Abstract
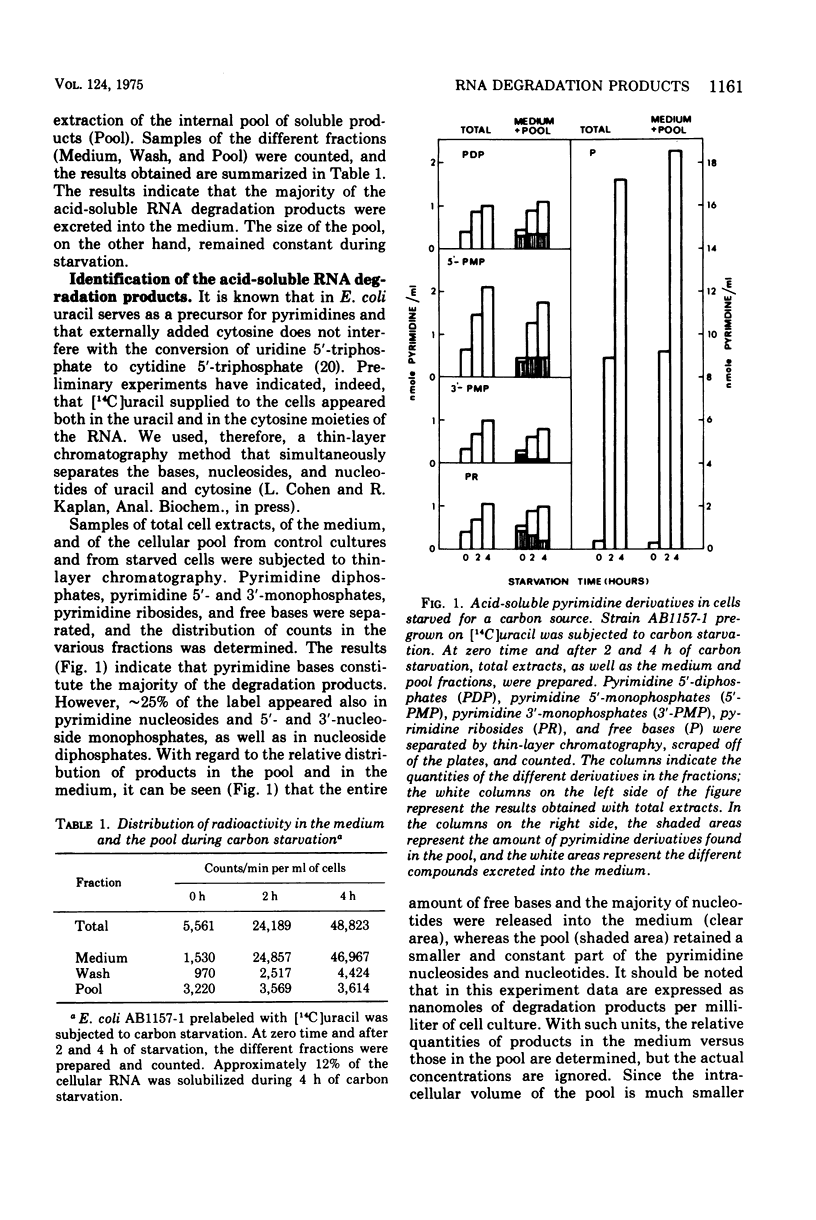
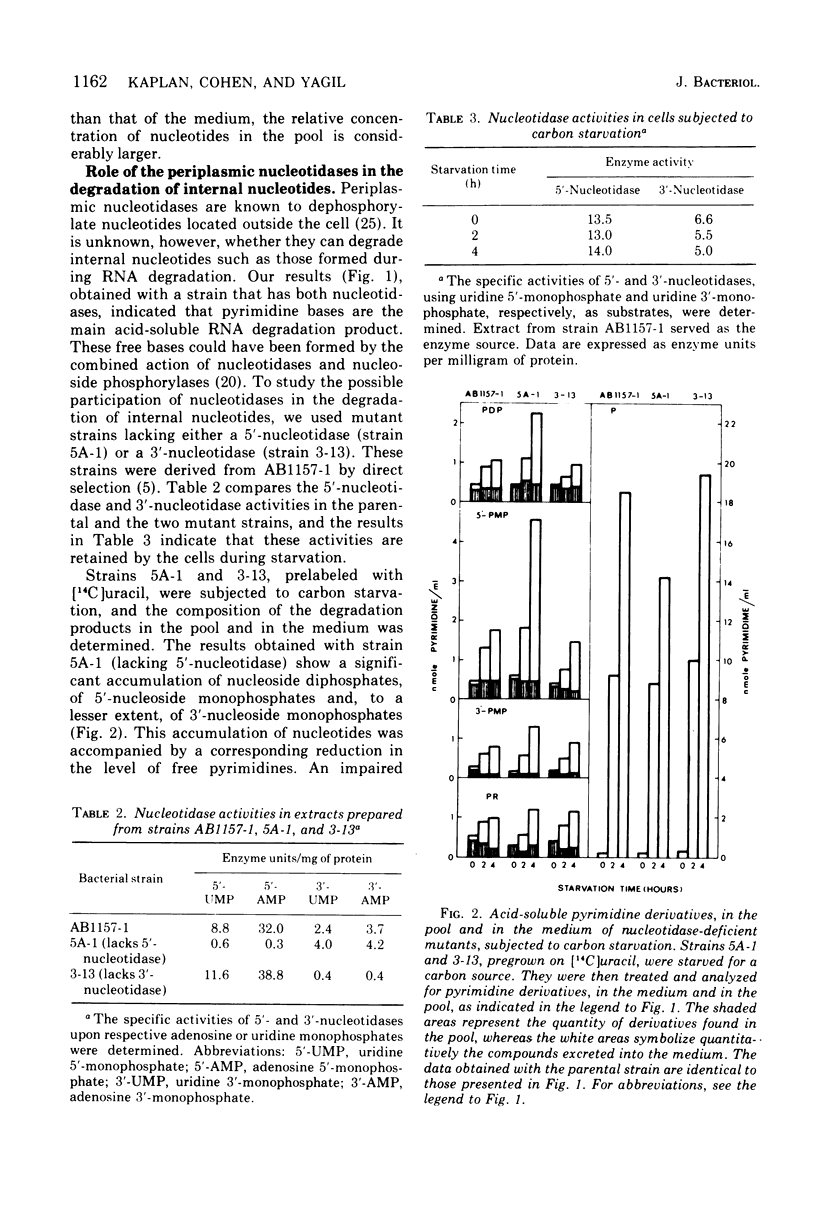
The fate of the internally formed nucleotides resulting from the degradation of ribonucleic acid was studied. Prelabeled Escherichia coli cells were submitted to carbon starvation, and the acid-soluble products were separated by thin-layer chromatography. It was determined that free bases constitute some 75% of the end product, the balance consisting of nucleoside diphosphates, 5'-nucleoside monophosphates, 3'-nucleoside monophosphates, and nucleosides. The majority of degradation products, including phosphorylated derivatives, were excreted into the medium. The amount of products in the pool remained constant. The soluble products formed by E. coli mutants lacking either 5'-nucleotidase (Ush-) or 3'-nucleotidase (Cpd-) were compared with those produced by the parental strain with both enzymes. The results obtained indicated that 5'-nucleotidase is involved in the degradation of internally foromed nucleotides but that 3'-nucleotidase takes no part in the process.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMES B. N., DUBIN D. T. The role of polyamines in the neutralization of bacteriophage deoxyribonucleic acid. J Biol Chem. 1960 Mar;235:769–775. [PubMed] [Google Scholar]
- ANRAKU Y. A NEW CYCLIC PHOSPHODIESTERASE HAVING A 3'-NUCLEOTIDASE ACTIVITY FROM ESCHERICHIA COLI B. II. FURTHER STUDIES ON SUBSTRATE SPECIFICITY AND MODE OF ACTION OF THE ENZYME. J Biol Chem. 1964 Oct;239:3420–3424. [PubMed] [Google Scholar]
- Anderson E. H. Growth Requirements of Virus-Resistant Mutants of Escherichia Coli Strain "B". Proc Natl Acad Sci U S A. 1946 May;32(5):120–128. doi: 10.1073/pnas.32.5.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmann B. J. Pedigrees of some mutant strains of Escherichia coli K-12. Bacteriol Rev. 1972 Dec;36(4):525–557. doi: 10.1128/br.36.4.525-557.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beacham I. R., Kahana R., Levy L., Yagil E. Mutants of Escherichia coli K-12 "cryptic," or deficient in 5'-nucleotidase (uridine diphosphate-sugar hydrolase) and 3'-nucleotidase (cyclic phosphodiesterase) activity. J Bacteriol. 