Abstract
Immunohistochemical staining for FHIT and PCNA proteins was carried out in 451 breast lesions showing nonproliferative benign breast disease (BBD) (n=263), proliferative BBD without atypia (n=128), proliferative BBD with atypia (n=11), carcinoma in situ (n=15) or invasive carcinoma (n=34) and for EGFR protein in a subset of 71 of these cases. FHIT underexpression was not detected in nonproliferative lesions, but occurred in 2% of proliferative BBD without atypia, 10% proliferative BBD with atypia, 27% of carcinoma in situ and 41% of invasive carcinoma, which suggests that it could be useful in assessing those carcinoma in situ lesions (ductal, DCIS and lobular, LCIS) that are more likely to progress to malignancy. Preliminary microarray comparisons on DCIS and invasive carcinoma samples dissected from formalin-fixed paraffin sections showed a consistent downregulation of two previously identified FHIT-related genes, caspase 1 and BRCA1 in lesions underexpressing FHIT.
Keywords: FHIT, PCNA, EGFR, BBD, CIS, Ca
Benign breast disease (BBD) and carcinoma in situ (ductal, DCIS and lobular, LCIS) represent a group of histologically heterogeneous lesions, some of which are associated with increased risk for invasive breast cancer. In moderate to florid usual type ductal hyperplasia (UDH) and in papillomas, the relative risk (RR) of developing invasive breast cancer is small (RR=1.5–2.0), but this rises in atypical ductal hyperplasia (ADH, RR=4.0–5.0) (Page and Dupont, 1989) and in carcinoma in situ (DCIS or LCIS, RR=8.0–10.0) (Page, 1991; Page et al, 2000). Pathological and clinical evidence suggests that different BBD lesions have different magnitudes of risk for the subsequent development of malignancy and one of the key challenges is to identify at an early stage those BBD lesions which could progress to cancer. Certain pathologies have been associated with higher risk of malignancy, for example atypical ductal hyperplasia (ADH) and LCIS, but their diagnosis is subject to considerable interobserver variability (Elston et al, 2000) and therefore any independent molecular attribute could help in improving consistency as well as providing insight into the underlying biology.
Underexpression of the FHIT gene has frequently been linked to human cancer including breast cancer (Pekarsky et al, 2002). The FHIT gene is located at chromosome 3p14.2. It spans 1.8 Mb and has 10 exons. Exons 5–9 code for a small mRNA of 1.1 kb, which is susceptible to modification by alternative splicing and downregulation by promoter methylation. The FHIT protein (16.8 kDa) is mainly localised in the cytoplasm of epithelial cells complexed with tubulin and a ubiquitin conjugating enzyme, UBC9 (Shi et al, 2000; Golebiowski et al, 2004). Experimental results have shown that FHIT protein functions as a hydrolase for intracellular diadenosine triphosphate that is involved in the control of cell growth. It is associated with check point proteins ATR and CHK1 (Hu et al, 2005) and is a target for Src protein kinase, suggesting that FHIT protein is also involved more directly in the cell cycle (Pekarsky et al, 2004). In addition, FHIT is known to be a proapoptotic protein closely associated with FADD, caspase-8 (Dumon et al, 2001; Ishii et al, 2001; Roz et al, 2002, 2004), MDM2 and p53 (Nishizaki et al, 2004).
In the breast, loss of heterozygosity (LOH) at the FHIT locus has been observed in 45% of invasive cancers (Ca) and in 11% of unspecified preneoplastic lesions including usual type ductal hyperplasia (UDH), aprocrine metaplasia, DCIS and intraductal papilloma (Maitra et al, 2001). In another study, reduction or loss of FHIT protein expression was found in 40–80% of Ca and in 60% of CIS (Gatalica et al, 2000; Yang et al, 2001). Univariate analysis of disease-free survival showed FHIT to be a significant prognostic factor in patients with early breast cancer (Yang et al, 2001; Ginestier et al, 2003). Although the frequency of abnormalities in the FHIT gene is comparable to, if not in excess of those found for currently used biomarkers for breast cancer such as p53 or HER2 (Stark et al, 2000; Arun et al, 2005), the potential role for FHIT in predicting malignant progression in BBD has not been fully assessed. We report here the expression of FHIT and PCNA proteins in 451 cases with benign or malignant breast disease and EGFR in a subset of 71 of these cases. Our results show that underexpression of FHIT may have some use as a marker of breast disease progression in BBD. As the incidence of FHIT underexpression increases at the stage of carcinoma in situ, we have undertaken a preliminary examination of the cell environment associated with normal and abnormal FHIT expression in DCIS and invasive carcinoma using expression microarrays to evaluate the possible function of this protein in breast tissues. The use of FHIT protein detection for the monitoring of breast disease progression is discussed.
MATERIALS AND METHODS
Formalin-fixed and paraffin-embedded (FFPE) sections were obtained from 787 archival blocks from 532 women. Biopsies from 451 women were tested and reported in this study, made up of the following groups: nonproliferative BBD (n=263), proliferative BBD without atypia (n=128), proliferative BBD with atypia (n=11), carcinoma in situ (n=15) and invasive carcinoma (n=34). Biopsies from 10 women with incomplete histology data and from 71 women whose tissue sections were damaged during staining were excluded from the study. The routine and reviewed histology results were combined. To classify the biopsy and select a lesion from several co-existing lesions, preference was given to lesion categories in the order of severity: Invasive carcinoma >carcinoma in situ >proliferative lesions with atypia >proliferative lesions without atypia >nonproliferative lesions (Page et al, 2000). Among coexisting lesions within the same category, preference was given in the order of : extent >severity.
Sections for immunohistochemical staining (IHS) were 3 μm thick and were stored at 4°C. Sections for microarray analysis were 10 μm thick and stored at −80°C. Ethics approval was obtained from Guy's Hospital NHS Trust Ethics Committee for the use of this archival material.
