Abstract
There is no consensus on the management of locally advanced pancreatic cancer, with either chemotherapy or combined modality approaches being employed (Maheshwari and Moser, 2005). No published meta-analysis (Fung et al, 2003; Banu et al, 2005; Liang, 2005; Bria et al, 2006; Milella et al, 2006) has included randomised controlled trials employing radiation therapy. The aim of this systematic review was to compare the following: (i) chemoradiation followed by chemotherapy (combined modality therapy) vs best supportive care (ii) radiotherapy vs chemoradiation (iii) radiotherapy vs combined modality therapy (iv) chemotherapy vs combined modality therapy (v) 5FU-based combined modality treatment vs another-agent-based combined modality therapy. Relevant randomised controlled trials were identified by searching databases, trial registers and conference proceedings. The primary end point was overall survival and secondary end points were progression-free survival/time-to-progression, response rate and adverse events. Survival data were summarised using hazard ratio (HR) and response-rate/adverse-event data with relative risk. Eleven trials involving 794 patients met the inclusion criteria. Length of survival with chemoradiation was increased compared with radiotherapy alone (two trials, 168 patients, HR 0.69; 95% confidence interval (CI) 0.51–0.94), but chemoradiation followed by chemotherapy did not lead to a survival advantage over chemotherapy alone (two trials, 134 patients, HR 0.79; CI 0.32–1.95). Meta-analyses could not be performed for the other comparisons. A survival benefit was demonstrated for chemoradiation over radiotherapy alone. Chemoradiation followed by chemotherapy did not demonstrate any survival advantage over chemotherapy alone, but important clinical differences cannot be ruled out due to the wide CI.
Keywords: pancreas cancer, radiotherapy, chemoradiation, chemotherapy, combined modality
Pancreatic cancer is a difficult condition to treat, evidenced by the fact that the annual mortality figures are close to the incidence rate (Jemal et al, 2006). Ninety per cent of patients have unresectable disease at diagnosis, of whom 40–50% have locally advanced disease (White et al, 1999), and reported to have better median survival of 6–10 months compared to the 3–6 months noted in metastatic disease (American Cancer Society, 2003). Radiotherapy approaches, with or without chemotherapy, have been frequently used in this subset (Maheshwari and Moser, 2005).
Previous meta-analyses in this area have looked at chemotherapy and novel agents (Fung et al, 2003; Banu et al, 2005; Liang, 2005; Bria et al, 2006; Milella et al, 2006), and the Cochrane Collaboration (Yip et al, 2006) have done a recent systematic review on advanced pancreatic cancer that included a qualitative overview of trials involving radiotherapy, but there has been no meta-analyses performed to date addressing this treatment option. Evaluating this approach is important, as currently there is no uniformly agreed standard of care in the management of patients with locally advanced disease.
We have attempted an up-to-date analysis of the different radiotherapeutic options employed in locally advanced pancreatic cancer, thereby including an area not covered by previous meta-analyses. Furthermore, we have adopted the most appropriate statistical methods for meta-analysis of time to event data extracted from published reports (Parmar et al, 1998).
METHODS
Aims
To review systematically the published and unpublished literature, comparing the following therapies:
Chemoradiotherapy, followed by chemotherapy vs best supportive care
Radiotherapy vs chemoradiotherapy
Radiotherapy vs chemoradiotherapy, followed by chemotherapy
Chemotherapy vs chemoradiotherapy, followed by chemotherapy (combined modality therapy)
5FU-based chemoradiotherapy followed by chemotherapy vs another agent-based chemoradiotherapy, followed by chemotherapy
Search strategy
Trials were identified by searching MEDLINE, OLDMEDLINE (1950–1965), EMBASE (1974 to date), ISI Web of Science (incorporating Science Citation Index 1945 to date; ISI Science and Technology Proceedings 1990 to date; CancerLit (1960 to date) and Current contents databases (from 1996 to date) as far back as they go. In addition, trial registries (Registries of the National Cancer Institute Physician Data Query, the UK Co-ordinating Committee on Cancer Research, National Clinical Trials Registry, and the Cochrane Controlled Trials Register) and conference proceedings (American Society of Clinical Oncology, American Association of Cancer Research and the European Cancer Conference, European Society of Medical Oncology, American Gastroenterological Association, European Pancreatic Club, American Association of Pancreatology, British Society of Gastroenterology, and the United European Gastroenterology Week) were searched. References of selected papers and previous systematic reviews were scanned for any other relevant trials, and original trialists were contacted for possible unpublished trials.
Selection criteria
Randomised controlled trials were selected based on their abstract, or if that was unclear, the paper. Inclusion criteria were randomised controlled trials involving patients with advanced pancreatic cancer of the exocrine pancreas, comparing the therapies listed above. The exclusion criteria were trials which were nonrandomised, or included surgical resection of tumours and cancers other than pancreas cancers, wherein data was not available for the pancreas cancer subset. The study selection was done by two independent assessors, with any difference of opinion sorted by discussion.
End points
Overall survival (OS) defined as time from randomisation to death, was the primary outcome measure. Alternative definitions such as time from initiation of therapy to death were also included and noted as a potential source of heterogeneity.
Progression free survival (PFS) or time to progression (TTP), overall response rate (ORR) and adverse events (AE) were the secondary outcome measures. PFS was defined as time from randomisation to progression or death. Time to progression (TTP) was defined as the time from randomisation to disease progression. PFS was analysed separately from TTP as the former accounts for all deaths as well as progression events, whereas the latter only accounts for progression events. Overall response rate (ORR) was defined as the number of partial and complete responses and adverse events defined as side effects occurring from the date of randomisation till either end of study or death.
