Abstract
Preferential phosphorylation of specific proteins by cAMP-dependent protein kinase (PKA) may be mediated in part by the anchoring of PKA to a family of A-kinase anchor proteins (AKAPs) positioned in close proximity to target proteins. This interaction is thought to depend on binding of the type II regulatory (RII) subunits to AKAPs and is essential for PKA-dependent modulation of the α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid/kainate receptor, the L-type Ca2+ channel, and the KCa channel. We hypothesized that the targeted disruption of the gene for the ubiquitously expressed RIIα subunit would reveal those tissues and signaling events that require anchored PKA. RIIα knockout mice appear normal and healthy. In adult skeletal muscle, RIα protein levels increased to partially compensate for the loss of RIIα. Nonetheless, a reduction in both catalytic (C) subunit protein levels and total kinase activity was observed. Surprisingly, the anchored PKA-dependent potentiation of the L-type Ca2+ channel in RIIα knockout skeletal muscle was unchanged compared with wild type although it was more sensitive to inhibitors of PKA–AKAP interactions. The C subunit colocalized with the L-type Ca2+ channel in transverse tubules in wild-type skeletal muscle and retained this localization in knockout muscle. The RIα subunit was shown to bind AKAPs, although with a 500-fold lower affinity than the RIIα subunit. The potentiation of the L-type Ca2+ channel in RIIα knockout mouse skeletal muscle suggests that, despite a lower affinity for AKAP binding, RIα is capable of physiologically relevant anchoring interactions.
Following the hormonal elevation of cAMP, phosphorylation of protein substrates by cAMP-dependent protein kinase (PKA) influences many physiological processes, including cellular differentiation, metabolism, and ion channel activity. PKA is a holoenzyme consisting of two regulatory (R) and two catalytic (C) subunits. Multiple subunits have been identified for R (RIα, RIβ, RIIα, and RIIβ) and C (Cα, Cβ, and Cγ) subunits. In general, the α isoforms are constitutively expressed in most tissues, whereas the β isoforms are highly expressed in the brain with a lower and more selective expression in other tissues (1). Despite the ubiquitous expression of PKA, cells are capable of specific responses to hormones and neurotransmitters. It has been proposed that the selective phosphorylation of specific proteins by PKA is achieved in part by the subcellular compartmentalization of PKA (2).
The RII subunits (RIIα and RIIβ) are postulated to anchor PKA holoenzyme to subcellular compartments thereby positioning PKA in close proximity to its substrates (3). In support of this, RII subunits have been shown to bind to a family of A-kinase anchor proteins (AKAPs) (4, 5). These AKAPs contain a conserved amphipathic helix that binds to the amino terminus of the RII subunit (5, 6). In addition to binding to the RII subunit of PKA, AKAP 79 interacts with the calcium and calmodulin-dependent protein phosphatase 2B (calcineurin) and protein kinase C through sites distinct from the RII binding site (7, 8). It is therefore possible that AKAPs act as a scaffold for multiple kinases and phosphatases, adding another level of complexity to cAMP-mediated signal transduction.
The interaction between RII subunits and AKAPs is required for certain intracellular signaling events. For instance, disruption of RII-AKAP binding with Ht31 peptide, a 24 amino acid peptide from a human thyroid AKAP, prevents the PKA-dependent increase in synaptic current through α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid/kainate glutamate receptors (9). Activation of skeletal muscle Ca2+ channels is enhanced by activation of PKA, and potentiation of Ca2+ channel activity by single 50–200 ms depolarizations or trains of 3 ms depolarizations requires phosphorylation by PKA (10). Ht31 peptide blocked the voltage-dependent potentiation of the skeletal muscle L-type Ca2+ channel, suggesting that close proximity between PKA and the L-type Ca2+ channel is necessary for the rapid phosphorylation of this channel during trains of brief depolarizations (11). Ht31 peptide has also been shown to block the stimulation by ATP of the calcium-activated potassium channel (KCa), a finding which suggests that the ATP stimulation is mediated by phosphorylation by anchored PKA (12). These reports demonstrate the functional importance of anchoring PKA near membrane-bound protein substrates and support the hypothesis that the subcellular localization of PKA is physiologically significant.
In this study, we tested the hypothesis that mice deficient in the ubiquitously expressed type II regulatory subunit (RIIα) would reveal defects in signaling events that require anchored PKA. We report that the targeted disruption of the RIIα gene resulted in viable mice with no obvious physiological defects. Furthermore, the potentiation of the L-type Ca2+ channel in skeletal muscle cells from RIIα knockout mice was retained, and this potentiation was sensitive to inhibitors of PKA anchoring. These findings suggest that PKA can be anchored by RIα in RIIα knockout skeletal muscle.