Immunohistochemical staining
All sections were de-waxed, processed for epitope retrieval and stained as previously described (Terry et al, 2004). Rabbit anti-FHIT antibody (Zymed Laboratories Inc., California, USA) and horse-radish peroxidase (HRP)-conjugated anti-rabbit antibody (Abcam Ltd, Cambridgeshire, UK) were used for detection of FHIT protein. Mouse anti-PCNA antibody (Sigma-Aldrid Ltd, Dorset, UK) and alkaline phosphatase (ALP)-conjugated goat anti-mouse (Abcam) were used for PCNA protein. FHIT and PCNA proteins were stained simultaneously. Mouse anti-EGFR antibody (Abcam) and HRP-conjugated anti-mouse antibody (Abcam) were used for the detection of EGFR protein, with haematoxylin as counter-stain. HRP and ALP were detected using the corresponding detection kits (Abcam).
The results were scored ‘blind’ and independently by three laboratory scientists. A lesion was scored positive if the stained section from any of the associated paraffin blocks was scored positive by two or more observers. The scoring system used for each protein was as follows:
FHIT. Cytoplasmic staining was graded as normal (↔) or underexpressed (↓). Underexpression was assigned if >30% of cells in the designated histological category showed absent or reduced staining when compared to adjacent normal breast tissue.
PCNA. Nuclear staining of 500 nuclei in the designated histological category were counted. Overexpression (↑) was assigned if >10% of the nuclei showed either (i) red trabeculated, (ii) intensely red or (iii) cloudy red stain. Otherwise, the lesion was scored as normal (↔).
EGFR. Membrane staining for EGFR. Overexpression (↑) was assigned if complete membrane staining was present in the designated histological category. Sections obtained from lesions, which showed EGFR gene amplification, was used as control. Lesions with no staining were scored as normal (↔). Of the 79 stained lesions, eight (two with FHIT underexpression and six with normal FHIT expression) showed either cytoplasmic staining only or cytoplasmic and membrane staining combined and were excluded from the analysis. Intense cytoplasmic stain in some cells can lead to a false impression of a positively stained membrane.
EGFR gene amplification analysis by polymerase chain reaction
DNA extracts from 14 available paraffin sections were analysed. Quantitation of polymerase chain reaction (PCR) fragments was carried out by AlfExpress as previously described (Terry et al, 2004). The primers used were as follows:
EGFR exon 8 forward 5′-CGCAAGTGTAAGAAGTGCGAA-3′, reverse 5′-CGTAGCATTTATGGAGAGTGAGTCT-3′.
GAPDH exon 9 forward 5′-CCCCCACCACACTGAATCT-3′, reverse 5′-CTAGGCCCCTCCCCTCTT-3′.
Microarray analysis using human cancer arrays (MWG Biotech, Ebensberg, Germany)
RNA extraction and amplification (1.5 cycles) from paraffin-embedded sections were carried out using the Paradise Reagent System from Arcturus Bioscience Inc., California, USA, in accordance with the manufacturer's instruction.
Incorporation of Cy3- or Cy5-UTP (Perkin-Elmer Life and Analytical Sciences) was carried out during the IVT stage of the final amplification half-cycle using MEGAscript reagents (Ambion Inc., Cambridgeshire, UK) in accordance with the manufacturer's instructions and the amplified RNA (aRNA) purified using Arcturus Paradise kit reagents.
aRNA analysis. aRNA was quantitated spectroscopically using an ND-1000 spectrophotometer (NanoDrop Technologies, Delaware, USA) and its size measured by electrophoresis in formaldehyde-containing agarose gels (Maniatis et al, 1982). In total, 15 μg each of Cy3- and Cy5-labelled aRNAs were mixed and used for hybridisation to each array.
Hybridisation to MWG human cancer gene arrays spotted with 50-mer oligonucleotides specific for 1853 human genes involved in cancer development was carried out according to the manufacturer's protocol (MWG Biotech, Ebersberg, Germany). Arrays were scanned by MWG Scanning Service (Ebersberg, Germany) using an Affymetrics 428 scanner. Each channel (Cy3 or Cy5) was scanned at 10 μm resolution at three different photomultiplier gain settings. Fluorescence intensity values from each channel were processed using Imagene 4.2 software (Biodiscovery, Inc., California, USA). To obtain maximal signal intensities without saturation effects, intensity values from Tiff images were integrated into one value per probe by the MAVI software (Version Pro 2.6.0, MWG Biotech, Ebersberg, Germany). A negative control threshold was calculated from the mean fluorescence intensities obtained for 28 control Arabidopsis oligonucleotides. Fluorescence intensity values for cellular genes that exceeded this threshold by two standard deviations were regarded as significant expression signals.
Analysis of the signals was carried out by MWG Analysis Service (Ebersberg, Germany). Essentially, signals were calculated as median intensity minus median background. Sample intensities were normalised by the 50th percentile (median) method using all of the spot-filtered genes. Spots flagged bad or not found were excluded from further analysis.
Analysis of trend and variance was carried out using STATA Statistics and Data Analysis package v 8.2.
RESULTS
Staining for FHIT, PCNA and EGFR
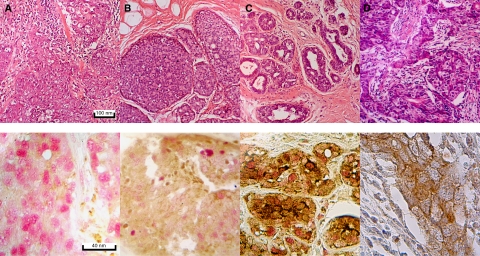
Figure 1 shows H&E and dual staining for FHIT and PCNA proteins of (A) an FHIT-negative cancer, (B) an FHIT-reduced low-grade DCIS and (C) an FHIT-positive florid hyperplasia. A cancer stained with H&E and EGFR is shown in (D). Arrows indicate PCNA-positive trabeculated staining, PCNA-positive intensive staining and PCNA-positive cloudy staining nuclei.
