Abstract
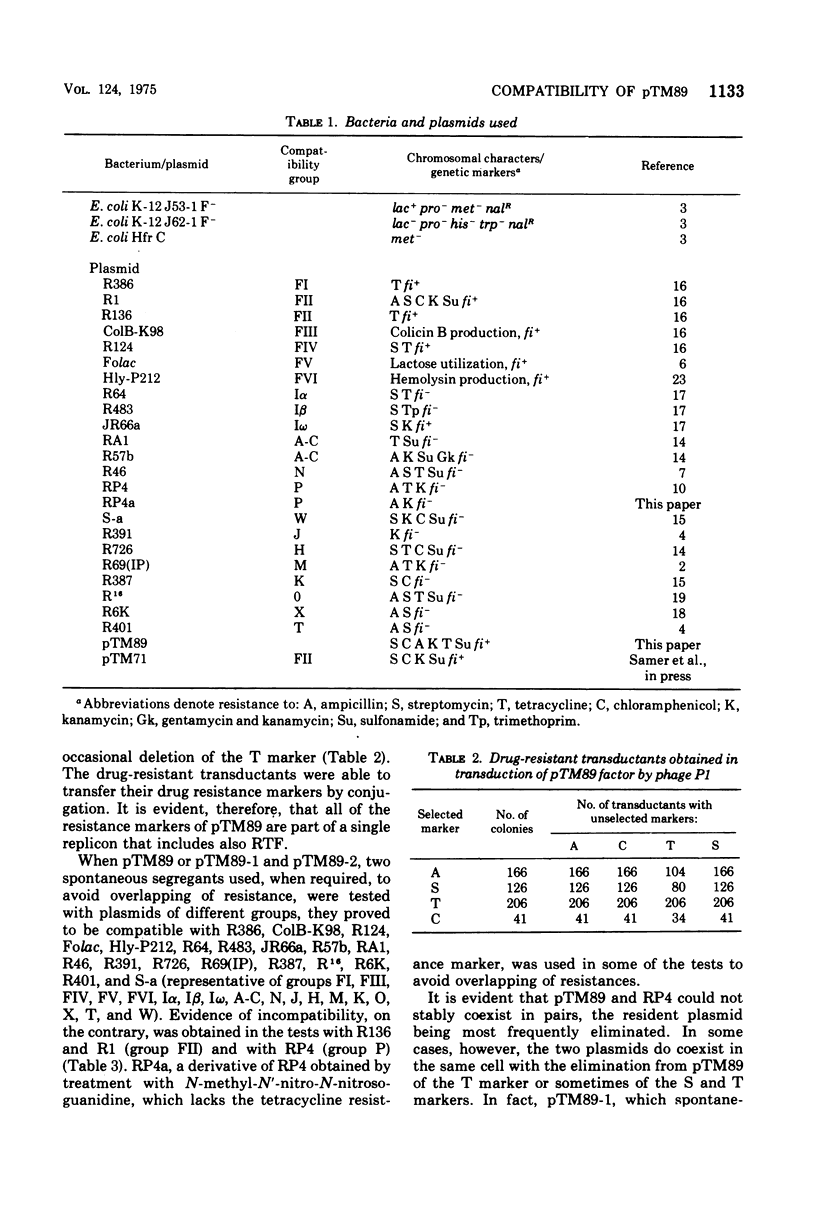
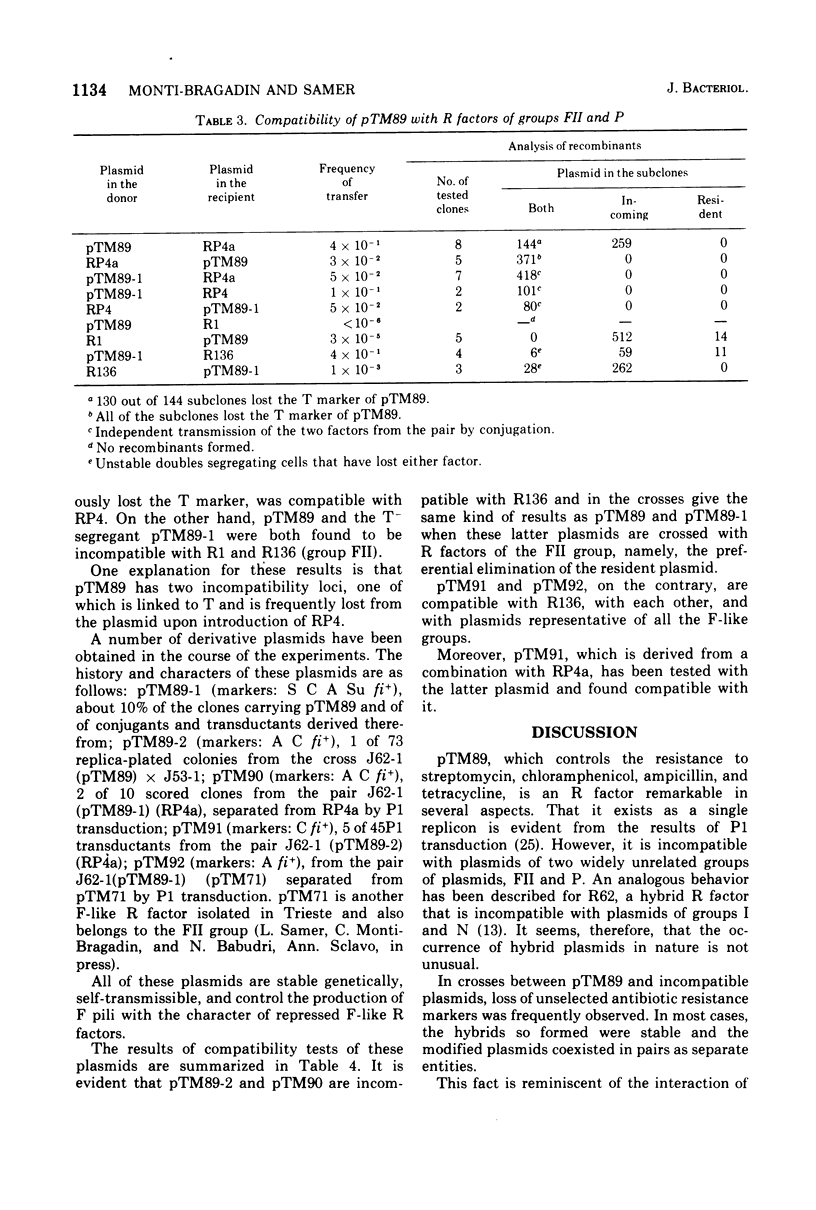
pTM89, an fi+ R factor that controls the production of repressed F-type pili, is incompatible with plasmids belonging to the FII and P groups. The results of P1 transduction show that all of the resistance markers of pTM89 are part of a single replicon, which also includes RTF. When the compatibility of different derivative plasmids was investigated, it was found that they fall into two classes. Those of the first class have lost the compatibility of pTM89 for the P group but are still incompatible with FII group, whereas those of the second class are compatible with plasmids of both groups. Plasmids of the latter class that are also compatible with each other and, therefore, apparently lack any determinant for compatibility are genetically stable and self-transmissible. It appears, therefore, that compatibility between plasmids cannot be explained by the hypothesis of competition for a maintenance site.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson E. S. Recombination between unrelated bacterial plasmids. Ann Microbiol (Paris) 1974 Apr;125A(3):251–259. [PubMed] [Google Scholar]
- Chabbert Y. A., Scavizzi M. R., Witchitz J. L., Gerbaud G. R., Bouanchaud D. H. Incompatibility groups and the classification of fi - resistance factors. J Bacteriol. 1972 Nov;112(2):666–675. doi: 10.1128/jb.112.2.666-675.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coetzee J. N., Datta N., Hedges R. W. R factors from Proteus rettgeri. J Gen Microbiol. 1972 Oct;72(3):543–552. doi: 10.1099/00221287-72-3-543. [DOI] [PubMed] [Google Scholar]
- Cooper P. Interaction of a colicinogenic factor with a resistance factor and with the fertility factor F in Escherichia coli K-12. Genet Res. 1971 Apr;17(2):151–159. doi: 10.1017/s0016672300012143. [DOI] [PubMed] [Google Scholar]
