Abstract
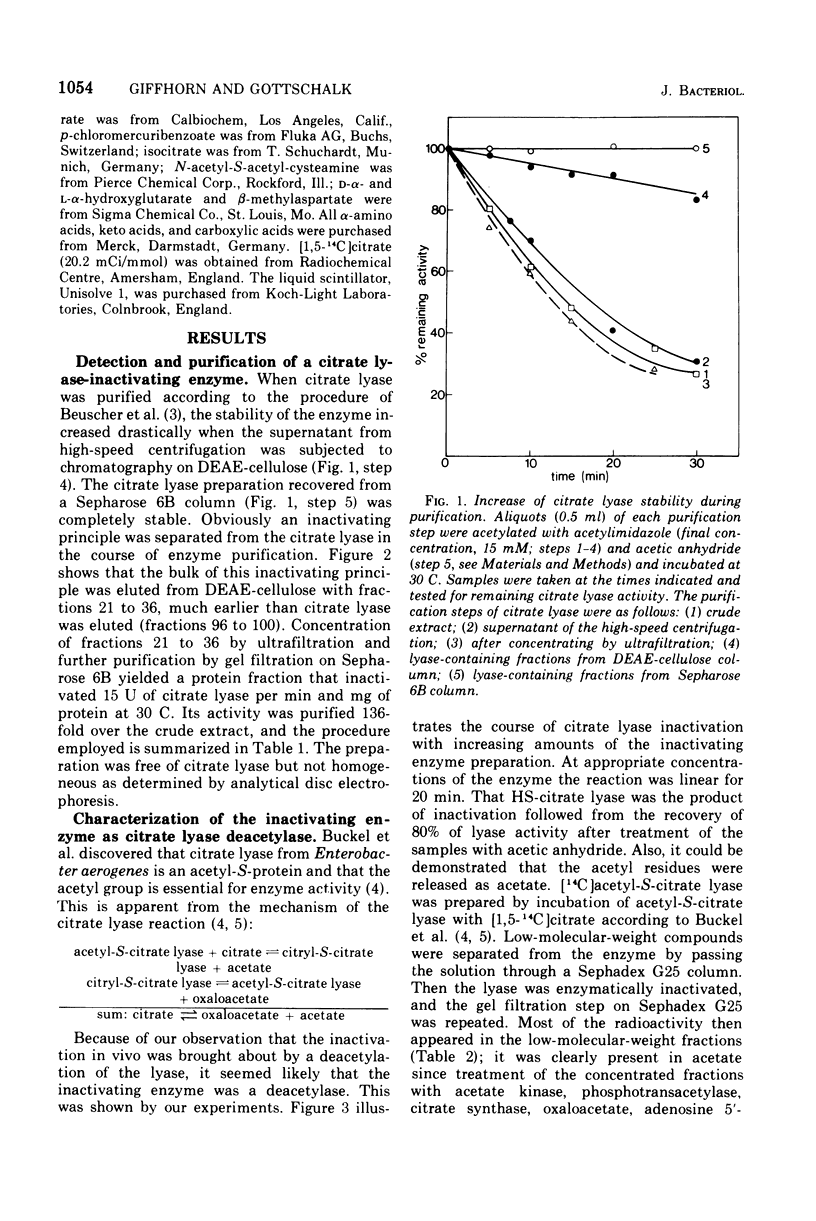
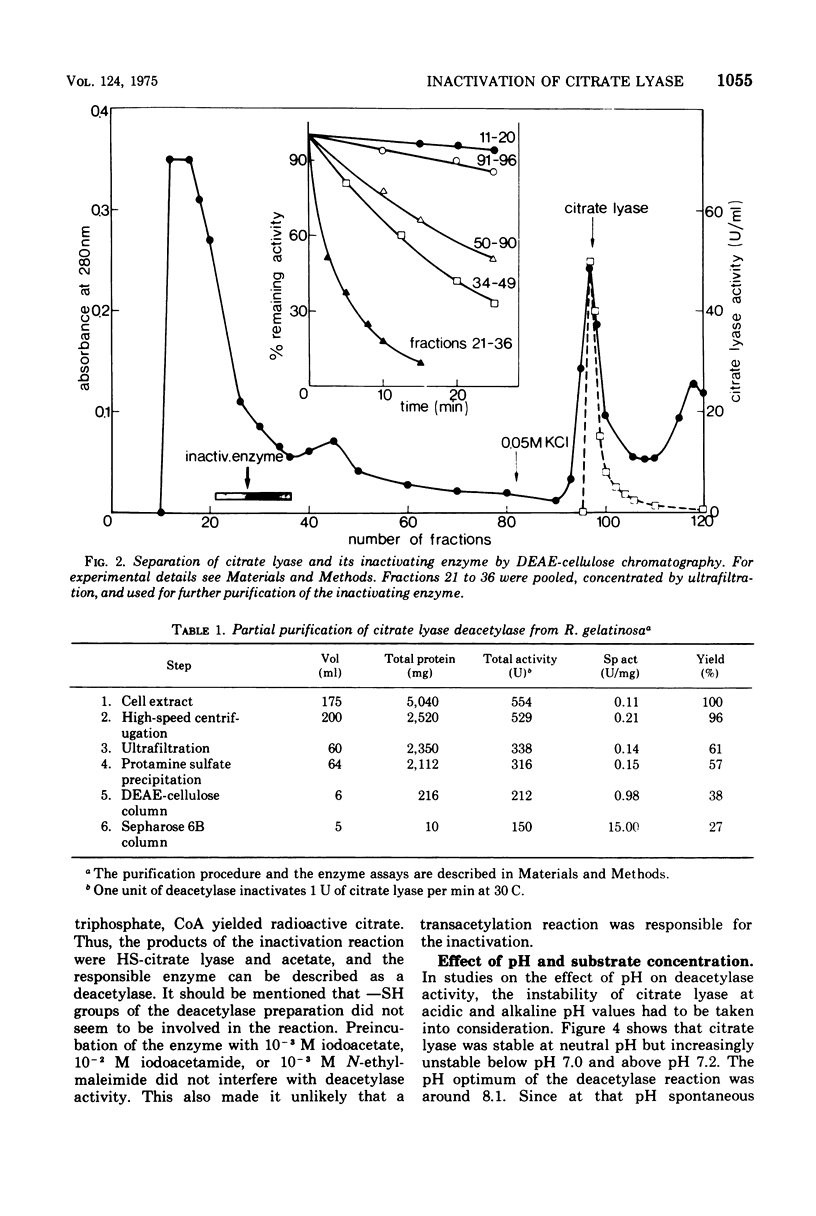
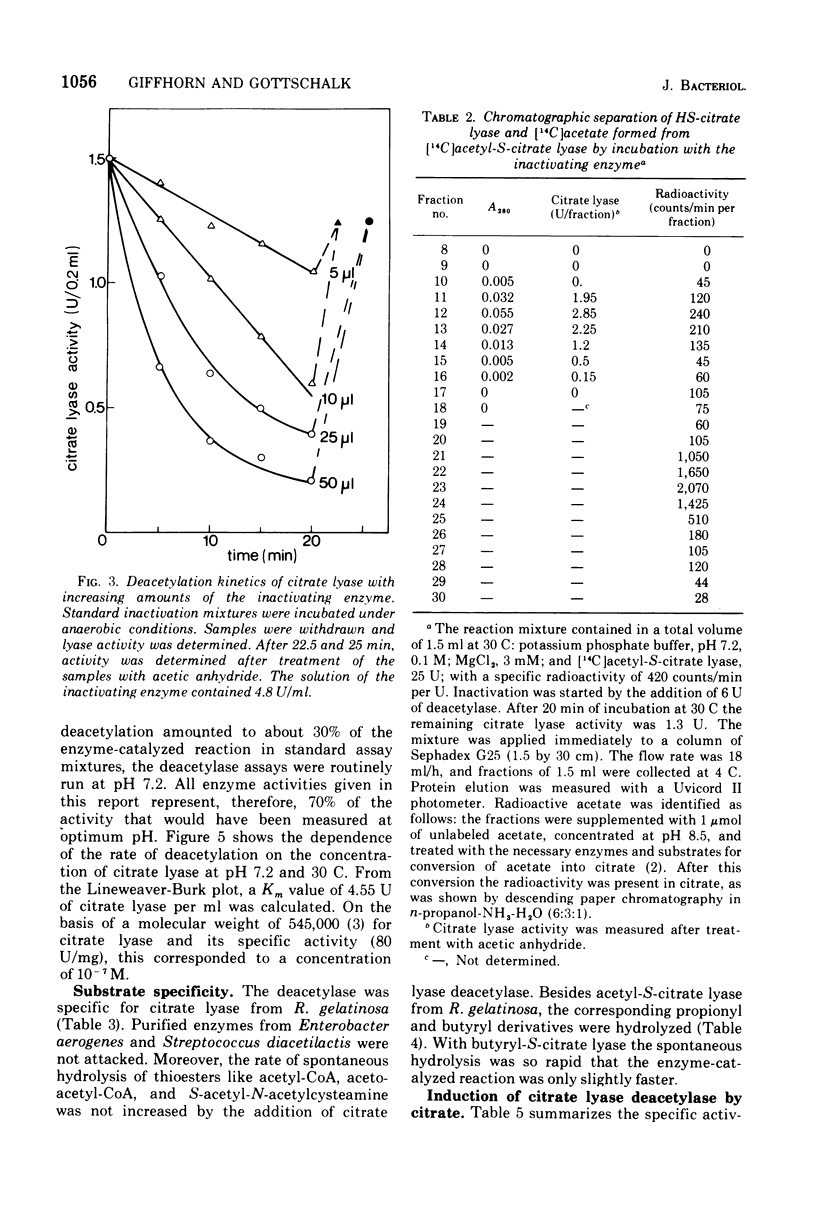
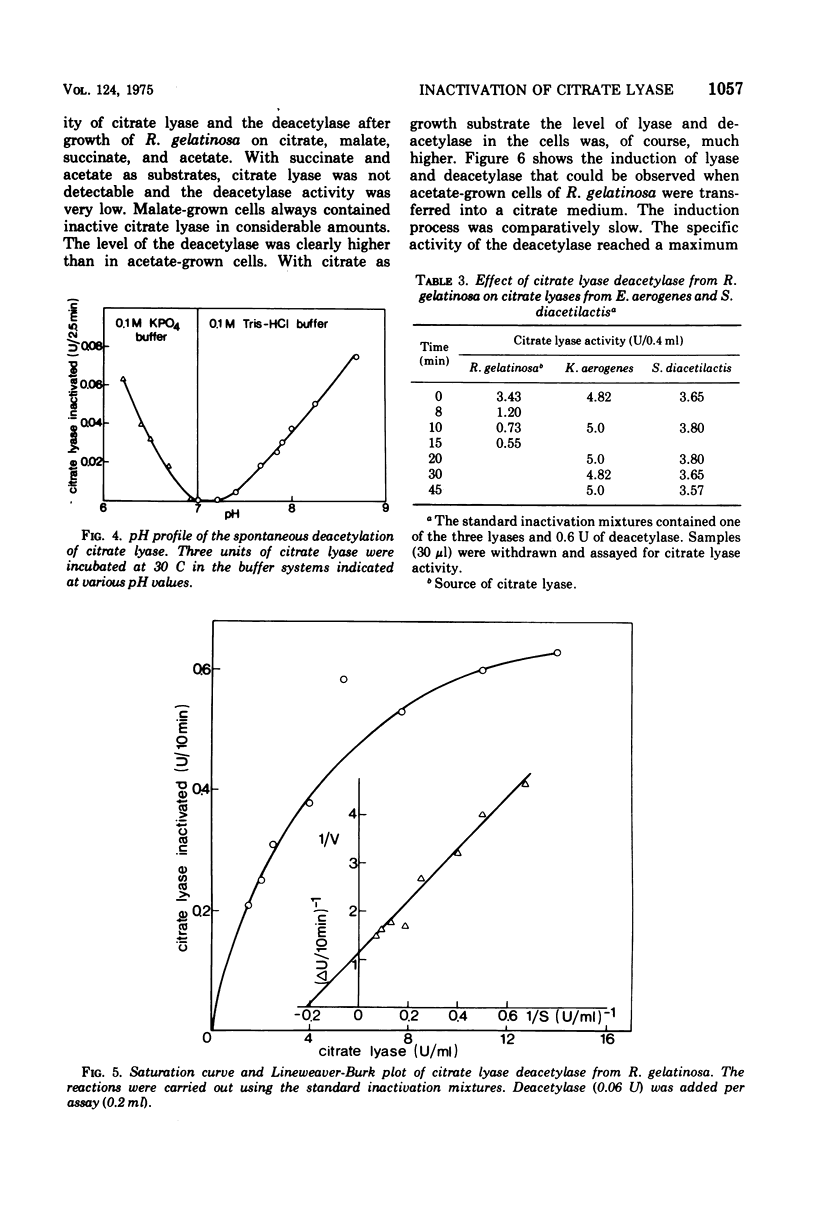
A previously unrecognized enzyme, citrate lyase deacetylase, has been purified about 140-fold from cell extracts of Rhodopseudomonas gelatinosa. It catalyzed the conversion of enzymatically active acetyl-S-citrate lyase into the inactive HS-form and acetate. The enzyme exhibited an optimal rate of inactivation at pH 8.1. Because of the instability of acetyl-S-citrate lyase at acidic and alkaline pH values, all assays were carried out at pH 7.2, where the spontaneous hydrolysis of the acetyl-S-citrate lyase was negligible and deacetylase showed 70% of the activity at pH 8.1. The apparent Km value for citrate lyase was 10(-7) M at pH 7.2 and 30 C. The activity of the deacetylase was restricted to the citrate lyase from R. gelatinosa. The corresponding lyases from Enterobacter aerogenes (formerly Klebsiella aerogenes) and Streptococcus diacetilactis were not deacetylated; likewise, thioesters such as acetyl-S coenzyme A, acetoacetyl-S coenzyme A, and