Quality assessment
Methodological quality was assessed based on the method of allocation generation (method of randomisation), allocation concealment (where the randomisation was carried out), blinding and losses to follow up. These were classified as adequate, inadequate or unknown, and the results of the different components discussed qualitatively.
Data extraction
Data extraction was performed independently by two reviewers using a standardised data extraction sheet. Disagreements were resolved by discussion and any data uncertainties forwarded to the original trialist for clarification.
Statistical analysis
Individual trial level time to event data (OS and PFS/TTP) were summarised by the log hazard ratio (HR) and its variance. As many trials do not report this information directly (Altman et al, 1995), appropriate data such as log rank test results were extracted to allow estimation of the log HR and its variance using previously reported methods (Parmar et al, 1998; Williamson et al, 2002). One of these approaches relies on extracting data from published survival curves (Parmar et al, 1998). The software we used (version 3.0; 28 September, 2004) to estimate the trial level log HR and variance-based on summary data extracted from published survival curves was developed by Matthew Sydes and Jayne Teirney of the MRC Clinical Trials Unit, London. Trial-level log HRs and their variances were entered into RevMan version 4.2 (a Windows-based software package used by the Cochrane Collaboration for writing systematic reviews and undertaking meta-analysis Sterne et al, 2001) and pooled using an inverse variance weighted average with results presented as a HR and 95% confidence interval (CI).
Dichotomous data (response rate/adverse events) were summarised using relative risks and 95% CIs with the Mantel–Haensel method used for pooling results across trials (Deeks et al, 2001).
Heterogeneity was assessed by visual inspection of the forrest plot, the Cochran's χ2 test (using a 10% significance level) and interpretation of the I2 statistic (percentage of variation due to heterogeneity with higher values indicating a greater degree of heterogeneity) (Deeks et al, 2004). The factors set out a priori to investigate heterogeneity were age, gender, performance status, previous treatment, site of the cancer (head, body or tail), and the chemotherapy/radiation used with the dose, combinations, and frequency. A fixed effect (FE) approach was adopted unless there was evidence of significant heterogeneity that could not be adequately explained, in which case a random effects (RE) approach was used.
Publication bias was assessed by visual inspection of funnel plots (Light and Pillemer, 1984).
RESULTS
Eleven trials involving 794 patients met the reviews' inclusion criteria and six of these trials involving 451 patients were included in the meta-analyses. The quality of included studies is described in Table 1.
Table 1. Quality of included studies.
| Comparison | Trial | Allocation sequence generation | Allocation concealement | Blinding | Follow-up |
|---|---|---|---|---|---|
| Chemoradiotherapy followed by chemotherapy vs BSC | Shinchi et al (2002) | Unclear | Unclear | Not performed | Adequate |
| Radiotherapy vs chemoradiotherapy, followed by chemotherapy | Moertel et al (1981) | Adequate | Adequate | Not performed | Adequate |
| 5FU-based chemoradiotherapy followed by chemotherapy vs other agent-based chemoradiotherapy, followed by chemotherapy | GITSG (1985) | Unclear | Adequate | Not performed | Adequate |
| Li et al (2003) | Unclear | Unclear | Not performed | Adequate | |
| Wilkowski et al (2006) | Unclear | Unclear | Not performed | Unclear | |
| Radiotherapy vs chemoradiotherapy | Cohen et al (2005) | Adequate | Adequate | Not performed | Adequate |
| Moertel et al (1969) | Unclear | Unclear | Adequate | Adequate | |
| Chemotherapy vs chemoradiotherapy, followed by chemotherapy | GITSG (1988) | Adequate | Adequate | Not performed | Adequate |
| Klassen et al (1985) | Adequate | Unclear | Not performed | Adequate | |
| Hazel et al (1981) | Unclear | Unclear | Not performed | Adequate | |
| Chauffert et al (2006) | Unclear | Unclear | Not performed | Unclear |
BSC=best supportive care; FU=fluorouracil.
As there was only one study identified in two comparisons viz., chemoradiation, followed by chemotherapy vs best supportive care (BSC) comparison (Table 2) (Shinchi et al, 2002), and radiotherapy vs chemoradiation, followed by chemotherapy comparison (Table 3) (Moertel et al, 1981), a meta-analysis could not be undertaken for these comparisons.
Table 2. Study included in comparison of chemoradiation, followed by chemotherapy vs BSC.
| Trial | Group | Mean age and gender | Chemotherapy/radio-therapy used and dose |
|---|---|---|---|
| Shinchi et al (2002) | Chemoradiation, followed by chemotherapy (n=16) | 62.9 years; 36% women, 64% men | 50.4 Gy per 28 fractions and continuous-infusion 5FU 200 mg m−2 day−1 |
| BSC (n=15) | 64.6 years; 67% women, 33% men | — |
Table 3. Study included in comparison of chemoradiation, followed by chemotherapy vs radiation.
| Trial | Group | Mean age and gender | Chemotherapy/radio-therapy used and dose |
|---|---|---|---|
| Moertel et al (1981) | Chemoradiation, followed by chemotherapy (n=31) | 62.9 years; 36% women, 64% men | 6000 rad, given as 2000 rad over 2 weeks and separated by a 2 weeks' rest period. A total of 5FU – 500 mg m−2 day−1 on days 1–3 of each 2000 rad radiotherapy course |
| Radiation alone (n=25) | 64.6 years; 67% women, 33% men | 6000 rad |
In the single randomised controlled trial examined (Shinchi et al, 2002), there was survival advantage for chemoradiation followed by chemotherapy, over BSC (1 trial, 31 patients, HR 0.28; 95% CI 0.13–0.60). The median time-to-progression was 6.1 months, with overall response rate of 31% (5 out of 16) in the treated group but corresponding data were not provided for the BSC group. A quarter of treated patients (4 out of 16) developed complications secondary to the chemoradiation, with nausea occurring in three patients and one experiencing grade 2 leucopenia.
