Abstract
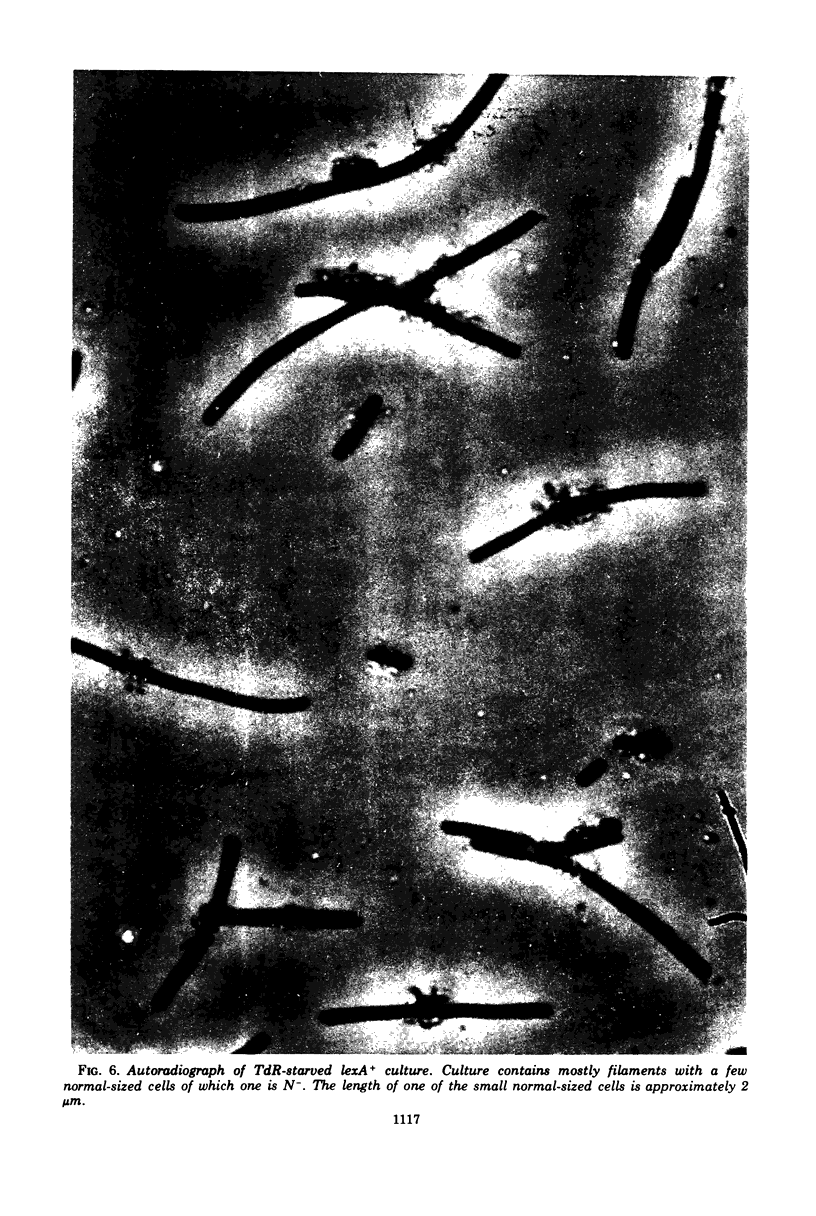
When thymidine-requiring lexA- strains were starved for thymidine, the kinetics of survival were similar to those of a nearly isogenic lexA+ strain. The size distribution of cells in the lexA- and lexA+ cultures were, however, quite different. Whereas most of the cells in the starved lexA+ cultures grew into long filamentous forms (longer than 4.0 mum), many of the lexA- cells were found to have a normal rod shape (4.0 mum or shorter). It was shown that lexA- cells undergo more divisions during thymidine starvation than lexA+ cells. Furthermore, using an autoradiographic method to analyze deoxyribonucleic acid (DNA) distribution in the starved cells, we demonstrated that cells without DNA are produced in both normal and starved lexA- cultures at a much higher frequency than in lexA+ cultures. Some of these cells may be produced by breakdown of DNA, but we favor the hypothesis that they result from an abnormal cell division process. Since lexA mutations are dominant, we conclude that a diffusible product decreases the synthesis or activity of an inhibitor of cell division in lexA- strains when DNA synthesis is blocked by thymidine starvation.