MATERIALS AND METHODS
Targeted Disruption of the Genomic Locus.
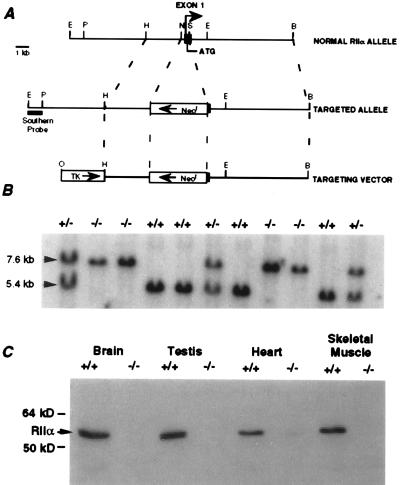
A NotI–SalI fragment containing both the promoter sequences and the first 27 amino acids of the RIIα protein was deleted and replaced with a neomycin phosphotransferase gene driven by the simian virus 40 early promoter and containing the simian virus 40 T antigen polyadenylation site. A viral thymidine kinase expression cassette was added to the end of the targeting construct for double selection. Embryonic stem cells (REK3) were transfected and selected with G418 and ganciclovir (13). Four cell lines were used to make chimeric mice. A single cell line produced germ-line chimeras that were bred to C57BL/6 mice to produce heterozygotes. The studies were conducted on mice with a genetic background of 75% C57BL/6 and 25% 129 Sv/J. For Southern blot analysis, total nucleic acid from tail biopsies was digested with EcoRI and, following hybridization with the Southern probe, yielded the 5.4-kb wild-type and the 7.6-kb knockout alleles.
Western Blot Analysis.
Protein (40 μg per lane) from tissue homogenates was separated by SDS/10% polyacrylamide gel electrophoresis (PAGE) and transferred to nitrocellulose. The blot was probed with a polyclonal antiserum against the following: murine RIIα at 1:10,000; murine RI at 1:2,000; murine Cα at 1:10,000 (provided by S. S. Taylor, University of California, San Diego). Protein was visualized by using an enhanced chemiluminescence kit (Amersham). Optical density measurements were done using the National Institutes of Health image program to evaluate scanned autoradiograms.
Kinase Assay.
The kinase assay was performed in triplicate on fore- and hindlimb skeletal muscle homogenates as described (14). Tissue was pooled from neonatal (2-day-old) litters. Nonspecific kinase activity that remained in the presence of the PKA inhibitor, PKI, was subtracted from both basal and total PKA activity.
Electrophysiological Recordings.
Fore- and hindlimb mouse skeletal muscle was harvested from 1- to 5-day-old pups, trypsinized (0.01%) overnight at 4°C, and collagenase (0.008%) treated for 45 min at 37°C. Myotubes were differentiated from myoblasts in DMEM supplemented with 10% horse serum and 5% fetal calf serum and cultured for 5–10 days (15). Electrophysiological recordings and analysis were performed blind on coded cell cultures as follows. Barium currents through Ca2+ channels were recorded using the whole-cell configuration of the patch clamp technique. Voltage-dependent currents have been corrected for leak using an online P/4 subtraction paradigm. The extracellular saline (bath) contained 150 mM Tris, 2 mM MgCl2, 10 mM BaCl2, with pH adjusted to 7.3 with methanesulfonic acid. Patch pipette saline (intracellular) contained 130 mM N-methyl-d-glucamine, 10 mM EGTA, 60 mM Hepes, 2 mM MgATP, 1 mM MgCl2, with pH adjusted to 7.3 with methanesulfonic acid. All experiments were performed at room temperature (20–23°C). Ht31 and Ht31P peptides (11, 16) were synthesized and purified by HPLC in the University of Washington Molecular Pharmacology Facility with the following sequence: Ht31, Asp-Leu-Ile-Glu-Glu-Ala-Ala-Ser-Arg-Ile-Val-Asp-Ala-Val-Ile-Glu-Gln-Val-Lys-Ala-Ala-Gly-Ala-Tyr; Ht31P, Pro substituted for Ile at positions 10 and 15.
RIIα(1–45) Peptide Synthesis.
The coding region of the first 45 amino acids of mouse RIIα was subcloned into Pet 16b (Novagen). This construct creates a N-terminal histidine tag for purification. The protein was expressed in BL21DE3 (1 liter) and induced with isopropyl β-d-thiogalactoside for 18 hr at 37°C. The protein was unfolded from the insoluble fraction in 6 M guanidine chloride then diluted 3 times with 1 M imidazole. The protein was then dialyzed into His binding buffer and put over a nickel charge resin. The protein was concentrated to 10 ml and exchanged into 20 mM Tris, 100 mM NaCl, 2 mM CaCl2, pH 8.0, using a PD10 column. The protein was then cleaved overnight with excess Factor Xa (NEB, Beverly, MA). The 1–45 fragment was separated from the histidine tag using a superdex gel filtration column.