There was survival advantage with chemoradiotherapy followed by chemotherapy over radiation alone (1 trial, 56 patients, HR 0.50; 95% CI 0.29–0.84) in the trial conducted by Moertel et al (1981). Time to progression was also better in the chemoradiotherapy followed by chemotherapy arm over radiotherapy alone arm (HR 0.51; 95% CI 0.32–0.81).
Three trials (256 patients) met the inclusion criteria for the comparison of 5FU-based multimodality therapy vs another-agent-based multimodality therapy (Table 4). However, meta-analyses were not performed, as the studies were too clinically heterogeneous to be grouped together in a clinically meaningful analysis. The agents used for radio sensitisation in the non-5FU arm were different in all three trials, with gemcitabine alone (Li et al, 2003), gemcitabine+cisplatin (Wilkowski et al, 2006) and adriamycin (Gastrointestinal Tumour Study Group, 1985) being used.
Table 4. Study included in comparison of 5FU-based chemoradiation, followed by chemotherapy vs another chemotherapy agent-based chemoradiation, followed by chemotherapy.
| Authors | Group (number randomised) | Median age and gender | Chemotherapy/radio-therapy used and dose |
|---|---|---|---|
| Wilkowski et al (2006) | 5FU chemoradiotherapy (n=32) | NA | 5FU 350 mg m−2 irradiation day−1+50 Gy conventional radiation |
| Gemcitabine+cisplatin chemoradiotherapy (n=33) | NA | Gemcitabine 300 mg m−2 day−1 30 min infusion, cisplatin 30 mg m−2 day−1 60 min infusion on days 1, 8, 22 and 29+50 Gy conventional radiation | |
| Li et al (2003) | 5FU chemoradiotherapy (n=16) | 69 years; 12 men, 4 women | 500 mgm−2 day−1 for 3 days, repeated every 2 weeks for 6 weeks+3D conformal radiotherapy 50.4–61.2 Gy |
| Gemcitabine chemoradiotherapy (n=18) | 68.5 years; 13 men, 5 women | 600 mg m−2 week−1 for 6 weeks+3D conformal radiotherapy 50.4–61.2 Gy | |
| GITSG (1985) | 5FU chemoradiotherapy (n=79) | 5FU 500 mg m−2 on first 3 days of each radiotherapy course+6000 rad double split course, followed by weekly maintenance with 5FU 500 mg m−2 till progression | |
| Adriamycin chemoradiotherapy (n=78) | Adriamycin 15 mg m−2 on day 1; thereafter 10 mg m−2 week−1, for a minimum of five doses+4000 rad continuous course, followed by weekly maintenance with 5FU 500 mg m−2 till progression |
FU=fluorouracil.
Adriamycin-based multimodality therapy, using split course radiotherapy given via two portals, did not demonstrate a significant survival advantage over 5FU-based treatment (HR for 5FU vs Adriamycin=0.97 95% CI 0.73–1.29), accompanied by the drawback of significantly increased adverse events (P<0.05) (Gastrointestinal Tumour Study Group, 1985).
A randomised controlled trial of 34 patients found significantly improved overall survival (14.5 vs 6.7 months), time to progression (7.1 vs 2.7 months) and response rate (50 vs 13%) in patients treated with gemcitabine-based chemoradiation (600 mg m−2week−1 for 6 weeks), followed by gemcitabine, in comparison to a control arm of 5FU-based chemoradiation (500 mg m−2 day−1 for 3 days repeated every 2 weeks for 6 weeks), followed by gemcitabine (Li et al, 2003). Toxicity between the two arms was similar and radiation had been given using three-dimensional conformal radiotherapy. These results were not borne out in a recent randomised controlled trial of 65 patients (preliminary results), which did not find improvement in 9 month survival for a group treated with gemcitabine and cisplatin chemoradiation, vs another treated with protracted venous 5-FU infusion chemoradiation (Wilkowski et al, 2006).
Comparison of radiotherapy vs chemoradiotherapy
Two randomised controlled trials with 168 patients were included in this analysis (Table 5) (Moertel et al, 1969; Cohen et al, 2005). One study described adequate methods of allocation generation, one described adequate methods of concealment, and both described adequate losses to follow-up. One trial was blinded (Table 1).
Table 5. Studies included in comparison of radiotherapy vs chemoradiotherapy.
| Trial | Group | Median age and gender | Chemotherapy/radiotherapy used and dose |
|---|---|---|---|
| Moertel et al (1969) | Radiotherapy (n=32) | NA | 3500–4000 rad by cobalt 60 teletherapy unit |
| Chemoradiotherapy (n=32) | NA | 3500–4000 rad by cobalt 60 teletherapy unit, 5FU 45 mg kg−1 on first 3 days of radiotherapy | |
| Cohen et al (2005) | Radiotherapy (n=49) | 62 years; 55 men, 45 women | Radiotherapy 59.4 Gy |
| Chemoradiotherapy (n=55) | 64 years; 67 men, 33 women | Radiotherapy 59.4 Gy, 5FU 1000 mg m−2 day−1 on days 2–5 and 28–31 of radiotherapy MMC 10 mg m−2 on day 2 |
MMC=mitomycin; 5FU=5-fluorouracil.
