Abstract
We performed a meta-analysis of studies of the association between excess body weight and risk of gallbladder cancer identified from MEDLINE and EMBASE databases from 1966 to February 2007 and the references of retrieved articles. A random-effects model was used to combine results from eight cohort studies and three case–control studies, with a total of 3288 cases. Compared with individuals of ‘normal weight’, the summary relative risk of gallbladder cancer for those who were overweight or obese was 1.15 (95% CI, 1.01–1.30) and 1.66 (95% CI, 1.47–1.88) respectively. The association with obesity was stronger for women (relative risk, 1.88; 95% CI, 1.66–2.13) than for men (relative risk, 1.35; 95% CI, 1.09–1.68). There was no statistically significant heterogeneity among the results of individual studies. This meta-analysis confirms the association between excess body weight and risk of gallbladder cancer.
Keywords: body mass index, case–control studies, cohort studies, gallbladder cancer, meta-analysis, obesity
Cancer of the gallbladder is a highly fatal malignancy, usually diagnosed at an advanced stage, with a 5-year survival rate of less than 10% (Misra et al, 2003). Although rare in most parts of Europe and in the United States, relatively high incidence and mortality rates are found in northern India, Chile, Pakistan, Korea and some eastern European countries (Randi et al, 2006). Gallbladder cancer is about two to five times more common in women than in men. Little is known about the aetiology of this neoplasm besides a strong link with gallstones (Randi et al, 2006). Obesity increases the risk for gallstones, and thus may be a risk factor of gallbladder cancer. Because the epidemiologic evidence on excess body weight and risk of gallbladder cancer has not yet been quantitatively summarised, we conducted a meta-analysis of cohort and case–control studies on this subject.
METHODS
Study selection
We conducted a computerised search of the MEDLINE and EMBASE databases for studies that were published from 1966 to February 2007, using the Medical Subject Headings or text words ‘body mass index’, ‘BMI’, ‘obesity’, or ‘weight’ combined with ‘gallbladder cancer’ or ‘gallbladder neoplasm’. In addition, we manually searched the reference lists of retrieved articles for additional relevant studies. No language restrictions were imposed.
Studies were included in the meta-analysis if they met the following criteria: (1) cohort or case–control study in which gallbladder cancer incidence or mortality was an outcome; (2) the exposure of interest was overweight and/or obesity defined by body mass index (BMI) (the weight in kilograms divided by the square of height in metres) and (3) estimates of relative risk (rate ratio, odds ratio, or standardised incidence ratio) with their corresponding 95% confidence intervals were reported. When there were multiple publications from the same study population, we included the one with the largest sample size.
We identified eight cohort studies (Møller et al, 1994; Wolk et al, 2001; Calle et al, 2003; Samanic et al, 2004, 2006; Engeland et al, 2005; Kuriyama et al, 2005; Oh et al, 2005) and four case–control studies (Zatonski et al, 1992, 1997; Strom et al, 1995; Serra et al, 2002) that reported results on overweight/obesity and risk of gallbladder cancer. One case–control study (Zatonski et al, 1992) was excluded because this study was included in a multicentre case–control study (Zatonski et al, 1997); the multicentre case–control study was included in this meta-analysis.
Data extraction
For each study, the following data were extracted: first author's last name; publication year; country in which the study was performed; study design; source of controls (in case–control studies); sample size; method of assessment of weight and height (self-reported or measured); type of outcome (incidence or mortality); covariates for adjustments in the analysis and the risk estimates with corresponding 95% confidence intervals. From each study, we extracted the risk estimates that reflected the greatest degree of adjustment for potential confounders.
Statistical analysis
Relative risk was used as the measure of the association between BMI and gallbladder cancer. Because gallbladder cancer is a rare disease, odds ratios and standardised incidence ratio provide a valid estimate of the relative risk; we report all results as relative risk for simplicity. The relative risks and corresponding standard errors (derived from the confidence intervals) from individual studies were logarithmically transformed to stabilise variances and normalise the distributions. We computed summary relative risk estimates for two categories of BMI as defined by the World Health Organisation (WHO) for adults: overweight (BMI 25–30 kg m−2) and obesity (BMI ⩾30 kg m−2) compared with ‘normal’ weight (BMI 18.5–24.9 kg m−2) as the reference category. Where nonstandard categories of BMI were used, we chose the category that was most similar to those defined by the WHO. Study-specific estimates were combined using the DerSimonian and Laird random-effects model, which incorporates both within- and between-study variation (DerSimonian and Laird, 1986).