Immunohistochemistry.
Forelimb skeletal muscle was removed from 1- to 4-day-old pups. The muscle was minced and digested in 0.05% trypsin for 20 min at 37°C. Cells were plated at multiple dilutions in F10C media supplemented with 15% horse serum on plates coated with 0.67% gelatin. Cells were fed two times per day with 2 ng/ml basic fibroblast growth factor (FGF) for 7 days and then cultured without FGF for 3–4 days. Plates containing cells that exhibited cross-striations and <25% fibroblasts were used for immunohistochemistry.
After day 10–11 the growth medium was removed and the cells were rinsed with 0.1 M phosphate buffer for 3 min, fixed for 30 min in 4% paraformaldehyde buffered in 0.1 M phosphate buffer, pretreated with 2% avidin and 2% biotin as described (17), and then blocked for 30 min in TBS containing 5% normal goat serum. The cultures were then incubated overnight at 4°C in anti-C (diluted 1:500), or in no primary antibody. The diluent for all the antibodies was a solution of TBS containing 5% normal goat serum and 0.025% Triton X-100. The cultures were then rinsed, incubated in biotinylated goat anti-rabbit IgG and then avidin D fluorescein as described (17). Labeled samples were viewed using a Bio-Rad MRC 600 confocal microscope located in the W. M. Keck Imaging Facility at the University of Washington, Seattle.
Double-labeling experiments were done in two separate rounds of staining as described above. Between the two rounds of staining, sections were treated as described (18) and in the Jackson ImmunoResearch catalog. Briefly, the cultures were rinsed in TBS (3 × 10 min) following the first round of staining, incubated in TBS containing 5% normal rabbit serum for 1 hr at 37°C, rinsed with TBS (3 × 10 min), incubated in 10 μg/ml affinity-purified goat anti-rabbit IgG Fab fragments diluted in TBS for 1 hr at 37°C, rinsed with TBS (3 × 10 min), and then the blocking steps using avidin and biotin were repeated. The cultures were then incubated in anti-CP11 diluted 1:10, as described (19) overnight at 4°C. The cultures were then incubated in biotinylated goat anti-rabbit IgG, followed by avidin D Texas red (Vector Laboratories, diluted 1:200) and then prepared for viewing.
RIα Purification.
Following overexpression in Escherichia coli strain DH1 and induction with 1 mM isopropyl β-d-thiogalactoside for 4 hr (20), recombinant RIα protein was purified as described (21). RIα protein was resolved by DEAE ion exchange chromatography (20). The purity of the protein was estimated by SDS/PAGE.
Surface Plasmon Resonance Measurements.
Neutraavidin (Pierce) at 100 μg/ml was coupled to a carboxymethyldextran IAsys cuvette as described (22). After washing with phosphate- buffered saline with 0.5% Tween 20, 17 μM recombinant RIα was added to the cuvette and no binding was observed under these conditions. Biotinylated Ht31 peptide was then bound to the cuvette and binding experiments were performed over a range of RIα concentrations from 425 nM to 12 μM in 70 μl. The binding surface was regenerated between binding measurements using 6 M guanidine chloride with no decrease in extent measurements over the duration of an experiment. Data were collected over 2-sec intervals and were analyzed using the fastfit software.
RESULTS
The gene for the RIIα subunit of PKA was disrupted with a replacement type vector (Fig. 1A). A region of exon 1 containing both the transcription and translation start sites was deleted by homologous recombination in embryonic stem cells (13). The generation of wild-type, heterozygous, and homozygous offspring (Fig. 1B) was in the expected Mendelian ratio of 1:2:1, indicating that the mutation caused no embryonic lethality. RIIα protein was shown to be absent in RIIα knockout mice (Fig. 1C). RIIα knockout mice exhibited normal growth and appeared healthy. No apparent deficits in locomotor activity, muscle strength, or exploratory behavior were observed.
Figure 1.
Generation and analysis of RIIα knockout mice. (A) RIIα gene, disrupted allele, targeting vector and Southern probe. Arrow indicates transcription start site. E, EcoRI; N, NotI; S, SalI; B, BamHI; P, PstI; H, HindIII. (B) Genomic Southern blot analysis to determine the genotype of the offspring from an RIIα +/− cross. (C) Western blot analysis of adult wild-type and RIIα knockout tissue in which the absence of RIIα protein in RIIα knockout mice is confirmed.
