cells accumulating in the G2 phase amounted to 19.4% (Figure 4A), whereas the same cells treated with Metafectene alone (MOCK transfection) as a control had only 6.7% G2 phase proportion. Conversely, the G1 fraction decreased from 73.4% in MOCK-treated cells to 48% in LASP-1-silenced SKOV-3 cells. The S phase fraction for siRNA LASP-1-treated cells was 32.6% and for MOCK transfection 19.9%, respectively. Similar results were obtained in three independent experiments indicating that in LASP-1-silenced SKOV-3 cells mitotic progression cannot proceed normally. However, immunfluorescence staining of α-tubulin and DNA in the cells reveald no reduced tubulin polymerisation in the LASP-1-silenced cells arrested in G2/M phase (Figure 4C).
Knock-down of LASP-1 results in protein changes of glycolytic metabolism and cell cycle regulation
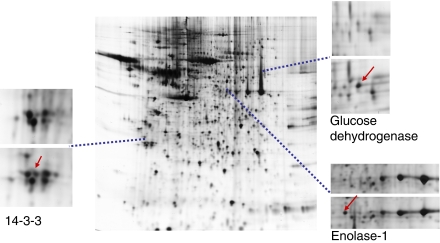
Under LASP-1 knock-down conditions it might be necessary for the maintenance of cellular steady state to upregulate alternative proteins to overcome functionally the loss of LASP-1. We used 2D gel electrophoreses to resolve the homogenate of SKOV-3 cells before and after LASP-1 silencing. Subtractive analysis of the two gels showed a high degree of similarity, however, at least five proteins have been identified to become up-, or respectively downregulated by LASP-1 silencing in three independent experiments: pyruvate kinase (up), enolase-1 (down), glucose dehydrogenase (down), 14-3-3 (up) and heat-shock protein (Hsp) 27 (up) (Figure 5).
Figure 5.
Two-dimensional gel of SKOV-3 proteins after LASP-1 silencing. Sections are showing differences between cellular proteins from control (upper panel) and LASP-1 knock-down (lower panel) SKOV-3 cells. Proteins were separated on nonlinear IPG-strips, pH 3–10. Two dimensional gels were stained with Coomassie blue. Proteins were identified following tryptic digestion and analysis of the resulting peptides by ESI-MS/MS.
Silencing of LASP-1 results in reduced zyxin binding to focal adhesions
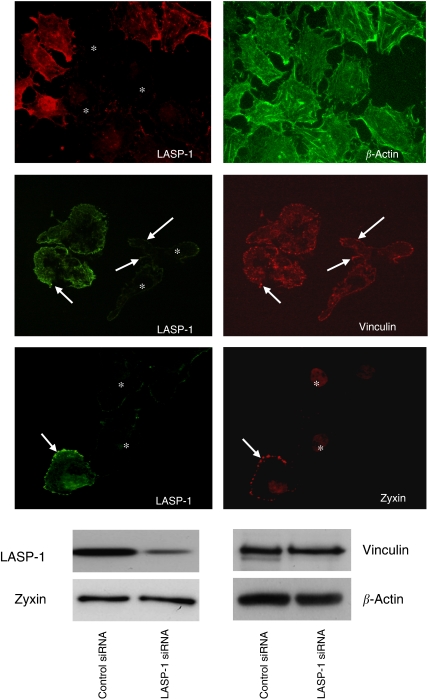
LASP-1 has previously been shown to localise to sites of cell adhesion and to interact with zyxin and actin (Chew et al, 2002; Li et al, 2004). To assess whether silencing of LASP-1 affects these binding partners, siRNA-treated SKOV-3 cells were stained with phalloidin green against actin or mouse anti-zyxin hybridoma supernatant. In LASP-1 siRNA-transfected cells, zyxin was absent from focal adhesions, whereas the cellular level of zyxin remained unchanged as confirmed by Western blot analysis (Figure 6). However, absence of zyxin from focal contacts did not lead to changes in focal adhesion morphology as visualized by vinculin staining (Figure 6). Likewise, actin filament assembly was not disturbed (Figure 6), despite less actin bundles, a blurred network of shorter filaments and some F-actin aggregates are typical for highly metastatic cancer cell lines (Liu et al, 2004).