Full text
PDF


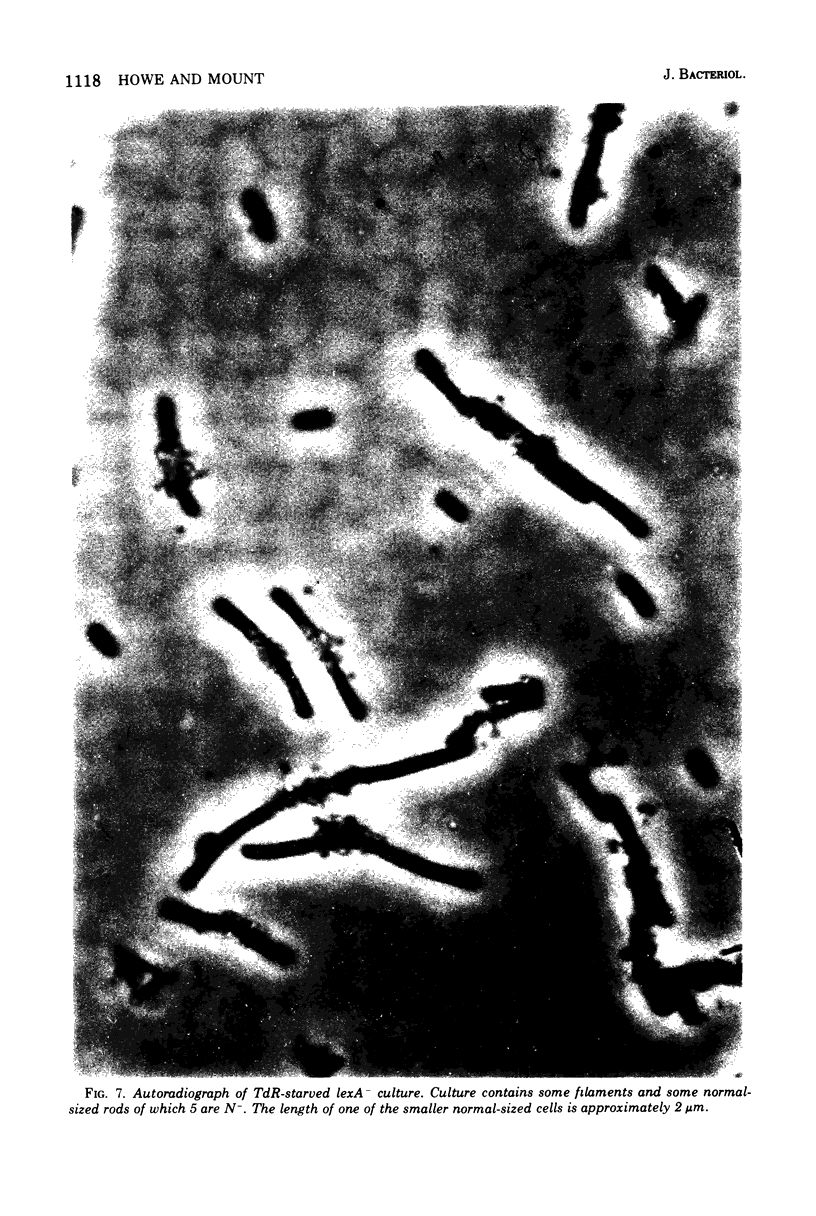
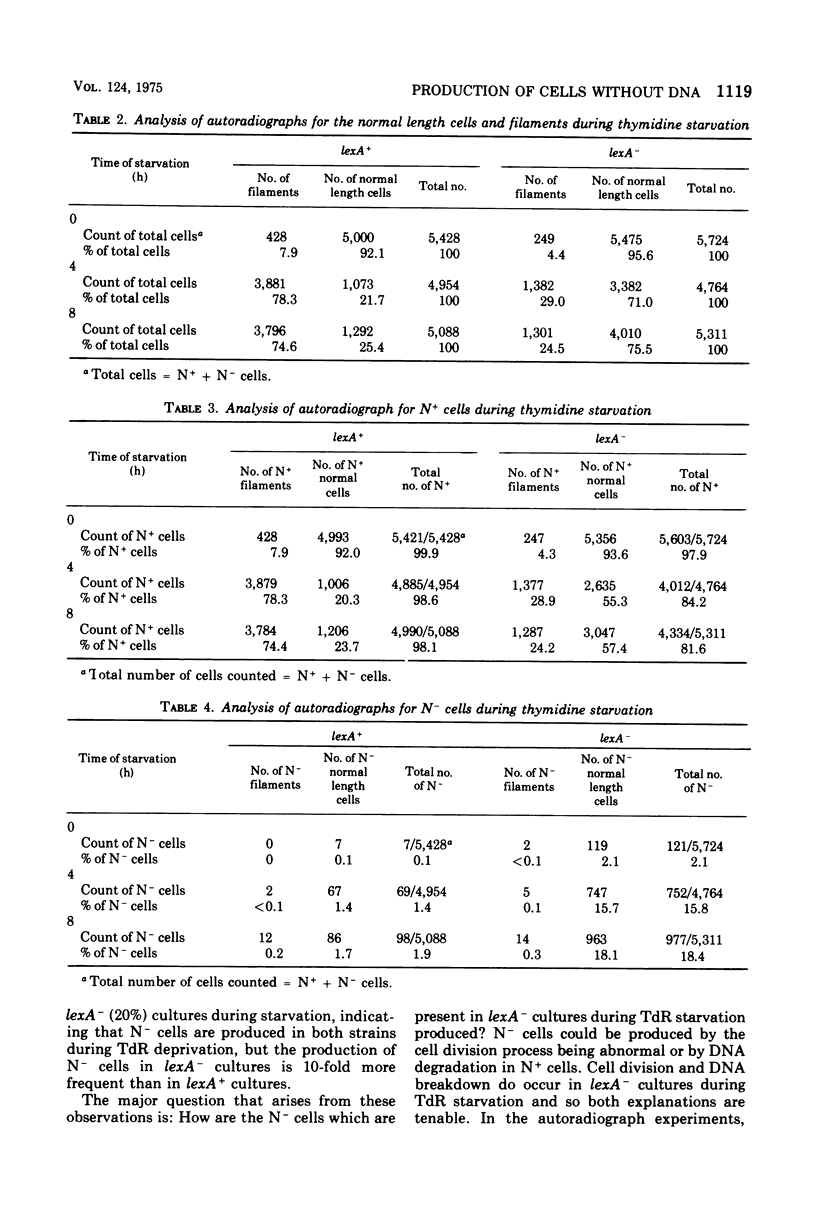
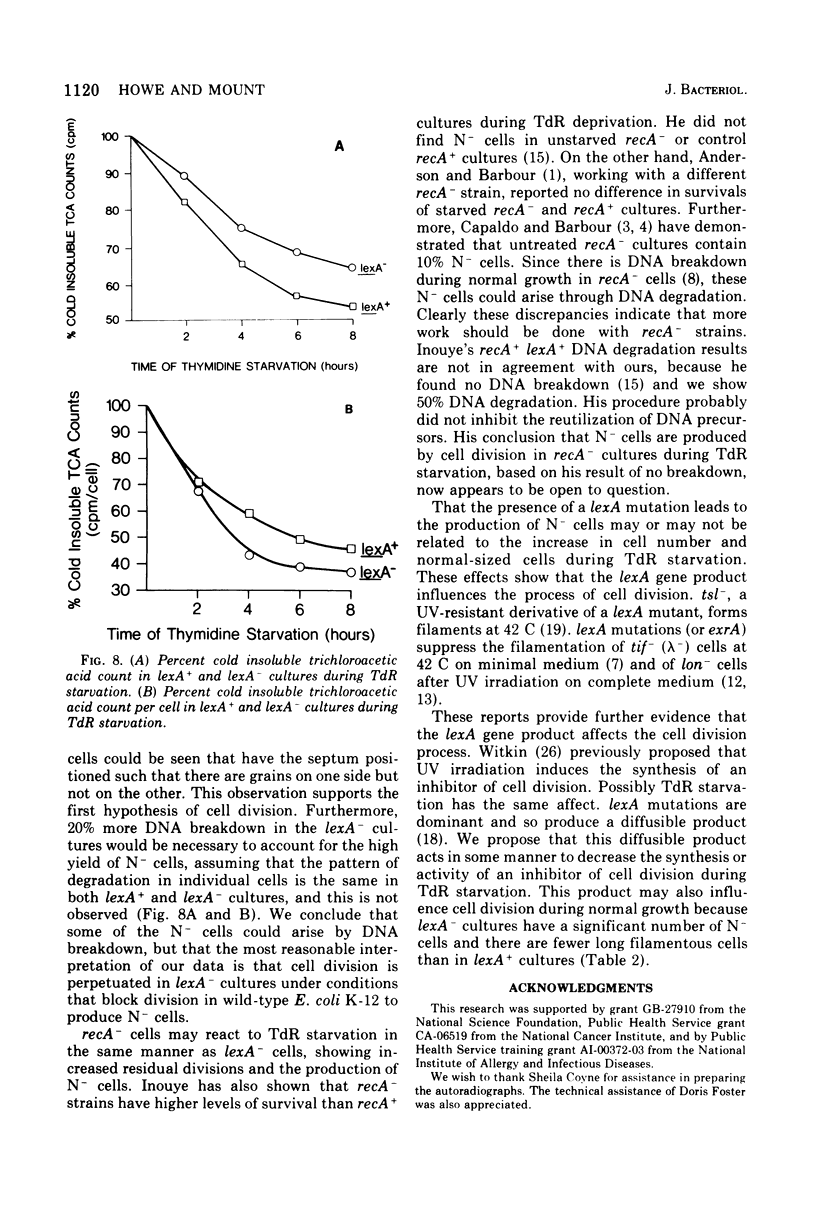

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson J. A., Barbour S. D. Effect of thymine starvation on deoxyribonucleic acid repair systems of Escherichia coli K-12. J Bacteriol. 1973 Jan;113(1):114–121. doi: 10.1128/jb.113.1.114-121.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breitman T. R., Maury P. B., Toal J. N. Loss of deoxyribonucleic acid-thymine during thymine starvation of Escherichia coli. J Bacteriol. 1972 Oct;112(1):646–648. doi: 10.1128/jb.112.1.646-648.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CARO L. G., VAN TUBERGEN R. P., KOLB J. A. High-resolution autoradiography. I. Methods. J Cell Biol. 1962 Nov;15:173–188. doi: 10.1083/jcb.15.2.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capaldo F. N., Barbour S. D. DNA content, synthesis and integrity in dividing and non-dividing cells of rec- strains of Escherichia coli K12. J Mol Biol. 1975 Jan 5;91(1):53–66. doi: 10.1016/0022-2836(75)90371-x. [DOI] [PubMed] [Google Scholar]
- Capaldo F. N., Ramsey G., Barbour S. D. Analysis of the growth of recombination-deficient strains of Escherichia coli K-12. J Bacteriol. 1974 Apr;118(1):242–249. doi: 10.1128/jb.118.1.242-249.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caro L. G., Schnös M. Tritium and Phosphorus-32 in High-Resolution Autoradiography. Science. 1965 Jul 2;149(3679):60–62. doi: 10.1126/science.149.3679.60. [DOI] [PubMed] [Google Scholar]