Statistical heterogeneity among studies was evaluated with the Q and I2 statistics (Higgins and Thompson, 2002). For the Q statistic, statistical significance was set at P<0.1. A meta-regression analysis was performed to investigate whether the association between obesity and risk of gallbladder cancer differed by study design or sex. We conducted a sensitivity analysis, in which one study at a time was removed and the rest analysed to assess whether the results were markedly affected by a single study. We used funnel plots (i.e., plots of study results against precision) to assess publication bias, and tested its symmetry (Egger et al, 1997).
Results are presented graphically, whereby squares represent study-specific estimates and diamonds represent summary estimates. The area of each square is proportional to the inverse of the variance of the logarithm of the relative risk; 95% confidence intervals for individual studies are represented by horizontal lines and for the summary estimates by the width of the diamonds. All statistical analyses were performed with Stata, version 9.0 (StataCorp, College Station, TX, USA).
Population-attributable risk (PAR) for gallbladder cancer was estimated for individuals with excess body weight (BMI ⩾25 kg m−2) compared with those of normal weight (BMI <25 kg m−2). The PAR describes the theoretical proportion of cases that would be prevented if all individuals were moved into the exposure level associated with the lowest risk for that factor. The PAR (PAR%) was calculated as PAR%=(p × (RR−1)/(p × (RR−1)+1)) × 100%, where p represents the prevalence in the population and RR the relative risk. Prevalence data were obtained from the National Health and Nutrition Examination Survey that assessed the prevalence of overweight and obesity in a representative sample of the US population (Ogden et al, 2006). In that survey, 39.7% of the men were overweight and 31.1% were obese. Among women, 28.6% were overweight and 33.2% were obese. PARs were calculated for the overweight and obese categories using the obtained summary relative risk estimates, and then summarised across the two categories for men and women separately.
RESULTS
The eight cohort studies (Møller et al, 1994; Wolk et al, 2001; Calle et al, 2003; Samanic et al, 2004, 2006; Engeland et al, 2005; Kuriyama et al, 2005; Oh et al, 2005) and three case–control studies (Strom et al, 1995; Zatonski et al, 1997; Serra et al, 2002) that were included in the meta-analysis involved a total of 3288 cases. Main characteristics of the studies are shown in Table 1. Weight and height were measured in three studies (Engeland et al, 2005; Oh et al, 2005; Samanic et al, 2006) and self-reported in five studies (Strom et al, 1995; Zatonski et al, 1997; Serra et al, 2002; Calle et al, 2003; Kuriyama et al, 2005); in three studies, obesity was defined by a discharge diagnosis of obesity (Møller et al, 1994; Wolk et al, 2001; Samanic et al, 2004). Among case–control studies, one used population-based controls (Zatonski et al, 1997) and two used hospital-based controls (Strom et al, 1995; Serra et al, 2002).
Table 1. Characteristics of the 11 studies included in the meta-analysis.
|
Relative riska
(95% CI) |
|||||||
|---|---|---|---|---|---|---|---|
| Study (country) | Study design | Cases (men/women) | Controls or cohort size (men/women) | BMI (kg m–2) categories | Men | Women | Adjustments |
| Møller et al, 1994 (Denmark) | Cohort | 2/26 | Cohort | Non-obese | 1.0 (reference) | 1.0 (reference) | Age |
| 14 531/29 434 | Obese | 0.5 (0.1–1.8) | 1.4 (0.9–2.1) | ||||
| Strom et al, 1995 (Mexico) | C–C | 65 | 110 | Men and women | Age, sex | ||
| <24.0 | 1.0 (reference) | ||||||
| 24.0–25.9 | 1.5 (0.5–4.6) | ||||||
| 26.0–28.0 | 2.2 (0.7–8.4) | ||||||
| >28.0 | 1.6 (0.4–6.1) | ||||||
| Zatonski et al, 1997 (Multicenterb) | C–C | 44/145 | 798/681 | Quartile 1 | 1.0 (reference) | 1.0 (reference) | Age, center, education, alcohol, smoking, response status |
| Quartile 2 | 1.0 (0.3–3.0) | 1.7 (0.9–3.1) | |||||
| Quartile 3 | 0.7 (0.3–2.0) | 1.5 (0.8–3.0) | |||||
| Quartile 4 | 1.0 (0.3–2.8) | 2.1 (1.2–3.8) | |||||