Figure 6.
LIM and SH3 protein 1 (LASP-1) is required for zyxin localisation at focal adhesions. Immunfluorescent images of LASP-1, zyxin, vinculin and β-actin in siRNA LASP-1-treated SKOV-3 cells. Focal adhesions are marked with white arrows. Positions of downregulated cells are marked with stars. Western blot (WB) analysis to assess LASP-1 and zyxin levels in the LASP-1 siRNA and control siRNA-treated SKOV-3 cells were performed from the corresponding immunofluorescent cell extracts.
Silencing of zyxin does not change LASP-1 localisation or focal adhesion morphology
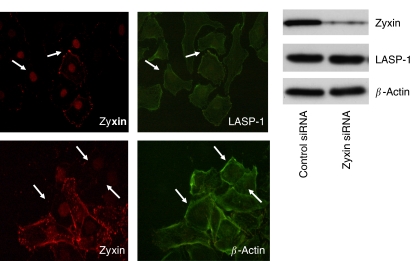
As knock-down of LASP-1 is altering zyxin localisation, we further assessed the interaction of both proteins in a reverse experiment by knocking down zyxin. Transfection of SKOV-3 with zyxin-specific siRNA dramatically reduced zyxin expression down to 10–20%, while the cellular level of LASP-1 and β-actin remained unchanged as confirmed by Western blot analysis (Figure 7). Immunofluorescence of the zyxin-silenced cells illustrated lack of zyxin at the focal adhesions without altering the position of LASP-1 (Figure 7), suggesting that LASP-1 is necessary for the positioning and recruiting of zyxin to focal adhesions. Other focal adhesion proteins, for example, β-actin (Figure 7) and vinculin (data not shown), were unaffected by the zyxin knock-down.
Figure 7.
Zyxin silencing is not influencing actin and LASP-1 localisation. Immunoflourescent images of SKOV-3 cells transfected with siRNA zyxin and stained with antibodies against LASP-1 and β-actin. Shown are representative sections of a mixed population of both, zyxin downregulated cells and nontransfected cells, demonstrating-no changes in actin and LASP-1 distribution of cells lacking zyxin.
Silencing of LASP-1, but not of zyxin, decreases cell migration
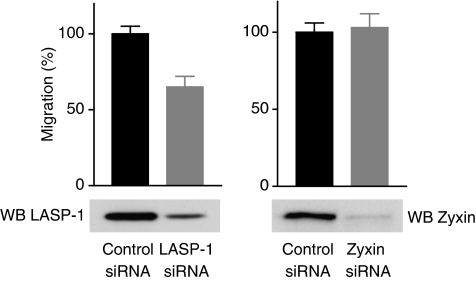
Although the exact function of LASP-1 is not known, recent results suggest an important role for this protein in cell adhesion and migration (Butt et al, 2003; Lin et al, 2004; Grunewald et al, 2006; Nakagawa et al, 2006). To directly examine the relevance of LASP-1 for cell motility we performed migration experiments in a modified Boyden chamber with SKOV-3 cells either transfected with LASP-1 siRNA or zyxin siRNA to downregulate the respective protein. Cells were seeded in the upper chamber of a transwell polycarbonate membrane. After 4 h, those cells that had migrated through the porous membrane were counted. Depletion of LASP-1 in SKOV-3 cells strongly reduced cell migration, while zyxin knock-down had no influence on cell migration (Figure 8) suggesting that LASP-1 acts as a positive regulator for cell motility.
Figure 8.
LIM and SH3 protein 1 is necessary for cell migration. SKOV-3 cells were transfected with LASP-1 siRNA, zyxin siRNA or control siRNA. Migration was measured over 4 h in a Transwell® cell culture chamber. At least four chambers from three different experiments were analysed (P-values significantly different from that of Control by t-test; P<0.001). Each bar represents the mean±s.d. Corresponding Western blots of control cells and LASP-1 siRNA-transfected or zyxin siRNA-transfected cells are shown in the lower panel.