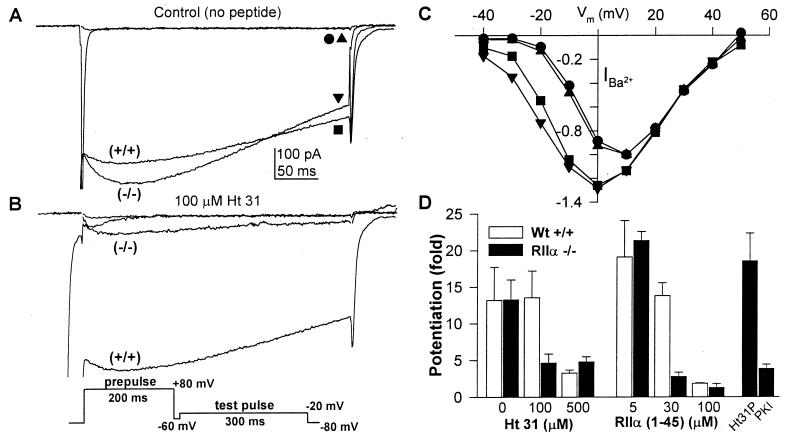
To test the model that the anchoring of PKA by RII subunits to AKAPs is essential for the potentiation of the L-type Ca2+ channel in skeletal muscle, we cultured neonatal skeletal muscle and measured the potentiation of Ca2+ channel activity in the absence and presence of two peptides that disrupt the anchoring of RII subunits to AKAPs: Ht31, a 24-amino acid peptide derived from the PKA binding domain of a human thyroid AKAP, and RIIα(1–45), a peptide fragment containing the first 45 amino acids of RIIα and encompassing the AKAP binding domain. Depolarization from the holding potential of −80 mV to a test potential near the threshold for channel activation (−20 mV) elicited little Ca2+ current (Fig. 2A, •, ▴), but large Ca2+ currents were recorded at this test potential following a 200-ms depolarization to +80 mV (Fig. 2A, ▪, ▾) for both wild-type and RIIα knockout myotubes. Moreover, the voltage dependence of activation of the Ca2+ current in wild-type and knockout myotubes was identical before the prepulse and was similarly shifted to more negative membrane potentials after the prepulse (Fig. 2C). However, in the RIIα knockout, potentiation of the Ca2+ current by a prepulse to +80 mV was blocked with a lower concentration of intracellular Ht31 (100 μM vs. 500 μM) (Fig. 2 B and D) and intracellular RIIα(1–45) peptide (30 μM vs. 100 μM) (Fig. 2D). The differences in the effects of Ht31 peptide and RIIα(1–45) peptide between wild-type and RIIα knockout skeletal muscle cells were observed for prepulse voltages from +40 to +100 mV and for prepulse durations from 50 to 300 ms using pulse protocols as described (11). As a control for nonspecific peptide effects, Ht31P, a proline-substituted Ht31 peptide that does not bind to RII proteins, was used (11, 23). At a concentration of 500 μM, Ht31P did not block potentiation in RIIα knockout cells (Fig. 2D). However, the PKA inhibitor, PKI (5–24) amide, blocked potentiation, demonstrating that potentiation requires PKA activity. These data suggest that PKA is anchored in RIIα knockout skeletal muscle because the potentiation of the Ca2+ channel requires anchored PKA. However, PKA anchoring in RIIα knockout skeletal muscle cells is more sensitive to peptides that disrupt interaction of PKA with AKAPs, suggesting that the affinity of the bound PKA for AKAPs is reduced.
Figure 2.
Voltage-dependent potentiation of the L-type Ca2+ channel and its inhibition by Ht31 and RIIα peptides in wild-type (+/+) and RIIα knockout (−/−) skeletal muscle cells. Neonatal mouse skeletal myotubes were voltage clamped in the whole cell patch clamp configuration with pipettes containing one of the following four synthesized peptides: (i) Ht31, consisting of 2-3 amino acids from the PKA binding domain from a human thyroid AKAP; (ii) Ht31P, in which the putative amphipathic helix formed by Ht31 is disrupted by replacing 2 isoleucines with prolines; (iii) RIIα(1–45), a truncated RIIα protein retaining the first 45 amino acids containing the AKAP-binding and the RII-dimerization domains; and (iv) PKI (5–24)-amide, a 20-residue inhibitor of PKA. (A and B) Potentiation of Ca2+ channel current (carried by Ba2+) under control conditions (A, no added peptide) or in the presence of 100 μM intracellular Ht31 (B). From a holding potential of −80 mV, test pulses to −20 mV were applied before and following a conditioning prepulse to +80 mV (Inset). (C) Comparison of Ca2+ channel current-voltage relationship without (wt, •; RIIα knockout, ▴) and with (wt, ▪; RIIα knockout, ▾) the conditioning prepulse under control conditions with no Ht31 present. (D) Mean potentiation ± SEM (n = 3–12) in the absence and presence of Ht31, RIIα(1–45), 500 μM Ht31P, or 10 μM PKI. Mean potentiation was measured as the ratio of the current at the end of test pulses taken after and before the prepulse.