| Wolk et al, 2001 (Sweden) | Cohort | 2/29 | Cohort | Non-obese | 1.0 (reference) | 1.0 (reference) | Age, calendar year |
| 8165/19 964 | Obese | 0.9 (0.1–3.4) | 1.7 (1.1–2.5) | ||||
| Serra et al, 2002 (Chile) | C–C | 114 | 114 | Men and women | Age, sex | ||
| <25.0 | 1.0 (reference) | ||||||
| 25.0–29.9 | 0.8 (0.4–1.4) | ||||||
| ⩾30.0 | 0.9 (0.4–1.8) | ||||||
| Calle et al, 2003 (United States) | Cohort | 180/304 | Cohort | 18.5–24.9 | 1.00 (reference) | 1.00 (reference) | Age, race, marital status, education, |
| 404 576/495 477 | 25.0–29.9 | 1.34 (0.97–1.84) | 1.12 (0.86–1.47) | smoking, physical activity, aspirin use, estrogen-replacement therapy (women), alcohol, dietary factors | |||
| ⩾30.0 | 1.76 (1.06–2.94) | 2.13 (1.56–2.90) | |||||
| Samanic et al, 2004 (United States) | Cohort | 291 whites/ | Cohort | White men | Black men | Age, calendar year | |
| 47 blacks | 366 8486 white | Non-obese | 1.00 (reference) | 1.00 (reference) | |||
| men/832 214 black men | Obese | 1.70 (1.13–2.57) | 0.93 (0.23–3.86) | ||||
| Kuriyama et al, 2005 (Japan) | Cohort | 9/24 | Cohort | 18.5–24.9 | 1.00 (reference) | 1.00 (reference) | Age, smoking, type of health |
| 12 485/15 054 | 25.0–27.4 | 0.46 (0.05–3.93)c | 0.83 (0.23–2.98) | insurance, intakes of alcohol, meat, | |||
| 27.5–29.9 | 3.43 (1.19–9.94) | fish, fruits, vegetables, bean-paste | |||||
| ⩾30.0 | 4.45 (1.39–14.2) | soupd | |||||
| Oh et al, 2005 (Korea) | Cohort | 182 | Cohort | 18.5–22.9 | 1.00 (reference) | Age, area of residence, smoking, | |
| 781 283/– | 23.0–24.9 | 1.55 (1.10–2.20) | exercise, alcohol | ||||
| 25.0–26.9 | 1.15 (0.74–1.80) | ||||||
| ⩾27.0 | 1.25 (0.70–2.24) | ||||||
| Engeland et al, 2006 (Norway) | Cohort | 628/1087 | Cohort | 18.5–24.9 | 1.00 (reference) | 1.00 (reference) | Age, birth cohort |
| 962 901/1037 077 | 25.0–29.9 | 1.00 (0.84–1.17) | 1.27 (1.10–1.47) | ||||
| ⩾30.0 | 1.38 (1.01–1.89) | 1.88 (1.60–2.21) | |||||
| Samanic et al, 2006 (Sweden) | Cohort | 109 | Cohort | 18.5–24.9 | 1.00 (reference) | Age, smoking | |
| 362 552/– | 25.0–29.9 | 0.93 (0.62–1.39) | |||||
| ⩾30.0 | 1.40 (0.73–2.70) | ||||||
BMI=body mass index; C–C=case–control study; CI=confidence interval.
Relative risks are rate ratios, odds ratios, or standardised incidence ratios.
Multicentre case–control study conducted in five centres located in Australia (Adelaide), Canada (Montreal and Toronto), The Netherlands (Utrecht), and Poland (Opole).
The highest BMI category for men was 25.0–27.4 kg m–2; there was only one case in this category.
Odds ratios for women were further controlled for age at menarche, age at end of first pregnancy, and menopausal status.
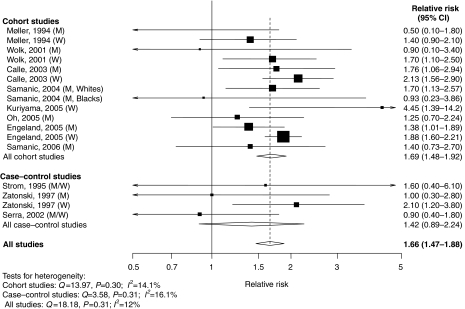
Relative risk estimates of gallbladder cancer for ‘obesity’ compared with ‘normal weight’ in individual studies (separately for men and women wherever this information was available) and all studies combined are shown in Figure 1. Of the 18 relative risk estimates from the 11 studies, 14 were above one, of which eight were statistically significant. Meta-analysis of all studies found that compared to individuals with ‘normal weight’, those who were ‘obese’ had a 66% increased risk with no statistically significant heterogeneity among individual studies (Figure 1). In a sensitivity analysis, no single study appreciably influenced the summary relative risk, which ranged from 1.59 to 1.73 when omitting individual studies one at a time. The relation with obesity did not differ – statistically – significantly by study design (P=0.56) (Figure 1). However, the summary estimate was higher (P=0.02) for women (RR, 1.88; 1.66–2.13) than for men (RR, 1.35; 95% CI, 1.09–1.68). Summary relative risks were 1.82 (95% CI, 1.37–2.42) for studies based on self-report, 1.60 (95% CI, 1.29–1.98) for those with weight and height measurements and 1.51 (95% CI, 1.20–1.91) for those based on a discharge diagnosis of obesity. There was some indication of publication bias on the funnel plot (data not shown), suggesting the relative absence of small studies with null results; the Egger's test yielded a P-value of 0.05.