DISCUSSION
Cell migration and controlled assembly and disassembly of focal adhesions are highly integrated multistep processes and a central feature in the molecular pathology of cancer (Ridley et al, 2003). To date, more than 50 different adhesion proteins that regulate the rate and organisation of actin polymerisation and focal adhesion turnover in protrusion have been identified.
In earlier publications, overexpression of LASP-1 mRNA in metastatic lymph nodes derived from breast cancer patients, as well as the co-amplification of the gene together with HER-2/neu (c-erbB2) were demonstrated (Chew et al, 1998; Legge et al, 2005). Two additional observations underscore the importance of LASP-1 in cancer. First, altered expression of LASP-1 is associated with the MLL gene in acute myeloid leukaemia (Strehl et al, 2003). Second, recent studies have shown LASP-1 to be transcriptionally upregulated in response to the morphogen Sonic Hedgehog (Ingram et al, 2002).
Consistent with these data, we just recently described the overexpression of LASP-1 to very high levels in breast carcinomas and lymph node metastases (Grunewald et al, 2006). The functional significance of LASP-1 in cancer metastasis is further supported by the presented data showing high LASP-1 expression in ovarian cancer tissue and reduced cell migration in ovarian cancer cells depleted of LASP-1. The absence of LASP-1 in cultures of primary ovarian cancer cells in contrast to established cell lines may reflect a downregulation of LASP-1 in the nonmigratory floating ascites cells which will be reverted after several passages of adherent cell culture. Comparable observations are published for LASP-1 in human mesenchymal stem cells showing an upregulation of the protein during later passages (Sun et al, 2006).
During LASP-1 silencing we observed reduced cell cycle progression and an induced G2/M phase accumulation of the cells without disrupted normal mitotic microtubule polymerisation. This was accompanied by the upregulation and downregulation of several proteins. The differentially expressed proteins pyruvate kinase, enolase-1 and glucose dehydrogenase are part of the glycolytic metabolism and their regulation correlates well with the cell cycle arrest in G2/M after LASP-1 silencing. Furthermore, pyruvate kinase and glucose dehydrogenase have been suspected to be highly important for tumour cell metabolism (Altenberg and Greulich, 2004). Pyruvat kinase is one of the proteins to be upregulated in cancer cells gaining energy by means of aerobic glycolysis, which is a characteristic of a number of cancer entities (Gatenby and Gillies, 2004). In addition, pyruvat kinase has been identified as a proteomic marker of cancer progression in breast cancer (Isidoro et al, 2005). The glycolytic enzyme enolase-1 as well as HSP27, two additional proteins identified in the 2D-gel experiments, are associated with high metastatic activity in breast cancer cells (Espana et al, 2005; Zhang et al, 2005).
14-3-3, found to be upregulated after LASP-1 depletion in ovarian cancer cells, has been implicated in cell cycle deregulation. The 14-3-3 proteins are a family of highly conserved DNA-binding proteins, which associate with the centrosomes during mitosis and are inhibitors of G2/M progression at the mitotic and G2 cell cycle checkpoint (Pietromonaco et al, 1996; Wang and Shakes, 1996; Hermeking et al, 1997; Peng et al, 1997; Alvarez et al, 2002). Overexpression of 14-3-3 led to cell cycle arrest in cell culture models (Tzivion et al, 2006) and, therefore, might contribute to the observed G2 arrest in ovarian cancer cells lacking LASP-1.
Heat-shock proteins are molecular chaperons and are induced during cellular stress. Upregulation of HSP27 after LASP-1 silencing correlates well with increased survival by inhibiting key effectors of the apoptotic pathway (Concannon et al, 2003).
So far, the identified proteins are regulated in response to cell cycle arrest, but do not substitute for LASP-1 after silencing.