- Castellazzi M., George J., Buttin G. [Prophage induction and cell division in E. coli. II. Linked (recA, zab) and unlinked (lex) suppressors of tif-1-mediated induction and filamentation]. Mol Gen Genet. 1972;119(2):153–174. doi: 10.1007/BF00269134. [DOI] [PubMed] [Google Scholar]
- Clark A. J., Chamberlin M., Boyce R. P., Howard-Flanders P. Abnormal metabolic response to ultraviolet light of a recombination deficient mutant of Escherichia coli K12. J Mol Biol. 1966 Aug;19(2):442–454. doi: 10.1016/s0022-2836(66)80015-3. [DOI] [PubMed] [Google Scholar]
- Cummings D. J., Mondale L. Thymineless death in Escherichia coli: strain specificity. J Bacteriol. 1967 Jun;93(6):1917–1924. doi: 10.1128/jb.93.6.1917-1924.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIS B. D., MINGIOLI E. S. Mutants of Escherichia coli requiring methionine or vitamin B12. J Bacteriol. 1950 Jul;60(1):17–28. doi: 10.1128/jb.60.1.17-28.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demerec M., Adelberg E. A., Clark A. J., Hartman P. E. A proposal for a uniform nomenclature in bacterial genetics. Genetics. 1966 Jul;54(1):61–76. doi: 10.1093/genetics/54.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donch J. J., Greenberg J. The effect of lex on UV sensitivity, filament formation and lambda induction in ion mutants of Escherichia coli. Mol Gen Genet. 1974;128(4):277–281. doi: 10.1007/BF00268515. [DOI] [PubMed] [Google Scholar]