Figure 1.
Staining patterns in four breast lesions. Upper row (H&E). Cancer (A and D), DCIS (B) and hyperplasia (C). Lower row (ISH). Cancer (A), DCIS (B) and hyperplasia (C) dually stained with antibodies to FHIT (brown) and PCNA (red). (D) Cancer stained with antibody to EGFR (brown) and haematoxylin (blue). Arrows indicate different staining patterns: PCNA (i) trabeculated, (ii) intense, (iii) cloudy, (iv) cloudy and EGFR (v) membrane-associated.
The expression of FHIT, PCNA and EGFR proteins in breast lesions
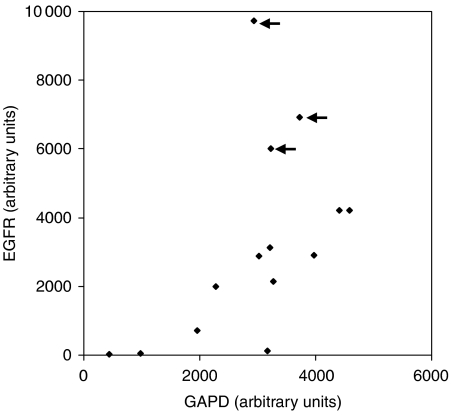
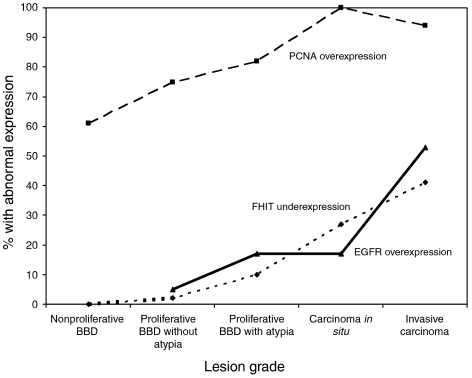
The expression of FHIT and PCNA in all 451 biopsies and EGFR in a subset of 71 biopsies showing proliferative changes is summarised in Table 1 and Figure 2. FHIT was expressed normally in all nonproliferative BBD lesions, but was underexpressed in 12% of all proliferative lesions particularly in those with atypia and cancerous changes (χ2trend=114.31, P=<0.0001). In all, 60–100% of all lesions overexpressed PCNA and 5–53% overexpressed EGFR in parallel with increasing lesion grades (χ2trend=13.02, P=0.00031). Only three of the 14 cases overexpressing EGFR showed EGFR gene amplification relative to the GAPDH housekeeping gene (Figure 3). All lesions, which underexpressed FHIT, also overexpressed PCNA (Table 2). Although FHIT underexpression and EGFR overexpression followed a overall similar distribution in proliferative BBD lesions and in invasive carcinomas, they were not always concordant in individual cases. Three DCIS and seven invasive carcinomas with normal EGFR expression underexpressed FHIT, whereas two DCIS and 11 invasive carcinomas with normal FHIT expression overexpressed EGFR (Table 2). Multivariate analysis showed correlation between the three markers together and increasing lesion grades (F=5.582, P=0.0017).
Table 1. FHIT, PCNA and EGFR proteins in nonproliferative and proliferative breast lesions.
|
Pathology
|
Relative risk of cancer (Page et al, 2000) | Breast lesions |
No. of cases with expression status as
|
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Lesion | Category (Stark et al, 2000) | FHIT | PCNA | EGFR | ||||||
| ↓ | ↔ | ↑ | ↓ | ↑ | ↔ | |||||
| BBD | (a) Nonproliferative | 1.0 | Normal | 0 | 60 | 24 | 36 | ND | ND | |
| Inflammation | 0 | 13 | 6 | 7 | ND | ND | ||||
| Cyst | 0 | 4 | 3 | 1 | ND | ND | ||||
| Metaplasia | 0 | 18 | 15 | 3 | ND | ND | ||||
| Blind duct adenosis | 0 | 60 | 35 | 25 | ND | ND | ||||
| Hyperplasia (usual type) | 0 | 24 | 17 | 7 | ND | ND | ||||
| Fibroadenoma | 0 | 84 | 60 | 24 | ND | ND | ||||
| Subtotal | 0 (0%) | 263 | 160 (61%) | 103 | NA | NA | ||||
| (b) Proliferative without atypia | 1.5–2.0 | Papilloma | 0 | 25 | 16 | 9 | ND | ND | ||
| Sclerosing adenosis | 0 | 36 | 29 | 7 | ND | ND | ||||
| Hyperplasia (moderate) | 1 | 47 | 35 | 13 | 0 | 4 | ||||
| Hyperplasia (florid) | 2 | 17 | 16 | 3 | 1a | 14 | ||||
| Subtotal | 3 (2%) | 125 | 96 (75%) | 32 | 1 (5%) | 18 | ||||
| (c) Proliferative with atypia | 4.0–5.0 | ADH/ALH | 1 (10%) | 10 | 9 (82%) | 2 | 1 (17%) | 5 | ||
| Carcinoma | (a) In situ | 8.0–10.0 | LCIS | 0 | 3 | 12 | 0 | 0 | 3 | |
| DCIS | 4 | 8 | 3 | 0 | 2 | 9 | ||||
| Subtotal | 4 (27%) | 11 | 15 (100%) | 0 | 2 (17%) | 12 | ||||
| (b) Invasive | NA | 14 (41%) | 20 | 32 (94%) | 2 | 17b(53%) | 15 | |||
| 22(5%) | 429 | 312 (69%) | 139 | 21 (30%) | 50 | |||||
| Total | n=451 | n=451 | n=71 | |||||||
Gene amplification detected in one case; ↔=normal level of expression; ↑=overexpression; ↓=underexpression; ND=not done; NA=not applicable.