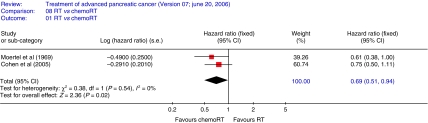
The HR summarises survival for chemoradiotherapy compared to radiotherapy with HR<1 indicating a survival advantage for chemoradiotherapy. Overall survival (Figure 1) was significantly better, with a 31% reduction in risk of death following chemoradiotherapy, compared to radiation alone (two trials 168 patients HR 0.69; CI 0.51–0.94 (FE)).
Figure 1.
Overall survival-radiotherapy vs chemoradiotherapy. The plot demonstrates a 31% reduction in risk of death following chemoradiotherapy, compared to radiation alone (two trials 168 patients HR 0.69; CI 0.51–0.94 (FE)).
AE data could only be assessed for two parameters, vomiting and haematological toxicity, and the latter was lower in the radiotherapy arm compared to the chemoradiation arm (Figure 2). Cohen et al (2005) did not find any difference in disease-free survival (HR 0.77; 95% CI 0.52–1.14) or response rate between the two treatments (RR 1.48; 95% CI 0.37–5.89).
Figure 2.
Adverse events radiotherapy vs chemoradiotherapy. The plot demonstrates vomiting and haematological toxicity adverse events, haematological toxicity was lower in the radiotherapy arm compared to the chemoradiation arm.
Comparison of chemotherapy to chemoradiotherapy, followed by chemotherapy
Four randomised controlled trials (Table 6) with 283 patients were included (Hazel et al, 1981; Klassen et al, 1985; Gastrointestinal Tumour Study Group, 1988; Chauffert et al, 2006), but overall survival data for time-to-event analysis was only available in two studies (134 patients) (Hazel et al, 1981; Klassen et al, 1985). Adequate methods of allocation generation were described in two studies, adequate methods of concealment in one study and adequate losses to follow-up in 3. No study was blinded (Table 1).
Table 6. Included studies – chemotherapy vs chemoradiotherapy, followed by chemotherapy.
| Authors | Group (number randomised) | Median age and gender | Chemotherapy/radiotherapy used and dose |
|---|---|---|---|
| Hazel et al (1981) | Chemo (n=15) | NA | 5FU 500 mg m−2 weekly, methyl CCNU 100 mg m−2 every 6 weeks |
| Combin rx (n=15) | NA | 5FU 500 mg m−2 weekly, radiotherapy 4600 rad in 4.5 weeks. After completion of chemoradiation, methyl CCNU added | |
| Klassen et al (1985) | chemo (n=44) | NA; 31 men, 13 women | 5FU 600 mg m−2 weekly |
| Combin rx (n=47) | NA; 22 men, 25 women | 5FU 600 mg m−2 on first days of radiotherapy 4000 rad radiotherapy over 4 weeks After completion of chemoradiation, 5FU 600 mg m−2 weekly | |
| GITSG (1988) | Chemo (n=21) | 60 years; 13 men, 8 women | 5FU 600 mg m−2 on days 1, 8, 29, 36, streptozocin 1 g m−2 every 8 weeks, mitomycin 10 mg m−2 on day 1 every 8 weeks |
| Combin rx (n=22) | 61 years; 14 men, 8 women | Radiotherapy 5400 rad over 6 weeks with 5FU 350 mg m−2 on first 3 days and last 3 days of radiotherapy. After completion of chemoradiation, chemo-SMF regimen: 5FU 600 mg m−2, streptozocin 1 g m−2 on days 1, 8, 29, 36 every 8 weeks, mitomycin 5 mg m−2 at first dose, then 10 mg m−2 every 8 weeks | |
| Chauffert et al (2006) | Chemotherapy (n=60) | Mean age=60.1 years | Gemcitabine 1000 mg m−2 7q8 weeks initially, then 3q4 weeks |
| Combination rx (n=59) | Mean age=62.7 years | 60 Gy in 6 weeks, with 5FU 300 mg m−2 24 h−1 on days 1–5 every week and cisplatin 20 mg m−2 day−1 on days 1–5 at week 1 and 5. After completion of chemoradiation, gemcitabine 1000 mg m−2 3q4 weeks |
C, CCNU=lomustine; chemo=chemotherapy; Combin rx=combination therapy (chemoradiotherapy, followed by chemotherapy); MMC=mitomycin; NA=data not available.
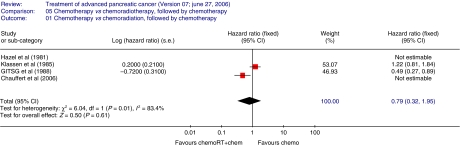
The HR summarises survival for chemoradiotherapy, followed by chemotherapy compared to chemotherapy with HR<1 indicating a survival advantage for chemoradiotherapy, followed by chemotherapy. Overall survival (Figure 3) was not significantly better in the chemoradiation, followed by chemotherapy arm compared to the chemotherapy only arm (two trials 134 patients HR 0.79; 95% CI 0.32–1.95 (RE)) but the wide CI includes clinically significant differences in both directions). There was significant heterogeneity between the two trials analysed (P=0.01; I2=83.4%).
Figure 3.
Overall survival-chemotherapy vs chemoradiotherapy, followed by chemotherapy. The plot demonstrates that overall survival was not significantly better in the chemoradiation followed by chemotherapy arm compared to the chemotherapy only arm (two trials 134 patients HR 0.79; 95% CI 0.32–1.95 (RE)) There was significant heterogeneity between the two trials analysed (P=0.01; I2=83.4%).