Recently, several LASP-1-binding partners have been identified. Along with zyxin (Li et al, 2004) and actin (Schreiber et al, 1998), LASP-1 interacts with Krp1 (Spence et al, 2006), palladin (Rachlin and Otey 2006), lipoma-preferred partner (LPP) and VASP (Keicher et al, 2004), which all can influence actin filament dynamics and pseudopodial elongation. In the case of palladin, LPP and zyxin, the binding occurs between the C-terminal SH3 domain of LASP-1 and the N-terminal proline-rich domains of these proteins, whereas in the case of Krp1, binding is observed between the nebulin-like repeats of LASP-1 and the N-terminal BTB/POZ domain of Krp1. The interaction of LASP-1 and Krp1 is crucial for pseudopodial elongation in fibroblasts in absence of fibronectin and results in their colocalisation with F-actin at the tips of extending pseudopodia (Spence et al, 2006).
Zyxin is localised primarily at focal adhesion plaques and plays a central role in actin filament polymerisation in mammalian cells (Beckerle, 1997).
Silencing of zyxin in HeLa cells resulted in significantly reduced actin stress fibres (Griffith et al, 2005), whereas under cyclic stretch zyxin only dissociated from focal contacts and accumulated in the nucleus, without affecting vinculin or actin filaments (Cattaruzza et al, 2004). Recent data show that in genetically zyxin-deficient fibroblasts, focal adhesions are depleted from Mena and VASP, and that cells lacking zyxin display deficits in actin cytoskeleton remodelling (Hoffman et al, 2006). In our immunflourescence experiments with LASP-1-deficient SKOV-3 cells, we observed a diffuse cytoplasmic localiation of zyxin without protein loss and without changes in neither vinculin distribution nor actin stress fibre organisation, emphasising the importance of LASP-1 for binding and recruiting zyxin to focal adhesions.
The loss of zyxin at the sites of focal contacts without changing cellular zyxin protein levels is not restricted to cancer cells, but was also observed in human umbilical vein endothelial cells (Grunewald et al, 2006). Interestingly, in these cells, zyxin could still be detected along the actin stress fibres, indicating the potential existence of another zyxin-recruiting protein along actin stress fibres since earlier results detected LASP-1 only in the focal adhesion plaques (Chew et al, 2002; Butt et al, 2003).
In our zyxin knock-down experiments, neither changes in LASP-1 localisation, actin cytoskeleton, microtubule polymerisation nor vinculin distribution were detectable suggesting that zyxin does not change focal adhesion morphology. This is concordant with the fact that genetically zyxin-deficient fibroblasts show even enhanced adhesion to surface and increased integrin expression (Hoffman et al, 2006). In synopsis, our LASP-1 and zyxin silencing studies have demonstrated that LASP-1 is necessary for recruiting zyxin to focal contacts.
The decreased cell motility after LASP-1 silencing can be explained by the functional loss of zyxin as a scaffolding protein that facilitates the formation of molecular complexes to promote site-specific actin assembly required for cell migration. This is in agreement with previous findings using a nongenetic approach and injecting a peptide derived from the N-terminus of zyxin to displace zyxin from its normal subcellular location thus leading to reduced cell migration (Drees et al, 1999). On the other hand, the knock-down of zyxin in SKOV-3 cells had no influence on cell migration while genetically zyxin-deficient fibroblasts display enhanced migration (Hoffman et al, 2006). These contrary effects have not been fully elucidated yet.
Recent work has shown that zyxin also shuttles through the nucleus – most likely by association with other LIM proteins – and may regulate gene transcription (Nix et al, 2001; Wang and Gilmore, 2003; Kadrmas and Beckerle, 2004). During mitosis, a fraction of zyxin becomes associated with the tumour suppressor h-warts at the mitotic apparatus (Hirota et al, 2000). h-warts is a key player in mitosis in mammalian cells and loss of its function disrupts normal cell cycle regulation, possibly leading to tumour development (Iida et al, 2004). In SKOV-3 cells transfected with LASP-1 siRNA, zyxin has been shown to dissociate from focal adhesion plaques and to distribute diffusely into the cytoplasm. It is, therefore, likely that part of zyxin enters the nucleus, binds to h-warts and leads to G2 cell cycle arrest and inhibition of proliferation as observed after LASP-1 silencing.