Gene amplification detected in 2 case.
Figure 2.
The detection of FHIT, PCNA and EGFR expression in nonproliferative and proliferative breast lesions.
Figure 3.
Quantitation of Cy5-labelled EGFR and GAPDH amplicons (in arbitrary units) from 14 EGFR-positive lesions by sequencing gel electrophoresis (AlfExpress) using Fragment Manager v.1.2 software (Pharmacia). ←, gene amplification.
Table 2. Corrleation of FHIT, PCNA and EGFR expression in nonproliferative and proliferative breast lesions.
| Category | Grade |
Expression status (no. of cases)
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| FHIT | ↔ | ↔ | ↓ | ↓ | FHIT | ↔ | ↓ | ↓ | ↔ | |||
| PCNA | ↑ | ↓ | ↑ | ↓ | ||||||||
| EGFR | ↑ | ↑ | ↔ | ↔ | ||||||||
| BBD | (a) Nonproliferative | Normal | 24 | 36 | 0 | 0 | ND | ND | ND | ND | ||
| Inflammation | 6 | 7 | 0 | 0 | ND | ND | ND | ND | ||||
| Cyst | 3 | 1 | 0 | 0 | ND | ND | ND | ND | ||||
| Metaplasia | 14 | 4 | 0 | 0 | ND | ND | ND | ND | ||||
| Blind duct adenosis | 35 | 25 | 0 | 0 | ND | ND | ND | ND | ||||
| Hyperplasia (usual type) | 17 | 7 | 0 | 0 | ND | ND | ND | ND | ||||
| Fibroadenoma | 60 | 24 | 0 | 0 | ND | ND | ND | ND | ||||
| (b) Proliferative | Papilloma | 16 | 9 | 0 | 0 | ND | ND | ND | ND | |||
| without atypia | Sclerosing adenosis | 29 | 7 | 0 | 0 | ND | ND | ND | ND | |||
| Hyperplasia (moderate) | 34 | 13 | 1 | 0 | 0 | 0 | 1 | 3 | ||||
| Hyperplasia (florid) | 14 | 3 | 2 | 0 | 1 | 0 | 0 | 14 | ||||
| (c) Proliferative | ADH/ALH | 8 | 2 | 1 | 0 | 0 | 1 | 0 | 5 | |||
| with atypia | ||||||||||||
| Carcinoma | (a) In situ | LCIS | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | ||
| DCIS | 8 | 0 | 4 | 0 | 2 | 0 | 3 | 6 | ||||
| (b) Invasive | 18 | 2 | 14 | 0 | 11 | 6 | 7 | 8 | ||||
| Total | 289 | 140 | 22 | 0 | 14 | 7 | 11 | 39 | ||||
| n=451 | n=71 | |||||||||||
↔=normal level of expression; ↑=overexpression; ↓=underexpression; ND=not done.
Comparison of FHIT-related gene expression in ductal carcinoma in situ and cancer lesions
RNA extracted from paired FHIT-positive and FHIT-negative lesions were amplified, labelled separately with either Cy3- or Cy5-UTP and pooled. The amounts of aRNA recovered after two amplification cycles was 16–20 μg and the predominant size was 300–600 bases, but extending up to 2000 bases (data not shown). Differentially labelled RNA from three DCIS pairs and one invasive carcinoma pair were hybridised to separate human cancer gene arrays.
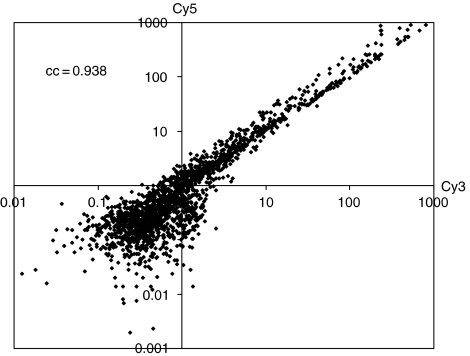
Figure 4 shows good pair-wise correlation between RNA extracted from an FHIT-positive DCIS labelled with Cy-3 and an FHIT-negative DCIS labelled with Cy-5 hybridised to 1853 cancer gene probes with a correlation coefficient of 0.938. A comparison of the Cy3/Cy5 ratios showed that two genes, caspase 1 and BRCA1, were underexpressed in all four lesions with reduced FHIT expression (Table 3).
Figure 4.
Correlation of Cy3-labelled FHIT-positive DCIS and Cy5-labelled FHIT reduced DCIS hybridised to MWG human cancer gene array.
Table 3. Cy3/Cy5 ratio in FHIT and/or breast cancer related genes.