Figure 1.
Relative risks of gallbladder cancer associated with obesity. Relative risk estimates are for comparison of individuals in the ‘obese’ category compared to those with ‘normal weight’. M=men; W=women.
Eight studies presented relative risk estimates for ‘overweight’ compared with ‘normal weight’ (Strom et al, 1995; Zatonski et al, 1997; Serra et al, 2002; Calle et al, 2003; Engeland et al, 2005; Kuriyama et al, 2005; Oh et al, 2005; Samanic et al, 2006). Meta-analysis of these studies found those in the overweight category had a statistically significant 15% increased risk (RR, 1.15; 95% CI, 1.01–1.30) compared to those of normal weight, with no statistically significant heterogeneity among studies (Q=14.93; P=0.19; I2=26.3). The summary relative risk did not differ – statistically – significantly by study design (P=0.86), but was higher (P=0.09) for women (RR, 1.28; 95% CI, 1.04–1.57) than men (RR, 1.05; 95% CI, 0.92–1.19). We found no evidence of publication bias on the funnel plot (data not shown) or by Egger's test (P=0.87).
The PAR for excess body weight was calculated using the estimates of prevalence in the United States (Ogden et al, 2006) and the obtained summary relative risks for overweight (1.05 for men and 1.28 for women) and obesity (1.35 for men and 1.88 for women). We estimated that 12% of the gallbladder cancer cases among men and 30% among women could be attributable to excess body weight (BMI ⩾25 kg m−2).
DISCUSSION
This meta-analysis of published studies indicates that the risk of gallbladder cancer increases with increasing BMI. Summary results showed that gallbladder cancer risk was 15 and 66% higher among those who were overweight and obese, respectively, as compared with those of normal weight. The association between obesity and gallbladder cancer risk was stronger in women than in men.
Several potential limitations should be considered when interpreting the results from this meta-analysis. First, because of the observational design of the included studies, we cannot rule out the possibility that the observed association between excess body weight and risk is owing to confounding from other risk factors. Second, five studies (Strom et al, 1995; Zatonski et al, 1997; Serra et al, 2002; Calle et al, 2003; Kuriyama et al, 2005) in this meta-analysis relied on self-reported weight and height and it is possible that weight has been under-reported, particularly by overweight or obese individuals (IARC, 2002). The summary relative risk estimate was higher for studies that relied on self-reported weight and height. In case–control studies, weight loss before the cancer diagnosis would attenuate the relative risk estimates. The summary relative risk estimate was slightly lower for case–control studies than for cohort studies. Findings from case–control studies could also be biased if individuals in the control group were more health conscious and thus less likely to be overweight or obese than the cases. Finally, there was some suggestion of publication bias. The presence of publication bias could have led to an overestimation of the true association between obesity and risk of gallbladder cancer.
Potential biological mechanisms for the association include increased concentrations of endogenous hormones (estrogens, insulin, and insulin-like growth factor-I) in overweight and obese individuals. Obesity may increase risk indirectly by increasing the risk of gallstones, which, in turn, increase the risk for gallbladder cancer (Randi et al, 2006). The reason for the stronger association observed with obesity in women than in men is unclear. The three studies that found that obesity was associated with an increased risk in men only were all based on a small number of male cases in the obese category (Møller et al, 1994; Zatonski et al, 1997; Wolk et al, 2001), so the stronger association observed in women may be due to chance.
In summary, this meta-analysis indicates that excess body weight is a risk factor for gallbladder cancer, suggesting that it may in part be prevented by maintaining a healthy body weight.
Acknowledgments
This work was supported by grants from the Swedish Cancer Society.