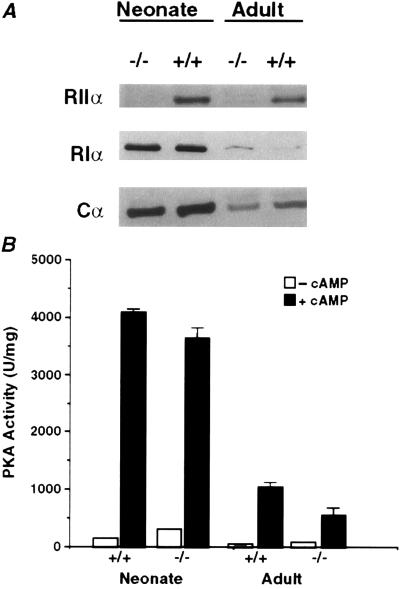
We next determined the PKA subunit composition and kinase activity in wild-type and RIIα knockout skeletal muscle comparing neonatal and adult tissues. In adult skeletal muscle, the loss of RIIα protein resulted in a 65% increase in RIα and a 35% decrease in Cα protein levels (Fig. 3A). As a result of the reduction in C subunit, total PKA activity is reduced by ≈46% in adult RIIα knockout skeletal muscle compared with wild type (Fig. 3B). In the neonate, where RIα is expressed at higher levels compared with the adult, there is no detectable change in the level of RIα in RIIα knockout skeletal muscle. Comparison of RIα and Cα protein levels between wild type and RIIα knockouts using various dilutions of protein extracts from neonates confirmed that RIα and Cα levels remain unchanged in neonatal RIIα knockout skeletal muscle (data not shown). In the neonatal skeletal muscle, total PKA activity is greater compared with adult and there is little change in the RIIα knockout. We have also demonstrated by Western blot analysis that neither RIIβ nor RIβ is expressed in adult or neonatal skeletal muscle in wild-type or RIIα knockout mice (data not shown). These results suggest that RIα is anchoring PKA in RIIα knockout skeletal muscle because it is the only regulatory subunit expressed.
Figure 3.
PKA subunit levels and total PKA activity in neonatal and adult skeletal muscle from wild-type and RIIα knockout mice. (A) Western blot analysis for RIIα, RIα, and Cα subunits. (B) Basal kinase activity (□, −cAMP) and total PKA activity (▪, +cAMP). Kinase activity reported as mean ± SEM.
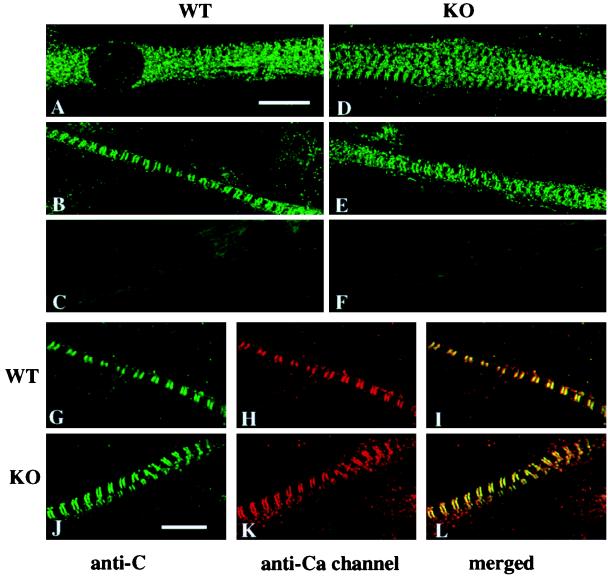
Immunohistochemical techniques were used to assess the subcellular localization of the C subunit of PKA and its possible colocalization with the L-type Ca2+ channel in wild-type and RIIα knockout skeletal muscle. In cultured neonatal wild-type myoblasts that have fused and formed myotubes, Cα was observed throughout the length of the myotube but was excluded from the nucleus (Fig. 4A). In myotubes that have differentiated and formed cross-striations, Cα (Fig. 4 B and G) and the L-type Ca2+ channel α1 subunit (Fig. 4H) were found colocalized in the transverse tubules (Fig. 4I). In RIIα knockout skeletal muscle, Cα (Fig. 4 D, E, and J) and the L-type Ca2+ channel (Fig. 4K) had a similar pattern of distribution compared with wild-type and were colocalized in the transverse tubules (Fig. 4L). The finding that Cα remains colocalized with the L-type Ca2+ channel in the transverse tubules in RIIα knockout skeletal muscle was unexpected and suggests that the remaining RIα holoenzyme is capable of specific subcellular association.