| Association | Genes |
Cy3/Cy5 ratio
|
|||
|---|---|---|---|---|---|
| Ca |
DCIS
|
||||
| F+/F− | F+/F± | F+/F± | F+/F± | ||
| Median value for all genes | 1.041/0.789 | 0.753/0.456 | 1.048/0.690 | 0.979/0.647 | |
| Median ratio for all genes | 1.319 | 1.651 | 1.519 | 1.513 | |
| FHIT-apoptosis | APAF1 | 0.022 | 1.228 | 0.528 | 0.698 |
| BAD | 0.394 | 1.530 | 0.737 | 0.629 | |
| BAX | 3.481 | 0.882 | 1.322 | 1.078 | |
| BCL2 (probe 1) | 0.973 | 0.649 | 0.744 | 0.912 | |
| BCL2 (probe 2) | 0.340 | 2.053 | 1.028 | 0.638 | |
| BIRC5 | 0.296 | 2.335 | 1.170 | 0.453 | |
| CASP1 | 1.666 | 2.263 | 8.992 | 4.758 | |
| CASP10 | 2.089 | 0.535 | 0.716 | 1.273 | |
| CASP8 | 2.998 | 1.035 | 0.940 | 0.939 | |
| CDC2 | ND | ND | 1.758 | 0.337 | |
| COX7A2L | 2.637 | 1.989 | 1.462 | 1.060 | |
| CFLAR | 0.627 | 1.172 | 0.988 | 0.903 | |
| FADD | 0.180 | 1.900 | 1.167 | 0.616 | |
| FAS | 0.685 | 1.044 | 3.079 | 4.253 | |
| FASLG | 0.114 | 0.578 | 1.213 | 0.641 | |
| IL1B | 0.030 | 0.554 | 1.058 | 0.660 | |
| IL1RN | 0.039 | 0.732 | 0.679 | 1.697 | |
| FHIT-cell cycle | ATM | 0.351 | 0.772 | 1.111 | 0.776 |
| WWOX | 3.112 | 0.902 | 1.122 | 1.185 | |
| Breast cancer prognostic markers | AKT1 | 1.254 | 0.734 | 0.759 | 0.850 |
| AR | 0.310 | 0.780 | 1.016 | 0.646 | |
| BRCA1 (probe 1) | 0.660 | 1.527 | 1.085 | 1.650 | |
| BRCA1 (probe 2) | 1.724 | 14.354 | 13.737 | 23.800 | |
| BRCA2 | 1.537 | ND | 1.267 | 1.338 | |
| EGFR | 2.858 | 0.527 | 1.318 | 0.998 | |
| ERBB2 | 1.975 | 0.751 | 0.617 | 0.520 | |
| ERBB3 | 0.683 | 1.539 | 0.863 | 3.632 | |
| ERBB4 | 2.036 | 0.980 | 0.808 | 1.064 | |
| ESR1 | 0.415 | 6.568 | 0.970 | 0.551 | |
| MLH1 | 0.089 | 0.869 | 2.323 | 0.952 | |
| MLH3 | 2.134 | 0.646 | 2.710 | 24.352 | |
| PCNA | 0.236 | 0.630 | 0.801 | 0.526 | |
| PGR | 0.991 | 1.737 | 20.092 | 10.050 | |
| SRC | 0.135 | 0.710 | 1.515 | 0.889 | |
| TNF | 1.583 | 0.934 | 0.684 | 0.708 | |
| TP53 | 0.933 | 5.030 | 0.689 | 0.607 | |
ND=instensity in one or both channels below the cutoff levels set by the MAVI software; F+=normal FHIT expression; F− or F±=FHIT underexpression.
DISCUSSION
Oncogenesis represents an interplay between cell proliferation and apoptosis. Markers for these biological activities have been extensively sought in breast cancer for prognosis, prediction of treatment effectiveness and development of new chemotherapeutic agents. Whether the same markers can be used for predicting risk of subsequent changes in BBD and precursor progression in DCIS has not been extensively studied. We have used the cell cycle protein PCNA and the mitogenic receptor EGFR as markers of proliferation, and FHIT as a marker associated with apoptosis. Table 1 and Figure 2 summarise the relationship between the proliferative activities observed in breast lesions and PCNA, EGFR or FHIT expression. Of the 451 cases studied, 312 (69%) were found to be positive for PCNA. The positivity rate is comparable to that reported previously (Steck and El-Naggar 1994; Fabian et al, 2002; Honrado et al, 2005). The synthesis of PCNA is closely associated with the normal G1/S transition of the cell cycle and the protein has a comparatively long half-life. It is, therefore, a good marker for measuring proliferative activity in breast lesions where cells are at different phases of growth and accounts for its apparent expression in 94–100% of in situ and invasive carcinomas and even 61% of histologically ‘nonproliferative’ BBD (Table 1, Figure 2). In contrast, overexpression of the mitogenic signalling mediator EGFR, indicative of abnormalities in the commitment of G1 to S phase in the cell cycle, was found in 30% of breast lesions, predominantly in invasive carcinoma (53%). Similar rates of EGFR overexpression have been reported previously in DCIS and invasive carcinoma (Knoop et al, 2001; Lebeau et al, 2003). The causes of EGFR overexpression are not well understood but aneusomy of chromosome 7 on which the EGFR gene is located does not relate directly to EGFR protein overexpression (Bhargava et al, 2005; Sauer et al, 2005). In our study, gene amplification could only account for three out of 14 cases (Table 1, Figure 3), but our method would not distinguish between aneusomy of chromosome 7 from any other mechanism for gene amplification.
Overall, our results suggest that most BBD lesions and carcinomas contain populations of rapidly dividing normal and/or abnormal cells and both are predisposed to accumulation of genomic changes. This could include the establishment of FHIT underexpression in a subset of proliferative BBD lesions and carcinomas.
FHIT underexpression was found in 14 out of 34 (41%) of invasive carcinomas and in four out of 12 (33%) of DCIS (Table 1, Figure 2). Previously, loss of FHIT expression in cancer has been found to mirror other poor prognosis markers, being positively correlated with Bcl-2 and p53 overexpression, proliferative activity and aggressive histological phenotype and negatively correlated with oestrogen and progesterone receptor status (Campiglio et al, 1999; Yang et al, 2001; Arun et al, 2005). Loss of FHIT expression has also been found to confer a significant disadvantage in disease-free survival (Yang et al, 2001; Ginestier et al, 2003). However, the incidence of FHIT expression in BBD lesions in the absence of invasive carcinoma was unclear. In a study involving 50 cancers, Gatalica et al (2000) found reduced FHIT protein expression in four out of 34 (12%) hyperplastic epithelium (grades not specified), 26 out of 47 (55%) atypical hyperplasia and DCIS combined and 33 out of 46 (72%) cancers. In a study of 45 cancers, LOH at the FHIT locus 3p14.2 was found in five out of 45 (11%) of cancer-associated pre-neoplasia (grades not specified but included UDH, apocrine metaplasia, DCIS and intraductal papilloma) and 20 out of 44 (45%) cancers (Maitra et al, 2001). In both studies, the BBD lesions examined were adjacent to cancer tissues and the staining characteristics observed may not be a reflection of the true FHIT status as this could have arisen from a local field effect (Cavalli et al, 2004). In our study, each of the cases tested was classified by the highest grade of lesion present. We found normal expression of FHIT protein in all nonproliferative lesions, but underexpression in 2% of proliferative lesions without atypia, 10% of proliferative lesions with atypia, 0% of lobular carcinoma in situ, 33% of ductal carcinoma in situ and 41% of invasive carcinomas (Table 1). The outcome was known for eight of the 12 DCIS lesions. Two of three lesions with FHIT underexpression and five of five lesions with normal FHIT expression progressed subsequently to invasive breast cancer, but the difference was not significant (P=0.375). Similarly, no relationship could be ascertained between FHIT underexpression and different pathological types of in situ or invasive carcinoma because of the small number of lesions analysed.