N-acetyl-S-acetyl-cysteamine were also not hydrolyzed. Citrate lyase deacetylase was present in very small amounts in cells of R. gelatinosa grown with acetate or succinate; it was induced by citrate along with the citrate lyase. L-(+)-Glutamate strongly inhibited the deacetylase. Fifty percent inhibition was obtained at a concentration of 1.4 X 10(-4) L-(+)-glutamate. D-(-)-Glutamate, alpha-ketoglutarate, L-alpha-hydroxyglutarate, L-(-)-proline, and other metabolites were less effective.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beuscher N., Mayer F., Gottschalk G. Citrate lyase from Rhodopseudomonas gelatinosa: purification, electron microscopy and subunit structure. Arch Microbiol. 1974;100(4):307–328. doi: 10.1007/BF00446325. [DOI] [PubMed] [Google Scholar]
- Buckel W., Buschmeier V., Eggerer H. Der Wirkungsmechanismus der Citrat-Lyase aus Klebsiella aerogenes. Hoppe Seylers Z Physiol Chem. 1971 Sep;352(9):1195–1205. [PubMed] [Google Scholar]
- Buckel W., Ziegert K., Eggerer H. Acetyl-CoA-dependent cleavage of citrate on inactivated citrate lyase. Eur J Biochem. 1973 Aug 17;37(2):295–304. doi: 10.1111/j.1432-1033.1973.tb02988.x. [DOI] [PubMed] [Google Scholar]