The Klassen study found no significant difference in time to progression between the two arms (HR 1.03; 95% CI 0.73–1.47). No other end points could be analysed for this comparison, owing to inadequate published data.
Publication bias
Despite our exhaustive searches, examination of the funnel plots revealed evidence of bias, possibly publication bias, for all comparisons assessed. However, due to the small number of trials included within most comparisons, interpretation of funnel plots is difficult.
DISCUSSION
This systematic review includes 11 studies that randomised 794 patients with locally advanced pancreas cancer and represents the only meta-analyses to date that examine the use of radiotherapeutic approaches in locally advanced pancreas cancer. Compared with the Cochrane Collaboration review (Yip et al, 2006), our review excluded two of their studies (Childs et al, 1965; Earle et al, 1994) but included three additional recent randomised controlled trials (Cohen et al, 2005; Chauffert et al, 2006; Wilkowski et al, 2006). The study conducted by Childs et al (1965) was reported in final form by Moertel et al (1969), and hence the exclusion of the duplicate former study, whereas the study by Earle et al (1994) did not fit into the comparisons that were being assessed. The most appropriate statistical methods for meta-analysis of time to event data extracted from published reports have been used in our report (Parmar et al, 1998).
We did not find any randomised controlled trials that compared radiation alone or chemoradiation alone to BSC. The basis for incorporating radiation therapy in pancreatic cancer was based on a Mayo clinic randomised controlled trial that randomised patients to receive radiotherapy or 5FU-based radiotherapy (Moertel et al, 1969) and an uncontrolled study of 23 patients who received radiotherapy (5040–6680 rad), with 13 patients also receiving 5FU (Haslam et al, 1973). Median survival in the study by Haslam et al (1973) was 7.5 months.
One small randomised controlled trial (Shinchi et al, 2002) of 31 patients compared chemoradiation, followed by chemotherapy, to BSC. 5FU (200 mg m−2 day−1) was administered for the duration of the radiation therapy, followed by 500 mg m−2per week thereafter, until progressive disease or unacceptable toxicity occurred. The regimen of daily 5FU concomitant with radiation differs from all the other randomised controlled trials using chemoradiation (Moertel et al, 1969; Hazel et al, 1981; Klassen et al, 1985; Gastrointestinal Tumour Study Group, 1988; Cohen et al, 2005; Chauffert et al, 2006), wherein weekly 5FU (500–1000 mg m−2 given either weekly or on first and last 3 days of radiotherapy) was used for radio sensitisation. A nonsignificant reduction in liver and peritoneal metastases was seen in the treatment arm. This finding, along with significant improvement in overall survival, may well be an effect of the chemotherapy rather than the radiation. The fact that the majority of patients died of local disease progression (62%) in the treatment arm supports this possibility.
Overall survival was better with chemoradiation compared to radiotherapy alone. Although there was no statistical heterogeneity between these two trials, both the inclusion criteria and radiation techniques differed. Moertel et al (1969) staged patients using clinical and surgical techniques, whereas Cohen used extensive imaging, in the form of CT scan of abdomen, chest X-ray and bone and brain scan, followed by surgical staging. Thus, selection criteria were more stringent in the latter study. Radiation techniques have also improved between the 1960s, when Moertel published his findings, to the 1980s, when the Cohen study was open to accrual. The latter study questions the merit of combining these two modalities, in the light of low response rate, poor survival and increased toxicity. Moreover, neither radiotherapy nor chemoradiation address the micro metastases present in patients labelled as locally advanced cancer (Liu and Traverso, 2004; Shoup et al, 2004).
The 1981 GITSG study was instrumental in popularising multimodality therapy in the treatment of locally advanced pancreas cancer, as it showed a doubling of survival duration over radiation alone (Moertel et al, 1981). This was at the price of greater toxicity, as myelosuppression was more frequent and severe, and two cases of gastrointestinal bleeding and one instance of moderate azotemia were reported in the combined modality arm.
Meta-analysis of chemotherapy vs chemoradiation, followed by chemotherapy in the two evaluable trials, found no significant difference between the two approaches, in the presence of inter-study heterogeneity. This could be owing to the following factors:
Difference in radiation. The GITSG study (Gastrointestinal Tumour Study Group, 1988) utilised 54 Gy, given via three or four fields whereas the Klassen study (Klassen et al, 1985) used 4000 rad given by parallel-opposed anterior and posterior portals.
The chemotherapy agents also differed, with the GITSG study using a combination of 5FU, streptozotocin and mitomycin C (SMF), whereas the Klassen study used single agent 5FU.
The difference in effects between the two studies could be due to the difference in the radiotherapy used, as the GITSG study concluded that the SMF regimen did not prove to be superior to single agent 5FU. The CIs here are very wide, with reduction in risk of death with chemoradiation followed by chemotherapy being, on one end of the spectrum, as much as 68%, whereas at the other end, the increase in risk of death being 95% greater compared to chemotherapy alone. Another point to be borne in mind was that the GITSG study had closed prematurely, owing to lack of funding, with the total number of patients accrued only a third of the planned sample size. Early stoppage of a trial could lead to an erroneous estimation of treatment effects, with a propensity for exaggeration, that is, a random high (Schulz and Grimes, 2005).