- Donch J., Green M. H., Greenberg J. Interaction of the exr and lon genes in Escherichia coli. J Bacteriol. 1968 Nov;96(5):1704–1710. doi: 10.1128/jb.96.5.1704-1710.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirota Y., Jacob F., Ryter A., Buttin G., Nakai T. On the process of cellular division in Escherichia coli. I. Asymmetrical cell division and production of deoxyribonucleic acid-less bacteria. J Mol Biol. 1968 Jul 14;35(1):175–192. doi: 10.1016/s0022-2836(68)80046-4. [DOI] [PubMed] [Google Scholar]
- Inouye M. Pleiotropic effect of the rec A gene of Escherichia coli: uncoupling of cell division from deoxyribonucleic acid replication. J Bacteriol. 1971 May;106(2):539–542. doi: 10.1128/jb.106.2.539-542.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson B. F., Greenberg J. Mapping of sul, the suppressor of lon in Escherichia coli. J Bacteriol. 1975 May;122(2):570–574. doi: 10.1128/jb.122.2.570-574.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mound D. W., Walker A. C., Kosel C. Suppression of lex mutations affecting deoxyribonucleic acid repair in Escherichia coli K-12 by closely linked thermosensitive mutations. J Bacteriol. 1973 Nov;116(2):950–956. doi: 10.1128/jb.116.2.950-956.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mount D. W., Low K. B., Edmiston S. J. Dominant mutations (lex) in Escherichia coli K-12 which affect radiation sensitivity and frequency of ultraviolet lght-induced mutations. J Bacteriol. 1972 Nov;112(2):886–893. doi: 10.1128/jb.112.2.886-893.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Donovan G. A., Neuhard J. Pyrimidine metabolism in microorganisms. Bacteriol Rev. 1970 Sep;34(3):278–343. doi: 10.1128/br.34.3.278-343.