In vitro experiments have shown that FHIT is a proapoptotic protein which operates via both the extrinsic (Roz et al, 2004) and intrinsic pathways (Dumon et al, 2001; Ishii et al, 2001). In a study involving 100 colorectal adenocarcinomas, Mady and Melhem (2002) found that overexpression of FHIT is directly proportional to the rate of apoptosis. It was therefore of interest to assess if underexpression of FHIT in our biopsies is likely to have any functional effect. To do this, we used expression microarray analysis to assess the expression levels of other apoptosis-associated and breast cancer prognostic genes (Table 3). A commercially available protocol (Paradise kit, Arcturus, Biosciences Inc., California, USA) was successful in amplifying RNA retrieved from formalin-fixed and paraffin-embedded breast lesions. Pair-wise analysis confirmed other reports (Ma et al, 2003; True et al, 2006) that good-quality aRNA could be reproducibly obtained for hybridisation to microarrays (Figure 4). This technique is invaluable for analysis of archival material. Although only three of the four DCIS lesions with underexpressed FHIT expression (Table 1) were available for testing in this study, consistent downregulation of two genes, namely caspase 1 and BRCA1, was noted. Caspase 1 is an important regulator of epithelial cell apoptosis and its downregulation has been reported in breast, gastric, colon and prostate cancers (Boudreau et al, 1995; Jarry et al, 1999; Winter et al, 2001; Jee et al, 2005). Loss of BRCA1 is associated with a more aggressive phenotype in sporadic breast cancer (Jarvis et al, 1998; Taylor et al, 1998) and concomitant loss of FHIT and BRCA1 alleles has also been reported in a number of repair-deficient cancers including breast cancer and ovarian cancers (Wilson et al, 1999; Turner et al, 2002; Santos et al, 2004).
In this study, coexpression of PCNA and FHIT was determined in the same cell population by dual staining, and EGFR was monitored in the same cell population in an adjacent section. This allows a simultaneous assessment of an interplay between the expression of the three proteins. Our results showed that the presence of PCNA does not clearly distinguish between nonproliferative BBD lesions and proliferative BBD lesions (with or without atypia), or between in situ carcinomas and invasive carcinomas (Table 1). In contrast, underexpression of FHIT was associated with lesions with increasing severity (χ2trend=114.31), including four out of 12 DCIS and 14 out of 34 invasive carcinomas. Three DCIS and seven invasive carcinomas with normal EGFR expression underexpressed FHIT (Table 2), which suggest that detection of FHIT expression could be of use either alone or with other markers such as EGFR, in identifying a subset of proliferative breast lesions with malignant potential. In contrast to the current lack of consensus in scoring EGFR expression by IHS, detection of FHIT is technically simple and the incorporation of a quantitation step by image analyser would provide an objective measure of its expression (Mady and Melhem, 2002). The loss of caspase 1 in DCIS lesions also merits further analysis since the effectiveness of a number of therapeutic drugs depends on its activation.
References
- Arun B, Kilic G, Yen C, Foster B, Yardley DA, Gaynor R, Ashfaq R (2005) Loss of FHIT expression in breast cancer is correlated with poor prognostic markers. Can Epid Biomarkers Prev 14: 1681–1685 [DOI] [PubMed] [Google Scholar]
- Bhargava R, Gerald WL, Li AR, Pan Q, Lal P, Ladanyi M, Chen B (2005) EGFR gene amplification in breast cancer: correlation with epidermal growth factor receptor mRNA and protein expression and HER-2 status and absence of EGFR-activating mutations. Mod Path 18: 1027–1033 [DOI] [PubMed] [Google Scholar]
- Boudreau N, Sympson CJ, Werb Z, Bissell MJ (1995) Suppression of ICE and apoptosis in mammary epithelial cells by extracellular matrix. Science 267: 891–893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavalli LR, Singh B, Issacs C, Dickson RB, Haddad BR (2004) Loss of heterozygosity in normal breast epithelial tissue and benign breast lesions in BRCA1/2 carriers with breast cancer. Cancer Gen Cytogen 149: 38–43 [DOI] [PubMed] [Google Scholar]
- Campiglio M, Pekarsky Y, Menard S, Tagliabue E, Pilotti S, Croce CM (1999) FHIT loss of function in human primary breast cancer correlates with advanced stage of the disease. Cancer Res 59: 3866–3869 [PubMed] [Google Scholar]
- Dumon KR, Ishii H, Vecchione A, Trapasso F, Baldassarre G, Chakrani F, Druck T, Rosato EF, Williams NN, Baffa R, During MJ, Huebner K, Croce CM (2001) Fragile histidine triad expression delays tumor development and induces apoptosis in human pancreatic cancer. Cancer Res 61: 4827–4836 [PubMed] [Google Scholar]