Interestingly, in Ewing tumour cells, zyxin is only expressed at very low levels and remains diffusely distributed throughout the cytoplasm instead of concentrating in actin-rich dynamic structures. Zyxin gene transfer into EWS-FLI1-transformed fibroblasts elicits reconstitution of zyxin-rich focal adhesions and leads to decreased cell motility and inhibition of anchorage independent tumour growth, indicating that zyxin has tumour suppressor activity in these cells (Amsellem et al, 2005).
Similar to findings in human breast cancer (Grunewald et al, 2006), our immunofluorescence experiments have shown that absence of LASP-1 in focal contacts dramatically influences zyxin distribution. In reverse, tumour cells, that are overexpressing LASP-1, could functionally inhibit zyxin from shuttling into the nucleus and acting as a tumour suppressor through increased recruiting of zyxin to focal contacts by LASP-1. In summary, our observations suggest an expanded role for LASP-1 in proliferation and cancer cell migration. Further studies will define the potential of LASP-1 as an independent marker for diagnosis of cancer, as well as a marker for prognosis of this disease.
Acknowledgments
We thank Richard Friedl for expert flow cytometric measurements, Michaela Kapp, Elfriede Schulze and Sabrina Jaspers for excellent technical assistance and Matthias Eck (Department of Pathology, University of Wurzburg) for pathohistological guidance. This study was supported by Deutsche Krebshilfe 106219 to EB and AH.
References
- Altenberg B, Greulich KO (2004) Genes of glycolysis are ubiquitously overexpressed in 24 cancer classes. Genomics 84: 1014–1020 [DOI] [PubMed] [Google Scholar]
- Alvarez D, Novac O, Callejo M, Ruiz MT, Price GB, Zannis-Hadjopoulos M (2002) 14-3-3 sigma is a crciform DNA binding protein and associates in vivo with origins of DNA replication. J Cell Biochem 87: 197–207 [DOI] [PubMed] [Google Scholar]
- Amsellem V, Kryszke MH, Hervy M, Subra F, Athman R, Leh H, Brachet-Ducos C, Auclair C (2005) The actin cytoskeleton-associated protein zyxin acts as a tumour suppressor in Ewing tumour cells. Exp Cell Res 304: 443–456 [DOI] [PubMed] [Google Scholar]
- Antoniou A, Pharoah PD, Narod S, Risch HA, Eyfjord JE, Hopper JL (2003) Avarage risk of breast and ovarian cancer associated wti BRCA1 and BRCA2 mutations detected in case series unselected for family history: a combined analysis of 22 studies. Am J Hum Genet 72: 1117–1130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auersperg N, Wong AS, Choi KC, Kang SK, Leung PC (2001) Ovarian surface epithelium: biology, endocrinology, and pathology. Endocr Rev 22: 255–288 [DOI] [PubMed] [Google Scholar]
- Beckerle MC (1997) Zyxin: zinc fingers at sites of cell adhesion. Bioessays 19: 949–957 [DOI] [PubMed] [Google Scholar]
- Boehm AM, Galvin RP, Sickmann A (2004) Extractor for ESI quadrupole TOF tandem MS data enabled for high throughput batch processing. BMC Bioinformatics 5: 162–166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butt E, Gambaryan S, Göttfert N, Galler A, Marcus K, Meyer HE (2003) Actin binding of human LIM and SH3 protein is regulated by cAMP- and cGMP-dependent protein kinase phosphorylation on serine 146. J Biol Chem 278: 15601–15607 [DOI] [PubMed] [Google Scholar]