1973 Nov;116(2):957–964. doi: 10.1128/jb.116.2.957-964.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaser L., Melo A., Paul R. Uridine diphosphate sugar hydrolase. Purification of enzyme and protein inhibitor. J Biol Chem. 1967 Apr 25;242(8):1944–1954. [PubMed] [Google Scholar]
- HURWITZ C., ROSANO C. L., PEABODY R. A. Excretion of ultraviolet-absorbing material by leaky and non-leaky strains of Escherichia coli exposed to streptomycin. Biochim Biophys Acta. 1963 May 28;72:80–86. [PubMed] [Google Scholar]
- Hartree E. F. Determination of protein: a modification of the Lowry method that gives a linear photometric response. Anal Biochem. 1972 Aug;48(2):422–427. doi: 10.1016/0003-2697(72)90094-2. [DOI] [PubMed] [Google Scholar]
- Kaplan R., Apirion D. Decay of ribosomal ribonucleic acid in Escherichia coli cells starved for various nutrients. J Biol Chem. 1975 Apr 25;250(8):3174–3178. [PubMed] [Google Scholar]
- Kaplan R., Apirion D. The fate of ribosomes in Escherichia coli cells starved for a carbon source. J Biol Chem. 1975 Mar 10;250(5):1854–1863. [PubMed] [Google Scholar]
- Kaplan R., Apirion D. The involvement of ribonuclease I, ribonuclease II, and polynucleotide phosphorylase in the degradation of stable ribonucleic acid during carbon starvation in Escherichia coli. J Biol Chem. 1974 Jan 10;249(1):149–151. [PubMed] [Google Scholar]
- Klee C. B., Singer M. F. The processive degradation of individual polyribonucleotide chains. II. Micrococcus lysodeikticus polynucleotide phosphorylase. J Biol Chem. 1968 Mar 10;243(5):923–927. [PubMed] [Google Scholar]
- LICHTENSTEIN J., BARNER H. D., COHEN S. S. The metabolism of exogenously supplied nucleotides by Escherichia coli. J Biol Chem. 1960 Feb;235:457–465. [PubMed] [Google Scholar]