- Elston CW, Sloane JP, Amendoeira I, Apostolikas N, Bellocq JP, Bianchi S, Boecker W, Bussolati G, Coleman D, Connolly CE, Dervan P, Drijkoningen M, Eusebi V, Faverly D, Holland R, Jacquemier J, Lacerda M, Martinez-Penuela J, de Miguel C, Moss S, Munt C, Peterse JL, Rank F, Reiner A, Sylvan M, Wells CA, Zafrani B, European Commission Working Group on Breast Screening Pathology (2000) Causes of inconsistency in diagnosing and classifying intraductal proliferations of the breast. Eur J Cancer 36: 1769–1772 [DOI] [PubMed] [Google Scholar]
- Fabian CJ, Kimler BF, Brady DA, Mayo MS, Chang CHJ, Ferraro JA, Zalles CM, Stanton AL, Masood S, Grizzle WE, Boyd NF, Arneson DW, Johnson KA (2002) A phase II breast cancer chemoprevention trial of oral difluoromethylornithine: breast tissue imaging and serum and urine biomarkers. Clin Cancer Res 8: 3105–3117 [PubMed] [Google Scholar]
- Gatalica Z, Lele SM, Rampy BA, Norris BA (2000) The expression of Fhit protein is related inversely to disease progression in patients with breast carcinoma. Cancer 88: 1378–1383 [PubMed] [Google Scholar]
- Ginestier C, Bardou V-J, Popovici C, Charafe-Jauffret E, Bertucci F, Geneix J, Adelaide J, Chaffanet M, Hassoun J, Viens P, Jacquemier J, Birnbaum D (2003) Loss of fhit protein expression is a marker of adverse evolution in good prognosis localized breast cancer. Int J Cancer 107: 854–862 [DOI] [PubMed] [Google Scholar]
- Golebiowski F, Szulc A, Szutowicz A, Pawelczyk T (2004) Ubc9-induced inhibition of diadenosine triphosphate hydrolase activity of the putative tumor suppressor protein Fhit. Arch Biochem Biophy 428: 160–164 [DOI] [PubMed] [Google Scholar]
- Honrado E, Osorio A, Palacios J, Milne RL, Sanchez L, Diez O, Cazorla A, Syrjakoski K, Huntsman D, Heikkila P, Lerma E, Kallioniemi A, Rivas C, Foulkes WD, Nevanlinna H, Benitez J (2005) Immunohistochemical expression of DNA repair proteins in familial breast cancer differentiate BRCA2-associated tumors. J Clin Oncol 23: 7503–7511 [DOI] [PubMed] [Google Scholar]
- Hu BC, Wang HY, Wang X, Lu HR, Huang CF, Powell SN, Huebner K, Wang Y (2005) Fhit and CHK1 have opposing effects on homologous recombination repair. Cancer Res 65: 8613–8616 [DOI] [PubMed] [Google Scholar]
- Ishii H, Dumon KR, Vecchione A, Fong LYY, Baffa R, Huebner K, Croce CM (2001) Potential cancer therapy with the fragile histidine triad gene. Review of the preclinical studies. JAMA 286: 2441–2449 [DOI] [PubMed] [Google Scholar]
- Jarry A, Vallette G, Cassagnau E, Moreau A, Bou-Hanna C, Lemarre P, Letessier E, Le Neel J-c, Galmiche J-P, Laboisse CL (1999) Interleukin 1 and interleukin 1β converting enzyme (caspase 1) expression in the human colonic epithelial barrier. Caspase 1 downregulation in colon cancer. Gut 45: 246–251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvis EM, Kirk JA, Clarke CL (1998) Loss of nuclear BRCA1 expression in breast cancers is associated with a highly proliferative tumor phenotype. Cancer Genet Cytogenet 101: 109–115 [DOI] [PubMed] [Google Scholar]
- Jee CD, Lee HS, Bae SI, Yang HK, Lee YM, Rho MS, Kim WH (2005) Loss of caspase-1 gene expression in human gastric carcinomas and cell lines. Int J Oncol 26: 1265–1271 [PubMed] [Google Scholar]
- Knoop AS, Bentzen SM, Nielsen MM, Rasmussen BB, Rose C (2001) Value of epidermal growth factor receptor, HER2, p53 and steroid receptors in predicting the efficacy of tamoxifen in high-risk postmenopausal breast cancer patients. J Clin Oncol 19: 3376–3384 [DOI] [PubMed] [Google Scholar]
- Lebeau A, Unholzer A, Amann G, Kronawitter M, Bauerfeind I, Sendelhofert A, Iff A, Lohrs U (2003. EGFR, Her-2/neu, cyclin D1, p21 and p53 in correlation to cell proliferation and steroid hormone receptor status in ductal carcinoma in situ of the breast. Br Caner Res Treat 79: 187–198 [DOI] [PubMed] [Google Scholar]
- Ma X-J, Salunga R, Tuggle T, Gaudet J, Enright E, McQuary P, Payette T, Pistone M, Stecker K, Zhang BM, Zhou Y-X, Varnholt H, Smith B, Gadd M, Charfield E, Kessler J, Baer TM, Erlander MG, Sgroi DC (2003) Gene expression profiles of human breast cancer progression. Proc Natl Acad Sci USA 100: 5974–5979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mady HH, Melhem MF (2002) FHIT protein expression and its relation to apoptosis, tumor histologic grade and prognosis in colorectal adenocarcinoma: an immunohistochemical and image analysis study. Clin Exp Metast 19: 351–358 [DOI] [PubMed] [Google Scholar]
- Maitra A, Wistuba II, Washington C, Virmani AK, Ashfaq R, Milchgrub S, Gazdar AF, Minna JD (2001) High-resolution chromosome 3p allelotyping of breast carcinomas and precursor lesions demonstrates frequent loss of heterozygosity and a discontinuous pattern of allele loss. Am J Path 159: 119–130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maniatis T, Fritsch EF, Sambrook J (1982) Molecular cloning. A Laboratory Manual. pp 202–203. Cold Spring Harbor Laboratory