For the two studies (Hazel et al, 1981; Chauffert et al, 2006) in this comparison wherein we were unable to calculate HR from the published data, the overall results did not support the use of chemoradiotherapy followed by chemotherapy, over chemotherapy alone. In the trials conducted by Hazel et al (1981) of 30 patients, there was no significant difference in median survival between the two arms (7.3 months in multimodality treatment arm vs 7.8 months in the chemotherapy arm). A recent randomised controlled trial, done nearly two decades after the GITSG study, found significant survival advantage (log rank P=0.014) with gemcitabine single agent chemotherapy (median survival 14.3 months) over multimodality therapy in locally advanced disease (median survival=8.4 months), necessitating early stoppage of the trial (Chauffert et al, 2006). All studies found greater haematological toxicity in the multimodality treatment arm, and the Chauffert study found a higher incidence of nonhaematological toxicity as well.
To conclude, survival benefit was demonstrated for the comparison of chemoradiotherapy over radiation alone, with evidence from a single randomised controlled trial demonstrating survival benefit for chemoradiotherapy followed by chemotherapy over radiation alone and chemoradiotherapy followed by chemotherapy over BSC. There is insufficient evidence to support the use of chemoradiation with follow on chemotherapy over chemotherapy alone in the absence of a survival advantage, coupled with greater toxicity. However, the wide CIs make it difficult to rule out important clinical differences. The results of the Intergroup study E4201, which aimed to compare gemcitabine alone to gemcitabine and radiation therapy would have helped settle the issue of whether there is a role, if at all, for multimodality therapy in locally advanced pancreatic cancer (Lockhart et al, 2005). Unfortunately, this trial was closed owing to poor accrual and hence the question remains unanswered (Cardenes et al, 2006).
There are missing links in the chain of evidence using radiation therapy in advanced pancreas cancer, in particular, the fact that at inception, radiation alone was not compared against BSC in a randomised setting, unlike with chemotherapy approaches. In addition, there are several small inadequately powered randomised controlled trials testing different hypothesis, with a missing golden thread in the evolution of these studies. Staging has improved over time, with significant advances in imaging in the last 5 years following the advent of multidetector row helical CT with or without positron emission tomography (Michl et al, 2005). The frontline approaches to staging today are contrast-enhanced multi-detector row helical CT, with its high sensitivity for identifying vascular invasion, and endoscopic ultrasound, which can pick up tumours as small as 2–3 mm. In the event of these modalities being equivocal, there are additional tools available in the form of MRI with MR-angiography, MRCP, PET/CT and staging laparoscopy. Radiotherapy has also evolved, from the two-dimensional split course radiation encompassing larger treatment volumes with resultant toxicity, to the newer, more targeted three-dimensional conformal radiation and the intensity modulated radiation therapy (IMRT) approaches (Garofalo et al, 2006). Image-guided radiation therapy (IGRT) takes into account the interfraction and intrafraction dose variation, as a consequence of organ motion. Better technology and the use of conformal treatment have led to higher tolerable radiation doses (Yang et al, 2005). With improvements in staging and radiation techniques future studies may re-evaluate the application of upfront chemoradiation or the use of early systemic therapy for the treatment of micro metastases followed by consolidation therapy within adequately powered studies.
To conclude, we advocate the use of chemotherapy in patients with locally advanced cancer, as currently there is insufficient evidence to endorse the use of chemoradiation, followed by chemotherapy, over chemotherapy alone. This recommendation is also supported by a recent meta-analysis, which demonstrated a significant survival benefit for chemotherapy over BSC and gemcitabine-based combinations over single agent gemcitabine in patients with advanced pancreatic cancer (Sultana et al, 2007). It is important to bear in mind that no randomised controlled trial has compared radiotherapy to chemotherapy and the single randomised controlled trials that compared chemoradiation, followed by chemotherapy to either BSC or radiation, are small. With improvements in staging and radiation techniques future trials may influence these recommendations.
Acknowledgments
The following trialists facilitated these meta-analyses by generously providing clarifications and/or extra data:
1. Dr Steven J Cohen, Department of Medical Oncology, Fox Chase Cancer Centre, Philadelphia, USA and Dr Paul Catalano, Statistician, ECOG (clarifications and extra data: Cohen et al, 2005)
2. Dr Andrew Maksymiuk, Department of Haematology/Oncology, University of Manitoba, Canada (clarifications (Hazel et al, 1981))
3. Prof Harold O Douglass, Former Chair, Gastrointestinal Tumour Study Group (clarifications:Gastrointestinal Tumour Study Group, 1988; Deeks et al, 2004)
4. Dr Hiroyuki Shinchi, Department of Surgical Oncology and HPB Surgery, Kagoshima University, Japan (clarifications: Shinchi et al, 2002)
5. Dr Joel Novak, Statistician for GITSG trials (clarifications: Moertel et al, 1981)
Footnotes
Research support: Cancer Research UK.