- Datta N., Hedges R. W. An I pilus-determining R factor with anomalous compatibility properties, mobilizing a gentamicin-resistance plasmid. J Gen Microbiol. 1973 Jul;77(1):11–17. doi: 10.1099/00221287-77-1-11. [DOI] [PubMed] [Google Scholar]
- Datta N., Hedges R. W., Becker D., Davies J. Plasmid-determined fusidic acid resistance in the Enterobacteriaceae. J Gen Microbiol. 1974 Jul;83(0):191–196. doi: 10.1099/00221287-83-1-191. [DOI] [PubMed] [Google Scholar]
- Datta N., Hedges R. W. Compatibility groups among fi - R factors. Nature. 1971 Nov 26;234(5326):222–223. doi: 10.1038/234222a0. [DOI] [PubMed] [Google Scholar]
- Datta N., Hedges R. W., Shaw E. J., Sykes R. B., Richmond M. H. Properties of an R factor from Pseudomonas aeruginosa. J Bacteriol. 1971 Dec;108(3):1244–1249. doi: 10.1128/jb.108.3.1244-1249.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVries J. K., Maas W. K. Description of an incompatibility mutant of Escherichia coli. J Bacteriol. 1973 Jul;115(1):213–220. doi: 10.1128/jb.115.1.213-220.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerry P., Falkow S., Datta N. R62, a naturally occurring hybrid R plasmid. J Bacteriol. 1974 Jul;119(1):144–151. doi: 10.1128/jb.119.1.144-151.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedges R. W., Datta N., Coetzee J. N., Dennison S. R factors from Proteus morganii. J Gen Microbiol. 1973 Aug;77(2):249–259. doi: 10.1099/00221287-77-2-249. [DOI] [PubMed] [Google Scholar]
- Hedges R. W., Datta N., Kontomichalou P., Smith J. T. Molecular specificities of R factor-determined beta-lactamases: correlation with plasmid compatibility. J Bacteriol. 1974 Jan;117(1):56–62. doi: 10.1128/jb.117.1.56-62.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedges R. W., Datta N. Plasmids determining I pili constitute a compatibility complex. J Gen Microbiol. 1973 Jul;77(1):19–25. doi: 10.1099/00221287-77-1-19. [DOI] [PubMed] [Google Scholar]