Figure 4.
Localization of the PKA catalytic subunit and Ca2+ channel α1 subunit in cultured myotubes. (A and B) Skeletal muscle cultures stained with anti-C subunit antibody showing the distribution of C subunit of PKA throughout the muscle fibers of wild-type mice. (D and E) Distribution of C subunit in fibers from RIIα knockout mice. (C and F) Control sections demonstrating the lack of staining in wild-type and knockout, respectively, when no primary antibody was used. (G–I) Double labeling in wild-type cultures using anti-C (G) and anti-CP11 against the calcium channel α1 subunit (H) and colocalization of C subunit and calcium channel α1 subunit in muscle from wild-type mice (I). (J–L) Double labeling of anti-C and anti-CP11 showing the distribution of labeling by anti-C (J) and anti-CP 11 (K) antibodies and their colocalization (L) in muscles from knockout mice. (Bar = 10 μm.)
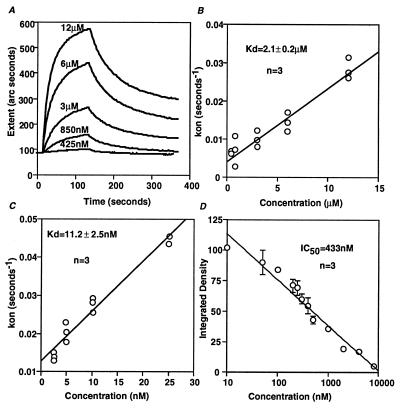
This hypothesis was further tested by measurements of the binding affinity (Kd) of RIα for Ht31 anchoring inhibitor peptide by surface plasmon resonance. The binding properties of RIα to immobilized Ht31 peptide were measured over a range of concentrations of recombinant RIα (425 nM to 12 μM) (Fig. 5 A and B). A dissociation constant (Kd) of 2.1 μM was calculated for the RIα-Ht31 peptide interaction. The binding affinity of RIIα for Ht31 peptide has been measured to be 4 nM by equilibrium dialysis (16), a value that is similar to the 11.2 nM determined by surface plasmon resonance (Fig. 5C). Using a quantitative overlay assay as described (6), we determined that RIα inhibits the binding of 1 nM RIIα to Ht31 protein with an IC50 of 433 nM (Fig. 5D). Both the surface plasmon resonance measurements and the overlay assay inhibition data suggest that RIα binds to AKAPs with ≈500-fold lower affinity than RIIα and that RIα can compete with RIIα for binding to AKAPs.
Figure 5.
Affinity of Ht31 peptide/RIα interaction. The binding affinity of RIα for the Ht31 peptide was measured by surface plasmon resonance (A and B). (A) Extent measurements (measured in arc seconds) showing the binding profiles for selected concentrations of RIα interacting with the immobilized biotinylated Ht31 peptide. (B) The measured on rates (M−1·s−1) are plotted against RIα concentration. (C) The measured on rates (M−1·s−1) are plotted against RIIα concentration. (D) A competition binding assay (6) between RIα (0–8,000 nM) and 32P-labeled RIIα (1 nM) for binding to immobilized Ht31 (25 ng).
DISCUSSION
Our results provide evidence that PKA remains anchored to AKAPs in vivo in RIIα knockout mouse skeletal muscle and that this anchoring sustains the PKA-dependent potentiation of the L-type Ca2+ channel. These results challenge the idea that type II regulatory subunits are required for anchoring of PKA in vivo and raise the possibility that RIα can also serve in this capacity. Immunocytochemical studies show that the C subunit of the type I holoenzyme in RIIα knockout skeletal muscle is colocalized in transverse tubules with the L-type Ca2+ channel, consistent with anchoring of type I PKA with Ca2+ channels. In vitro measurements of RIα binding affinity to Ht31 demonstrate that RIα binds to AKAPs with a 500-fold lower affinity than RIIα, but this low affinity is apparently sufficient for anchoring in vivo. Previous reports have estimated the intracellular concentration of PKA to be in the 1 μM range, which would lead to significant binding at the Kd measured by surface plasmon resonance. Because there is no detectable expression of RIIβ or RIβ in wild-type or RIIα knockout skeletal muscle, the only PKA regulatory subunit in RIIα knockout skeletal muscle is RIα. Thus, our working hypothesis is that RIα can serve to anchor PKA near the skeletal muscle calcium channel in RIIα knockout mice. Whether RIα contributes to the anchoring of PKA in wild-type skeletal muscle is unknown.