- ELLMAN G. L. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959 May;82(1):70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- Gancedo C., Holzer H. Enzymatic inactivation of glutamine synthetase in Enterobacteriaceae. Eur J Biochem. 1968 Apr 3;4(2):190–192. doi: 10.1111/j.1432-1033.1968.tb00192.x. [DOI] [PubMed] [Google Scholar]
- Giffhorn F., Gottschalk G. Effect of growth conditions on the activation and inactivation of citrate lyase of Rhodopseudomonas gelatinosa. J Bacteriol. 1975 Dec;124(3):1046–1051. doi: 10.1128/jb.124.3.1046-1051.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kümmel A., Behrens G., Gottschalk G. Citrate lyase from Streptococcus diacetilactis. Association with its acetylating enzyme. Arch Microbiol. 1975;102(2):111–116. doi: 10.1007/BF00428354. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lowry O. H., Carter J., Ward J. B., Glaser L. The effect of carbon and nitrogen sources on the level of metabolic intermediates in Escherichia coli. J Biol Chem. 1971 Nov;246(21):6511–6521. [PubMed] [Google Scholar]
- Moellering H., Gruber W. Determination of citrate with citrate lyase. Anal Biochem. 1966 Dec;17(3):369–376. doi: 10.1016/0003-2697(66)90172-2. [DOI] [PubMed] [Google Scholar]
- Robyt J. F., Ackerman R. J., Chittenden C. G. Reaction of protein disulfide groups with Ellman's reagent: a case study of the number of sulfhydryl and disulfide groups in Aspergillus oryzae -amylase, papain, and lysozyme. Arch Biochem Biophys. 1971 Nov;147(1):262–269. doi: 10.1016/0003-9861(71)90334-1. [DOI] [PubMed] [Google Scholar]
- Schmellenkamp H., Eggerer H. Mechanism of enzymic acetylation of des-acetyl citrate lyase. Proc Natl Acad Sci U S A. 1974 May;71(5):1987–1991. doi: 10.1073/pnas.71.5.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh M., Carpenter D. E., Srere P. A. Nonidentical subunits of citrate lyase from Klebsiella aerogenes. Biochem Biophys Res Commun. 1974 Aug 19;59(4):1211–1218. doi: 10.1016/0006-291x(74)90443-4. [DOI] [PubMed] [Google Scholar]
- Tempest D. W., Meers J. L., Brown C. M. Influence of environment on the content and composition of microbial free amino acid pools. J Gen Microbiol. 1970 Dec;64(2):171–185. doi: 10.1099/00221287-64-2-171. [DOI] [PubMed] [Google Scholar]