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiter H., Ramareddy G. Loss of DNA behind the growing point of thymine-starved Bacillus subtilis 168. J Mol Biol. 1970 Jun 14;50(2):533–548. doi: 10.1016/0022-2836(70)90210-x. [DOI] [PubMed] [Google Scholar]
- STACEY K. A., SIMSON E. IMPROVED METHOD FOR THE ISOLATION OF THYMINE-REQUIRING MUTANTS OF ESCHERICHIA COLI. J Bacteriol. 1965 Aug;90:554–555. doi: 10.1128/jb.90.2.554-555.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor A. L., Trotter C. D. Linkage map of Escherichia coli strain K-12. Bacteriol Rev. 1972 Dec;36(4):504–524. doi: 10.1128/br.36.4.504-524.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willetts N. S., Clark A. J., Low B. Genetic location of certain mutations conferring recombination deficiency in Escherichia coli. J Bacteriol. 1969 Jan;97(1):244–249. doi: 10.1128/jb.97.1.244-249.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willetts N. S., Mount D. W. Genetic analysis of recombination-deficient mutants of Escherichia coli K-12 carrying rec mutations cotransducible with thyA. J Bacteriol. 1969 Nov;100(2):923–934. doi: 10.1128/jb.100.2.923-934.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkin E. M. The radiation sensitivity of Escherichia coli B: a hypothesis relating filament formation and prophage induction. Proc Natl Acad Sci U S A. 1967 May;57(5):1275–1279. doi: 10.1073/pnas.57.5.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]