With the loss of RIIα protein, the levels of RIα and Cα protein are increased and decreased, respectively, in adult skeletal muscle, and there is a concomitant decrease in total kinase activity. A similar change in RIα and Cα protein levels was observed in brown adipose tissue of RIIβ knockout mice (24). The compensation in RIα levels is likely to be a result of protein stabilization in a holoenzyme complex to protect the cell from unregulated C subunit activity (25). The reduction in C subunit levels in RIIα knockout skeletal muscle is likely the result of either incomplete compensation by RIα or increased basal PKA activity leading to C subunit turnover (24, 25). This perturbation in RIα and Cα protein levels is not apparent in neonatal skeletal muscle from RIIα knockout mice, because it is likely that the RIIα containing holoenzyme contributes a much smaller proportion of the total PKA activity in neonatal muscle. Imaizumi-Scherrer et al. (26) report a decline in RIα and Cα protein levels during development of muscle and our results are in agreement.
The potentiation of the L-type Ca2+ channel is unchanged in the RIIα knockout skeletal muscle compared with wild-type, although it is blocked with lower concentrations of Ht31 and RIIα(1–45) peptides. These results were unexpected and suggest that RIα can anchor PKA in RIIα knockout skeletal muscle cells by binding to the same AKAP associated with or near the L-type Ca2+ channel. The identity of the AKAP mediating this interaction with RIIα and RIα is unknown. Huang et al. (27) identified a dual AKAP, D-AKAP1, which binds both RIIα and RIα and has a splice variant that is expressed in skeletal muscle. However, these authors suggest that D-AKAP1 in muscle is targeted to the mitochondria. Another AKAP, AKAP 100, was cloned and characterized in skeletal muscle, but was localized to the sarcoplasmic reticulum (28). Because D-AKAP1 and AKAP 100 have been localized to regions other than the transverse tubules, it is more likely that AKAP 15, a protein recently found to be associated with skeletal muscle L-type Ca2+ channels (21), mediates the anchoring of RIIα and RIα near these channels.
Studies localizing C subunit and the L-type Ca2+ channel in both wild-type and RIIα knockout cultured skeletal muscle cells support the conclusion that PKA is anchored near the L-type Ca2+ channel in skeletal muscle. In developing myotubes that are not cross-striated, the C subunit and L-type Ca2+ channel are diffusely distributed, similar to the pattern of the α1 subunit of the L-type Ca2+ channel described previously (29, 30). In longer term cultures of differentiated myotubes containing cross-striations, the C subunit and the L-type Ca2+ channel appear to be preferentially localized to the transverse tubules (Fig. 4; refs. 29 and 30). This subcellular compartmentalization of PKA at the transverse tubules in neonatal cultured myotubes suggests that PKA preferentially phosphorylates those proteins, including the L-type Ca2+ channel, present in the transverse tubules. In adult skeletal muscle, type I holoenzyme of PKA has been found at the neuromuscular junction (26). Whether the C subunit is also localized to the transverse tubules in adult skeletal muscle awaits further study.
In many cells, RIα is cytoplasmic. A few reports, however, have localized RIα to discrete subcellular compartments such as the plasma membrane in erythrocytes (31), and the TCR-CD3 complex (or the “cap” site) in lymphocytes following stimulation with anti-CD3 antibodies (32). Our data suggest that RIα is able to localize to the transverse tubules in skeletal muscle through its interaction with an AKAP. Based on the binding affinity measurements, the affinity of the RIα-AKAP interaction is much lower than the RIIα-AKAP interaction. This finding is corroborated by Huang et al. (27) who report a lower binding affinity of RIα compared with RIIα for D-AKAP1. Because the binding affinity between RIIα and known AKAPs is greater than between RIα and known AKAPs, RIIα will preferentially occupy this binding site on the AKAP in wild-type tissue. In tissues such as neonatal skeletal muscle where RIα levels are elevated, RIα may compete with RIIα for binding to AKAPs. Moreover, it remains possible that still undiscovered AKAPs also participate in localization of type I PKA.
Acknowledgments
We thank Thong Su, Kirstin Gerhold, and Barbara Treash for superb technical assistance. This work was supported by National Institutes of Health Grants 1 F32 HD08034–01-A1 (K.A.B.), PO1 HL44948 (G.S.M., W.A.C.), GM 32875 (G.S.M.), and DK 44239 (J.D.S.); American Heart Association Grant 95-WA-110 (K.A.B.); and a Research Grant and Postdoctoral Research Fellowship from the Muscular Dystrophy Association (W.A.C., B.D.J.).