- Cattaruzza M, Lattrich C, Hecker M (2004) Focal adhesion protein zyxin is a mechanosensitive modulator of gene expression in vascular smooth muscle cells. Hypertension 43: 726–730 [DOI] [PubMed] [Google Scholar]
- Chew CS, Chen X, Parente JA, Tarrer S, Okamoto C, Qin HY (2002) Lasp-1 binds to non-muscle F-actin in vitro and is localized within multiple sites of dynamic actin assembly in vivo. J Cell Sci 115: 4787–4799 [DOI] [PubMed] [Google Scholar]
- Chew CS, Parente JA, Chen X, Chaponnier C, Cameron RS (2000) The LIM and SH3 domain containing protein, lasp-1, may link the cAMP-signaling pathway with dynamic membrane restructuring activities in ion transporting epithelia. J Cell Sci 113: 2035–2045 [DOI] [PubMed] [Google Scholar]
- Chew CS, Parente JR, Zhou CJ, Baranco E, Chen X (1998) Lasp-1 is a regulated phosphoprotein within the cAMP-signaling pathway in the gastric parietal cell. J Physiol 275: C56–C67 [DOI] [PubMed] [Google Scholar]
- Concannon CG, Gorman AM, Samali A (2003) On the role of Hsp27 in regulating apoptosis. Apoptosis 8: 61–70 [DOI] [PubMed] [Google Scholar]
- Drees BE, Andrews KM, Beckerle MC (1999) Molecular dissection of zyxin function reveals its involvement in cell motility. J Cell Biol 147: 1549–1560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espana L, Martin B, Aragues R, Chiva C, Oliva B, Andreu D, Sierra A (2005) Bcl-x(L)-mediated changes in metabolic pathways of breast cancer cells: from survival in the blood stream to organ-specific metastasis. Am J Pathol 167: 1125–1137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatenby RA, Gillies RJ (2004) Why do cancers have high aerobic glycolysis? Nat Rev Cancer 4: 891–899 [DOI] [PubMed] [Google Scholar]
- Griffith E, Coutts AS, Black DM (2005) RNAi knock-down of the focal adhesion protein TES reveals its role in actin stress fibre organisation. Cell Mot Cytoskelt 60: 140–152 [DOI] [PubMed] [Google Scholar]
- Grunewald TG, Kammerer U, Schulze E, Schindler D, Honig A, Zimmer M, Butt E (2006) Silencing of LASP-1 influences zyxin localization, inhibits proliferation and reduces migration in breast cancer cells. Exp Cell Res 312: 974–982 [DOI] [PubMed] [Google Scholar]
- Hermeking H, Lengauer C, Polyak K, He TC, Zhang L, Thiagalingam S, Kinzler KW, Vogelstein B (1997) 14-3-3 sigma is p53-regulated inhibitor of G2/M progression. Mol Cell 1: 3–11 [DOI] [PubMed] [Google Scholar]
- Hirota T, Morisaki T, Nishiyama Y, Marumoto T, Tada K, Hara T, Masuko N, Inagaki M, Hatakeyama K, Saya H (2000) Zyxin, a regulator of actin filament assembly, targets the mitotic apparatus by interacting with h-warts/LATS1 tumour suppressor. J Cell Biol 5: 1073–1086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman LM, Jensen CJ, Kloeker S, Wang CL, Yoshigi M, Beckerle MC (2006) Gentic ablation of zyxin causes Mena/VASP mislocalization, increased motility, and deficits in actin remodelling. J Cell Biol 172: 771–782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iida S, Hirota T, Morisaki T, Marumoto T, Hara T, Kuninaka S, Honda S, Kosai K, Kawasuji M, Pallas DC, Saya H (2004) Tumour suppressor WARTS ensures genomic integrity by regulating both mitotic progression and tetraploidy checkpoint function. Oncogene 23: 5266–5274 [DOI] [PubMed] [Google Scholar]