References
- Altman D, De Stavola B, Love S, Stepniewska K (1995) Review of survival analysis published in cancer journals. Br J Cancer 72: 511–518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Cancer Society (2003) Cancer Facts and Figures 2003. American Cancer Society: Atlanta [Google Scholar]
- Banu E, Oudard S, Banu A, Fodor A, Landi B, Lecomte T, Laurent-Puig P, Cugnenc PJ, Andrieu JM (2005) Cumulative meta-analysis of randomised trials comparing gemcitabine-based chemotherapy vs gemcitabine alone in patients with advanced or metastatic pancreatic cancer. In ASCO Annual Meeting, abstract no. 4101, Orlando, USA
- Bria E, Carlini P, Gelibter A, Ruggeri E, Ceribelli A, Pino M, Terzoli E, Cognetti F, Giannarelli D, Milella M (2006) Current status of targeted agents in advanced pancreatic cancer (APC): A pooled analysis of 2,361 patients (pts) enrolled in six phase III trials. J Clin Oncol 24: 4126 [Google Scholar]
- Cardenes H, Chiorean E, DeWitt J, Schmidt M, Loehrer P (2006) Locally advanced pancreatic cancer: Current therapeutic approach. Oncologist 11: 612–623 [DOI] [PubMed] [Google Scholar]
- Chauffert B, Mornex F, Bonnetain F, Triboulet J, Bouche O, Rougier P, Bosset J, Aparicio T, Masskouri F, Bedenne L (2006) Phase III trial comparing initial chemoradiotherapy (intermittent cisplatin and infusional 5-FU) followed by gemcitabine vs gemcitabine alone in patients with locally advanced non metastatic pancreatic cancer: A FFCD-SFRO study. J Clin Oncol 24: 4008 [Google Scholar]
- Childs D, Moertel C, Holbrook M, Reitemeier RJ, Colby Jr MY (1965) Treatment of malignant neoplasms of the gastrointestinal tract with a combination of 5FU and radiation. Radiology 84: 843–848 [DOI] [PubMed] [Google Scholar]
- Cohen S, Dobelbower R, Lipsitz S, Catalano PJ, Sischy B, Smith TJ, Haller DG, Eastern Cooperative Oncology Group (2005) A randomised phase III study of radiotherapy alone or with 5-fluorouracil and mitomycin C in patients with locally advanced adenocarcinoma of the pancreas: Eastern Cooperative Oncology Group Study E8282. Int J Radiat Oncol Biol Phys 62: 1345–1350 [DOI] [PubMed] [Google Scholar]
- Deeks J, Altman D, Bradburn M (2001) Statistical methods for examining heterogeneity and combining results from several studies in meta-analysis. In Systematic Reviews in Health Care. Meta-Analysis in Context Egger M, Smith G and Altman D (eds) pp 285–312. London: BMJ, Publishing Group [Google Scholar]
- Deeks J, Higgins J, Altman D (2004) Analysing and presenting results. In Cochrane Reviewers Handbook 4.2.2 [updated March 2004]; Section 8 pp www.cochrane.org/resources/handbook/hbook.htm.
- Earle J, Foley J, Wieand H, Kvols LK, McKenna PJ, Krook JE, Tschetter LK, Schutt AJ, Twito DI (1994) Evaluation of external beam radiotherapy plus 5 Fluorouracil vs external beam radiotherapy plus hyacanthone in confined, unresectable pancreatic cancer. Int J Radiat Oncol Biol Phys 28: 207–211 [DOI] [PubMed] [Google Scholar]
- Fung M, Ishiguro H, Takayama S, Morizane T, Adachi S, Sakata T, Oncology Department Japan Clinical Research Eli Lilly Company Japan KK, Kobe Japan (2003) Survival benefit of chemotherapy treatment in advanced pancreatic cancer: a meta-analysis. In 2003 ASCO Annual Meeting, abstract no. 1155, USA
- Garofalo M, Flannery T, Regine W (2006) The case for adjuvant chemoradiation for pancreatic cancer. Best Practice Res Clin Gastroenterol 20: 403–416 [DOI] [PubMed] [Google Scholar]
- Gastrointestinal Tumour Study Group (1985) Radiation therapy combined with adriamycin or 5-Fluorouracil for the treatment of locally unresectable pancreatic carcinoma. Cancer 56: 2563–2568 [DOI] [PubMed] [Google Scholar]
- Gastrointestinal Tumour Study Group (1988) Treatment of locally unresectable carcinoma of the pancreas; comparison of combined-modality therapy (chemotherapy plus radiotherapy) to chemotherapy alone. J Natl Cancer Inst 80: 751–755 [PubMed] [Google Scholar]
- Haslam J, Cavanaugh P, Stroup S (1973) Radiation therapy in the treatment of unresectable adenocarcinoma of the pancreas. Cancer 32: 1341–1345 [DOI] [PubMed] [Google Scholar]
- Hazel J, Thirlwell M, Huggins M (1981) Multidrug chemotherapy with/without radiation for carcinoma of the stomach and pancreas: a prospective randomised trial. J Assoc Can Radiol 32: 164–165 [PubMed] [Google Scholar]
- Jemal A, Siegel R, Ward E, Murray T, Xu J, Smigal C, Thun M (2006) Cancer statistics. CA Cancer J Clin 56: 106–130 [DOI] [PubMed] [Google Scholar]
- Klassen D, MacIntyre J, Catton G, Engstrom PF, Moertel CG (1985) Treatment of locally unresectable cancer of the stomach and pancreas: A randomised comparison of 5FU alone with radiation plus concurrent and maintainence 5FU- An Eastern Cooperative Oncology Group Study. J Clin Oncol 3: 373–378 [DOI] [PubMed] [Google Scholar]