References
- Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ (2003) Overweight, obesity, and mortality from cancer in a prospectively studied cohort of US adults. N Engl J Med 348: 1625–1638 [DOI] [PubMed] [Google Scholar]
- DerSimonian R, Laird N (1986) Meta-analysis in clinical trials. Control Clin Trials 7: 177–188 [DOI] [PubMed] [Google Scholar]
- Egger M, Davey Smith G, Schneider M, Minder C (1997) Bias in meta-analysis detected by a simple, graphical test. BMJ 315: 629–634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engeland A, Tretli S, Austad G, Bjørge T (2005) Height and body mass index in relation to colorectal and gallbladder cancer in two million Norwegian men and women. Cancer Causes Control 16: 987–996 [DOI] [PubMed] [Google Scholar]
- Higgins JP, Thompson SG (2002) Quantifying heterogeneity in a meta-analysis. Stat Med 21: 1539–1558 [DOI] [PubMed] [Google Scholar]
- IARC (2002) IARC handbooks of cancer prevention. Weight control and physical activity Vol. 6. IARC Press: Lyon (France) [Google Scholar]
- Kuriyama S, Tsubono Y, Hozawa A, Shimazu T, Suzuki Y, Koizumi Y, Ohmori K, Nishino Y, Tsuji I (2005) Obesity and risk of cancer in Japan. Int J Cancer 113: 148–157 [DOI] [PubMed] [Google Scholar]
- Misra S, Chaturvedi A, Misra NC, Sharma ID (2003) Carcinoma of the gallbladder. Lancet Oncol 4: 167–176 [DOI] [PubMed] [Google Scholar]
- Møller H, Mellemgaard A, Lindvig K, Olsen JH (1994) Obesity and cancer risk: a Danish record-linkage study. Eur J Cancer 30A: 344–350 [DOI] [PubMed] [Google Scholar]
- Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM (2006) Prevalence of overweight and obesity in the United States, 1999–2004. JAMA 295: 1549–1555 [DOI] [PubMed] [Google Scholar]
- Oh SW, Yoon YS, Shin SA (2005) Effects of excess weight on cancer incidences depending on cancer sites and histologic findings among men: Korea National Health Insurance Corporation Study. J Clin Oncol 23: 4742–4754 [DOI] [PubMed] [Google Scholar]
- Randi G, Franceschi S, La Vecchia C (2006) Gallbladder cancer worldwide: geographical distribution and risk factors. Int J Cancer 118: 1591–1602 [DOI] [PubMed] [Google Scholar]
- Samanic C, Gridley G, Chow WH, Lubin J, Hoover RN, Fraumeni Jr JF (2004) Obesity and cancer risk among white and black United States veterans. Cancer Causes Control 15: 35–43 [DOI] [PubMed] [Google Scholar]
- Samanic C, Chow WH, Gridley G, Jarvholm B, Fraumeni JF (2006) Relation of body mass index to cancer risk in 362 552 Swedish men. Cancer Causes Control 17: 901–909 [DOI] [PubMed] [Google Scholar]
- Serra I, Yamamoto M, Calvo A, Cavada G, Baez S, Endoh K, Watanabe H, Tajima K (2002) Association of chili pepper consumption, low socioeconomic status and longstanding gallstones with gallbladder cancer in a Chilean population. Int J Cancer 102: 407–411 [DOI] [PubMed] [Google Scholar]
- Strom BL, Soloway RD, Rios-Dalenz JL, Rodriguez-Martinez HA, West SL, Kinman JL, Polansky M, Berlin JA (1995) Risk factors for gallbladder cancer. An international collaborative case-control study. Cancer 76: 1747–1756 [DOI] [PubMed] [Google Scholar]
- WHO. Physical status: the use and interpretation of anthropometry. Report of a WHO Expert Committee. World Health Organ 1995; 854: 1–452 [PubMed] [Google Scholar]
- Wolk A, Gridley G, Svensson M, Nyren O, McLaughlin JK, Fraumeni JF, Adam HO (2001) A prospective study of obesity and cancer risk (Sweden). Cancer Causes Control 12: 13–21 [DOI] [PubMed] [Google Scholar]
- Zatonski WA, La Vecchia C, Przewozniak K, Maisonneuve P, Lowenfels AB, Boyle P (1992) Risk factors for gallbladder cancer: a Polish case-control study. Int J Cancer 51: 707–711 [DOI] [PubMed] [Google Scholar]
- Zatonski WA, Lowenfels AB, Boyle P, Maisonneuve P, Bueno de Mesquita HB, Ghadirian P, Jain M, Przewozniak K, Baghurst P, Moerman CJ, Simard A, Howe GR, McMichael AJ, Hsieh CC, Walker AM (1997) Epidemiologic aspects of gallbladder cancer: a case-control study of the SEARCH Program of the International Agency for Research on Cancer. J Natl Cancer Inst 89: 1132–1138 [DOI] [PubMed] [Google Scholar]