- Isidoro A, Casado E, Redondo A, Acebo P, Espinosa E, Alonso AM, Cejas P, Hardisson D, Fresno Vara JA, Belda-Iniesta C, Gonzales-Baron M, Cuezva JM (2005) Breast carcinomas fulfill the Warburg hypothesis and provide metabolic markers of cancer prognosis. Carcinogenesis 26: 2095–2104 [DOI] [PubMed] [Google Scholar]
- Ingram WJ, Wicking CA, Grimmond SM, Forrest AR, Wainwright BJ (2002) Novel genes regulated by sonic Hedgehog in pluripotent mesenchymal cells. Oncogene 21: 8196–8205 [DOI] [PubMed] [Google Scholar]
- Kadrmas JL, Beckerle MC (2004) The LIM domain: from the cytoskeleton to the nucleus. Nat Rev Mol Cel Biol 5: 920–931 [DOI] [PubMed] [Google Scholar]
- Keicher C, Gambaryan S, Schulze E, Marcus K, Meyer HE, Butt E (2004) Phosphorylation of mouse LASP-1 on threonine 156 by cAMP-and cGMP-dependent protein kinase. Biochem Biophys Res Com 24: 308–316 [DOI] [PubMed] [Google Scholar]
- Legge F, Ferrandina G, Salutari V, Scambia G (2005) Biological characterization of ovarian cancer: prognostic and therapeutic implications. Ann Oncol 16: 95–101 [DOI] [PubMed] [Google Scholar]
- Li B, Zhuang LB, Trueb B (2004) Zyxin interacts with the SH3 domains of the cytoskeletal proteins LIM-nebulette and Lasp-1. J Bio Chem 279: 20401–20410 [DOI] [PubMed] [Google Scholar]
- Lin HY, Park ZY, Lin D, Brahmbhatt AA, Rio M, Yates JR, Klemke RL (2004) Regulation of cell migration and survival by focal adhesion targeting of LASP-1. J Cell Biol 165: 421–432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu CR, Ma CS, Ning JY, You JF Liao SL, Zheng J (2004) Differential thymosin beta 10 expression levels and actin filament organisation in tumour cell lines with different metastatic potential. Chin Med J 117: 213–218 [PubMed] [Google Scholar]
- Nakagawa H, Terasaki AG, Suzuki H, Ohashi K, Miyamoto S (2006) Short-term retention of actin filament binding proteins on lamellipodial actin bundles. FEBS Lett 580: 3223–3228 [DOI] [PubMed] [Google Scholar]
- Nix DA, Fradelizi J, Bockholt S, Menichi B, Louvard D, Friedrich E, Becherle MC (2001) Targeting of zyxin to sites of actin membrane interaction and to nucleus. J Biol Chem 276: 34759–34767 [DOI] [PubMed] [Google Scholar]
- Parkin DM, Bray F, Pisani P (2001) Estimating the world cancer burden: Globocan 2000. Int J Cancer 94: 153–156 [DOI] [PubMed] [Google Scholar]
- Peng CY, Graves PR, Thoma RS, Wu Z, Shaw AS, Piwnica-Worms H (1997) Mitotic and G2 checkpoint control: regulation of 14-3-3 protein binding by phosphorylation of Cdc25C on serine-216. Science 277: 1501–1505 [DOI] [PubMed] [Google Scholar]
- Perkins DN, Pappin DJ, Creasy DM, Cottrell JS (1999) Probability-based protein identification by searching sequence databases using mass spectrometry data. Electrophoresis 20: 3551–3567 [DOI] [PubMed] [Google Scholar]
- Pietromonaco SF, Seluja GA, Aitken A, Elias L (1996) Association of 14-3-3 proteins with centrosomes. Blood Cells Mol Dis 22: 225–237 [DOI] [PubMed] [Google Scholar]
- Quirk JT, Natarajan N (2005) Ovarian cancer incidnce in the United States, 1992–1999. Gynecol Oncol 97: 519–523 [DOI] [PubMed] [Google Scholar]
- Rachlin AS, Otey CA (2006) Identification of pallidin isoforms and characterization of an isoform-specific interaction between LASP-1 and pallidin. J Cell Sci 119: 995–1004 [DOI] [PubMed] [Google Scholar]
- Rauh-Adelmann C, Kin-Mang L, Sabeti N, Long JP, Mok SC, Ho S (2000) Altered expression of BRCA1, BRCA2 and newly identified BRCA2 exon 12 deletion variant in malignant human ovarian, prostate, and breast cancer cell lines. 28: 236–246 [DOI] [PubMed] [Google Scholar]