- Nishizaki M, Sasaki J, Fang B, Atkinson EN, Minna JD, Roth JA, Ji L (2004) Synergistic tumor suppression by coexpression of FHIT and p53 coincides with FHIT-mediated MDM2 inactivation and p53 stabilization in human non-small cell lung cancer cells. Cancer Res 64: 5745–5752 [DOI] [PubMed] [Google Scholar]
- Page DL (1991) Segregation analysis of breast cancer: histopathologic data. JNCI 83: 648. [DOI] [PubMed] [Google Scholar]
- Page DL, Dupont WD (1989) Benign breast diseases and premalignant breast disease. Arch Pathol Lab Med 122: 1048–1050 [PubMed] [Google Scholar]
- Page DL, Jensen RA, Simpson JF, Dupont WD (2000) Historical and epidemiologic background of human premalignant breast disease. J Mammary Gland Biol Neoplasia 5: 341–349 [DOI] [PubMed] [Google Scholar]
- Pekarsky Y, Garrison PN, Palamarchuk A, Zanesi N, Aqeilan RI, Huebner K, Barnes LD, Croce CM (2004) Fhit is a physiological target of the protein kinase Src. Proc Natl Acad Sci USA 101: 3775–3779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pekarsky Y, Zanesi N, Palamarchuk A, Huebner K, Croce CM (2002) FHIT: from gene discovery to cancer treatment and prevention. Lancet Oncol 3: 748–754 [DOI] [PubMed] [Google Scholar]
- Roz L, Gramegna M, Ishii H, Croce CM, Sozzi G (2002) Restoration of fragile histidine triad (FHIT) expression includes apoptosis and suppresses tumorigenicity in lung and cervical cancer cell lines. Proc Natl Acad Sci USA 99: 3615–3620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roz L, Andriani F, Ferreira CG, Giaccone G, Sozzi G (2004) The apoptotic pathway triggered by the Fhit protein in lung cancer cell lines is not affected by Bcl-2 or Bcl-x(L) overexpression. Oncogene 23: 9102–9110 [DOI] [PubMed] [Google Scholar]
- Santos SCL, Cavalli LR, Cavalli IJ, Lima RS, Haddad BR, Ribeiro EMSF (2004) Loss of heterozygosity of the BRCA1 and FHIT genes in patients with sporadic breast cancer from Southern Brazil. J Clin Pathol 57: 374–377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauer T, Beraki K, Noren T, Garred O, Naess O (2005) EGFR gene copy number heterogeneity in fine-needle aspiration cytology from breast carcinomas determined by chromogenic in situ hybridization. Diagn Cytopathol 33: 228–232 [DOI] [PubMed] [Google Scholar]
- Shi Y, Zou M, Farid NR, Paterson MC (2000) Association of FHIT (fragile histidine triad), a candidate tumour suppressor gene, with the ubiquitin-conjugating enzyme hUBC9. Biochem J 352: 443–448 [PMC free article] [PubMed] [Google Scholar]
- Stark A, Hulka BS, Joens S, Novotny D, Thor AD, Wold LE, Schell MJ, Melton LJ, Liu EY, Conway K (2000) Her-2/neu amplification in benign breast disease and the risk of subsequent breast cancer. J Clin Oncol 18: 267–274 [DOI] [PubMed] [Google Scholar]
- Steck K, El-Naggar AK (1994) Comparative flow cytometric analysis of Ki-67 and proliferating cell nuclear antigen (PCNA) in solid neoplasms. Cytometry 17: 258–265 [DOI] [PubMed] [Google Scholar]
- Taylor J, Lymboura M, Pace PE, A’Hern RP, Desai AJ, Shousha S, Coombes RC, Ali S (1998) An important role for BRCA1 in breast cancer progression is indicated by its loss in a large proportion of non-familial breast cancers. Int J Cancer 79: 334–342 [DOI] [PubMed] [Google Scholar]
- Terry G, Ho L, Londesborough P, Cross P, Lopes A, Monaghan J, Cuzick J (2004) The role of human papillomavirus type 16 and the fragile histidine triad gene in the outcome of cervical neoplastic lesions. Br J Cancer 91: 2056–2062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- True L, Coleman I, Hawley S, Huang CY, Gifford D, Coleman R, Beer TM, Gelmann E, Datta M, Mostaghel E, Knudsen B, Lange P, Vessella R, Lin D, Hood L, Nelson PS (2006) A molecular correlate to the Gleason grading system for prostate adenocarcinoma. Proc Natl Acad Sci USA 103: 10991–10996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner BC, Ottey M, Zimonjic DB, Potoczek M, Hauck WW, Pequignot E, Keck-Waggoner CL, Sevignani C, Aldaz CM, McCue PA, Palazzo J, Huebner K, Popescu NC (2002) The fragile histidine triad/common chromosome fragile site 3B locus and repair-deficient cancers. Cancer Res 62: 4054–4060 [PubMed] [Google Scholar]
- Wilson CA, Ramos L, Villasenor MR, Anders KH, Press MF, Clarke K, Karlan B, Chen J-J, Scully R, Livingston D, Zuch RH, Kanter MH, Cohen S, Calzone FJ, Slamon DJ (1999) Localization of human BRCA1 and its loss in high grade, non-inherited breast carcinomas. Nat Gen 21: 236–240 [DOI] [PubMed] [Google Scholar]
- Winter RN, Kramer A, Borkowski A, Kyprianou N (2001) Loss of caspase-1 and caspase-3 protein expression in human prostate cancer. Cancer Res 61: 1227–1232 [PubMed] [Google Scholar]
- Yang Q, Yoshimura G, Suzuma T, Tamaki T, Umemura T, Nakamura M, Nakamura Y, Wang X, Mori I, Sakurai T, Kakudo K (2001) Clinicopathological significance of fragile histidine triad transcription protein expression in breast carcinoma. Clin Cancer Res 7: 3869–3873 [PubMed] [Google Scholar]