- Li C-P, Chao Y, Chi K-H, Chan W-K, Teng H-C, Lee R-C, Chang F-Y, Lee S-D, Yen S-H (2003) Concurrent chemoradiotherapy treatment of locally advanced pancreatic cancer: gemcitabine vs 5-Fluorouracil, a randomised controlled trial. Int J Radiat Oncol Biol Phys 57: 98–104 [DOI] [PubMed] [Google Scholar]
- Liang H (2005) Comparing gemcitabine-based combination chemotherapy with gemcitabine alone in inoperable pancreatic cancer: a meta-analysis. In ASCO Annual Meeting, abstract no. 4110, Orlando, USA
- Light R, Pillemer D (1984) Summing Up: The Science of Reviewing Research. Harvard University Press: Cambridge, MA [Google Scholar]
- Liu R, Traverso L (2004) Laparoscopic staging should be used routinely for locally extensive cancer of the pancreatic head. J Gastrointest Surg 8: 923–924 [DOI] [PubMed] [Google Scholar]
- Lockhart A, Rothenberg M, Berlin J (2005) Treatment for pancreatic cancer: Current therapy and continued progress. Gastroenterology 128: 1642–1654 [DOI] [PubMed] [Google Scholar]
- Maheshwari V, Moser A (2005) Current management of locally advanced pancreatic cancer. Nat Clin Pract Gastroenterol Hepatol 2: 356–364 [DOI] [PubMed] [Google Scholar]
- Michl P, Pauls S, Gress TM (2005) Evidence-based diagnosis and staging of pancreatic cancer. Best Practice Res Clin Gastroenterol 20: 227–251 [DOI] [PubMed] [Google Scholar]
- Milella M, Carlini P, Gelibter A, Ruggeri E, Ceribelli A, Pino M, Terzoli E, Cognetti F, Giannarelli D, Bria E (2006) Gemcitabine-based polychemotherapy for advanced pancreatic cancer (APC): is it ready for prime time? pooled analysis of 3682 patients (pts) enrolled in 12 phase III trials. J Clin Oncol 24: 4118 [Google Scholar]
- Moertel C, Childs D, Reitmeier R, Colby Jr MY, Holbrook MA (1969) Combined 5FU and supervoltage RT for locally unresectable GI cancer. Lancet 2: 865–867 [DOI] [PubMed] [Google Scholar]
- Moertel C, Frytak S, Hahn RO'Connell MJ, Reitemeier RJ, Rubin J, Schutt AJ, Weiland LH, Childs DS, Holbrook MA, Lavin PT, Livstone E, Spiro H, Knowlton A, Kalser M, Barkin J, Lessner H, Mann-Kaplan R, Ramming K, Douglas Jr HO, Thomas P, Nave H, Bateman J, Lokich J, Brooks J, Chaffey J, Corson JM, Zamcheck N, Novak JW (1981) Therapy of locally unresectable pancreatic carcinoma: a randomised comparison of high dose (6000 rad) radiation alone, moderate dose radiation (4000 rad+5-Fluorouracil), and high dose radiation+5-Fluorouracil. Cancer 48: 1705–1710 [DOI] [PubMed] [Google Scholar]
- Parmar M, Valter T, Stewart L (1998) Extracting summary statistics to perform meta-analysis of the published literature for survival end points. Statist Med 17: 2815–2834 [DOI] [PubMed] [Google Scholar]
- Schulz K, Grimes D (2005) Multiplicity in randomised trials II: subgroup and interim analyses. Lancet 365: 1657–1661 [DOI] [PubMed] [Google Scholar]
- Shinchi H, Takao S, Noma H, Matsuo Y, Mataki Y, Mori S, Aikou T (2002) Length and quality of survival after external-beam radiotherapy with concurrent continuous 5-Fluorouracil infusion for locally unresectable pancreatic cancer. Int J Radiat Oncol Biol Phys 53: 146–150 [DOI] [PubMed] [Google Scholar]
- Shoup M, Winston C, Brennan M, Bassman D, Conlon K (2004) Is there a role for staging laparoscopy in patients with locally advanced, unresectable pancreatic adenocarcinoma? J Gastrointest Surg 8: 1068–1071 [DOI] [PubMed] [Google Scholar]
- Sterne J, Egger M, Sutton A (2001) Meta-analysis software. In Systematic Reviews in Health Care. Meta-Analysis in Context Egger M, Smith G and Altman D (eds) pp. 336–346. London: BMJ, Publishing Group [Google Scholar]
- Sultana A, Tudur SC, Cunningham D, Starling N, Neoptolemos JP, Ghaneh P (2007) Meta-analyses of chemotherapy for locally advanced and metastatic pancreatic cancer. J Clin Oncol (in press) [DOI] [PMC free article] [PubMed]
- White R, Lee C, Anscher M, Gottfried M, Wolff R, Keogan M, Pappas T, Hurwitz H, Tyler D (1999) Preoperative chemoradiation for patients with locally advanced adenocarcinoma of the pancreas. Ann Surg Oncol 6: 38–45 [DOI] [PubMed] [Google Scholar]
- Wilkowski R, Rau H, Bruns C, Wagner A, Sauer R, Hohenberger W, Koelbl O, Heinemann V (2006) Randomised phase II trial comparing gemcitabine/cisplatin-based chemoradiotherapy to 5-FU based chemoradiotherapy in patients with locally advanced pancreatic cancer. J Clin Oncol 24: 403816921063 [Google Scholar]
- Williamson P, Smith C, Hutton J, Marson A (2002) Aggregate data meta-analysis with time-to-event outcome. Statist Med 21: 3337–3351 [DOI] [PubMed] [Google Scholar]
- Yang G, Wagner T, Fuss M, Thomas C (2005) Multimodality approaches for pancreatic cancer. CA Cancer J Clin 55: 352–367 [DOI] [PubMed] [Google Scholar]
- Yip D, Karapetis C, Strickland A, Steer C, Goldstein D (2006) Chemotherapy and radiotherapy for inoperable pancreatic cancer. In Cochrane Database of Systematic Reviews 3, Art. No. CD002093. DOI: 10.1002/14651858.CD002093.pub2 [DOI] [PubMed] [Google Scholar]