- Ridley AJ, Schwartz MA, Burridge K, Firtel RA, Ginsberg MH, Borisy G, Parsons JT, Horwitz AR (2003) Cell migration: integrating signals from front to back. Science 302: 1704–1709 [DOI] [PubMed] [Google Scholar]
- Rottner K, Krause M, Gimona M, Small JV, Wehland J (2000) Zyxin is not colocalized with VASP at lamellipodial tips and exhibits different dynamics to vinculin, paxillin and VASP in focal adhesions. Mol Biol Cell 12: 3103–3113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindler D, Hoehn H (1999) Flow cytometric testing for syndromes with chromosomal instability. In Diagnostic Cytogenetics Wegner RD (ed) pp 269–281. Berlin: Springer [Google Scholar]
- Schreiber V, Moog-Lutz C, Regnier CH, Chenard MP, Boeuf H, Vonesch JL, Tomasetto C, Ri MC (1998) Lasp-1, a novel type of actin-binding protein accumulating in cell membrane extensions. Mol Med 4: 675–687 [PMC free article] [PubMed] [Google Scholar]
- Spence HJ, McGarry L, Chew CS, Carragher NO, Scott-Carrhager LA, Yuan Z, Croft DR, Olson MF, Frame M, Ozanne BW (2006) AP-1 differentially expressed proteins Krp1 and fibronectin cooperatively enhance Rho-ROCK-independent mesenchymal invasion by altering the function, localization, and activity of nondifferentially expressed proteins. Mol and Cell Biol 26: 1480–1495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strehl S, Borkhardt A, Slany R, Fuchs UE, König K, Haas OA (2003) The human LASP1 gene is fused to MLL in an acute myeloid leukemia with t(11;17)(q23;q21). Oncogene 22: 157–160 [DOI] [PubMed] [Google Scholar]
- Sun HJ, Bahk YY, Choi YR, Shim JH, Han SH, Lee JW (2006) A proteomic analysis during seriel subculture and osteogenic differentiation of human mesenchymal stem cells. J Orthop Res 24: 2059–2071 [DOI] [PubMed] [Google Scholar]
- Tomasetto C, Moog-Lutz C, Regnier CH, Schreiber V, Basset P, Rio MC (1995b) Lasp-1 (MLN 50) defines a new LIM protein subfamily characterized by the association of LIM and SH3 domains. FEBS Lett 373: 245–249 [DOI] [PubMed] [Google Scholar]
- Tomasetto C, Regnier C, Moog-Lutz C, Mattei MG, Chenard MP, Lidereau R, Basset P, Rio MC (1995a) Identification of four novel human genes amplified and overexpressed in breast carcinoma and localized to the q11–q21.3 region of chromosome 17. Genomics 28: 367–376 [DOI] [PubMed] [Google Scholar]
- Tzivion G, Gupta VS, Kaplun L, Balan V (2006) 14-3-3 proteins as potential oncogenes. Semin Cancer Biol 16: 203–213 [DOI] [PubMed] [Google Scholar]
- Wang W, Shakes DC (1996) Molecular evolution of the 14-3-3 protein family. J Mol Evol 43: 384–398 [DOI] [PubMed] [Google Scholar]
- Wang Y, Gilmore TD (2003) Zyxin and paxillin proteins: focal adhesion plaque LIM domain proteins go nuclear. Biochem Biophys Acta 1593: 115–120 [DOI] [PubMed] [Google Scholar]
- Yates III JR, Eng JK, McCormack AL, Schieltz D (1995) Method to correlate tandem mass spectra of modified peptides to amino acid sequences in the protein database. Anal Chem 67: 1426–1436 [DOI] [PubMed] [Google Scholar]
- Zhang D, Tai LK, Wong LL, Chiu LL, Sethi SK, Koay ES (2005) Proteomic study reveals that proteins involved in metabolic and detoxification pathways are highly expressed in HER-2/neu-positive breast cancer. Mol Cell Proteomics 4: 1686–1696 [DOI] [PubMed] [Google Scholar]