- Hedges R. W., Datta N. R124, an fi R factor of a new compatibility class. J Gen Microbiol. 1972 Jul;71(2):403–405. doi: 10.1099/00221287-71-2-403. [DOI] [PubMed] [Google Scholar]
- Hedges R. W. R factors from Providence. J Gen Microbiol. 1974 Mar;81(1):171–181. doi: 10.1099/00221287-81-1-171. [DOI] [PubMed] [Google Scholar]
- LENNOX E. S. Transduction of linked genetic characters of the host by bacteriophage P1. Virology. 1955 Jul;1(2):190–206. doi: 10.1016/0042-6822(55)90016-7. [DOI] [PubMed] [Google Scholar]
- Maas W. K., Goldschmidt A. D. A mutant of Escherichia coli permitting replication of two F factors. Proc Natl Acad Sci U S A. 1969 Mar;62(3):873–880. doi: 10.1073/pnas.62.3.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monti-Bragadin C., Samer L., Rottini G. D., Pani B. The compatibility of Hly factor, a transmissible element which controls alpha-haemolysin production in Escherichia coli. J Gen Microbiol. 1975 Feb;86(2):367–369. doi: 10.1099/00221287-86-2-367. [DOI] [PubMed] [Google Scholar]
- Smith H. R., Grindley N. D., Humphreys G. O., Anderson E. S. Interactions of group H resistance factors with the F factor. J Bacteriol. 1973 Aug;115(2):623–628. doi: 10.1128/jb.115.2.623-628.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe T., Furuse C., Sakaizumi S. Transduction of various R factors by phage P1 in Escherichia coli and by phage P22 in Salmonella typhimurium. J Bacteriol. 1968 Nov;96(5):1791–1795. doi: 10.1128/jb.96.5.1791-1795.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willetts N. Mapping loci for surface exclusion and incompatibility on the F factor of Escherichia coli K-12. J Bacteriol. 1974 Jun;118(3):778–782. doi: 10.1128/jb.118.3.778-782.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]