- MANDELSTAM J. The intracellular turnover of protein and nucleic acids and its role in biochemical differentiation. Bacteriol Rev. 1960 Sep;24(3):289–308. doi: 10.1128/br.24.3.289-308.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neu H. C., Ashman D. F., Price T. D. Effect of ethylenediaminetetraacetic acid-Tris(hydroxymethyl)aminomethane on release of the acid-soluble nucleotide pool and on breakdown of ribosomal ribonucleic acid in Escherichia coli. J Bacteriol. 1967 Apr;93(4):1360–1368. doi: 10.1128/jb.93.4.1360-1368.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neu H. C. The 5'-nucleotidase of Escherichia coli. I. Purification and properties. J Biol Chem. 1967 Sep 10;242(17):3896–3904. [PubMed] [Google Scholar]
- Nossal N. G., Singer M. F. The processive degradation of individual polyribonucleotide chains. I. Escherichia coli ribonuclease II. J Biol Chem. 1968 Mar 10;243(5):913–922. [PubMed] [Google Scholar]
- Nozawa R., Horiuchi T., Mizuno D. Degradation of ribosomal RNA in a temperature-sensitive Escherichia coli. Arch Biochem Biophys. 1967 Feb;118(2):402–409. doi: 10.1016/0003-9861(67)90367-0. [DOI] [PubMed] [Google Scholar]
- O'Donovan G. A., Neuhard J. Pyrimidine metabolism in microorganisms. Bacteriol Rev. 1970 Sep;34(3):278–343. doi: 10.1128/br.34.3.278-343.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piérard A., Glansdorff N., Yashphe J. Mutations affecting uridine monophosphate pyrophosphorylase or the argR gene in Escherichia coli. Effects on carbamoyl phosphate and pyrimidine biosynthesis and on uracil uptake. Mol Gen Genet. 1972;118(3):235–245. doi: 10.1007/BF00333460. [DOI] [PubMed] [Google Scholar]
- Pritchard R. H., Ahmad S. I. Fluorouracil and the isolation of mutants lacking uridine phosphorylase in Escherichia coli: location of the gene. Mol Gen Genet. 1971;111(1):84–88. doi: 10.1007/BF00286557. [DOI] [PubMed] [Google Scholar]
- Stebbing N. Precursor pools and endogenous control of enzyme synthesis and activity in biosynthetic pathways. Bacteriol Rev. 1974 Mar;38(1):1–28. doi: 10.1128/br.38.1.1-28.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yagil E., Beacham I. R. Uptake of adenosine 5'-monophosphate by Escherichia coli. J Bacteriol. 1975 Feb;121(2):401–405. doi: 10.1128/jb.121.2.401-405.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]