ABBREVIATIONS
- AKAP
A-kinase anchor protein
- C
catalytic subunit
- PKA
cAMP-dependent protein kinase
- R
regulatory subunit
- KCa
calcium-activated potassium channel
- PKI
PKA inhibitor
References
- 1.Cadd G, McKnight G S. Neuron. 1989;3:71–79. doi: 10.1016/0896-6273(89)90116-5. [DOI] [PubMed] [Google Scholar]
- 2.Harper J F, Haddox M K, Johanson R, Hanley R M, Steiner A L. Vitam Horm (San Francisco) 1985;42:197–252. doi: 10.1016/s0083-6729(08)60063-1. [DOI] [PubMed] [Google Scholar]
- 3.Lohmann S M, DeCamilli P, Einig I, Walter U. Proc Natl Acad Sci USA. 1984;81:6723–6727. doi: 10.1073/pnas.81.21.6723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rubin C S. Biochim Biophys Acta. 1994;1224:467–479. [PubMed] [Google Scholar]
- 5.Dell’Acqua M L, Scott J D. J Biol Chem. 1997;272:12881–12884. doi: 10.1074/jbc.272.20.12881. [DOI] [PubMed] [Google Scholar]
- 6.Hausken Z E, Coghlan V M, Schafer Hastings C A, Reimann E M, Scott J D. J Biol Chem. 1994;269:24245–24251. [PubMed] [Google Scholar]
- 7.Coghlan V, Perrino B A, Howard M, Langeberg L K, Hicks J B, Gallatin W H, Scott J D. Science. 1995;267:108–111. doi: 10.1126/science.7528941. [DOI] [PubMed] [Google Scholar]
- 8.Klauck T M, Faux M C, Labudda K, Langeberg L K, Jaken S, Scott J D. Science. 1996;271:1589–1592. doi: 10.1126/science.271.5255.1589. [DOI] [PubMed] [Google Scholar]
- 9.Rosenmund C, Carr D W, Bergeson S E, Nilaver G, Scott J D, Westbrook G L. Nature (London) 1994;368:853–856. doi: 10.1038/368853a0. [DOI] [PubMed] [Google Scholar]
- 10.Sculptoreanu A, Scheuer T, Catterall W A. Nature (London) 1993;364:240–243. doi: 10.1038/364240a0. [DOI] [PubMed] [Google Scholar]
- 11.Johnson B D, Scheuer T, Catterall W A. Proc Natl Acad Sci USA. 1994;91:11492–11496. doi: 10.1073/pnas.91.24.11492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang Z-W, Kotlikoff M I. Am J Physiol. 1996;271:L100–L105. doi: 10.1152/ajplung.1996.271.1.L100. [DOI] [PubMed] [Google Scholar]
- 13.Brandon E P, Gerhold K A, Qi M, McKnight G S, Idzerda R L. Recent Prog Horm Res. 1995;50:403–408. doi: 10.1016/b978-0-12-571150-0.50028-7. [DOI] [PubMed] [Google Scholar]
- 14.Clegg C H, Correll L A, Cadd G G, McKnight G S. J Biol Chem. 1987;262:13111–13119. [PubMed] [Google Scholar]
- 15.Koenig J, Bournaud R, Powell J A, Rieger F. Dev Biol. 1982;92:188–196. doi: 10.1016/0012-1606(82)90162-2. [DOI] [PubMed] [Google Scholar]
- 16.Carr D W, Hausken Z E, Fraser I D, Stofko-Hahn R E, Scott J D. J Biol Chem. 1992;267:13376–13382. [PubMed] [Google Scholar]
- 17.Westenbroek R E, Sakurai T, Elliot E M, Hell J W, Starr T V B, Snutch T P, Catterall W A. J Neurosci. 1995;15:6403–6418. doi: 10.1523/JNEUROSCI.15-10-06403.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bausch S B, Patterson T A, Ehrengruber M U, Lester H A, Davidson N, Chavkin C. Recept Channels. 1995;3:221–241. [PubMed] [Google Scholar]
- 19.De Jongh K S, Warner C, Colvin A A, Catterall W A. Proc Natl Acad Sci USA. 1991;88:10778–10782. doi: 10.1073/pnas.88.23.10778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Woodford T A, Correll L A, McKnight G S, Corbin J D. J Biol Chem. 1989;264:13321–13328. [PubMed] [Google Scholar]
- 21.Gray P C, Tibbs V C, Catterall W A, Murphy B J. J Biol Chem. 1997;272:6297–6302. doi: 10.1074/jbc.272.10.6297. [DOI] [PubMed] [Google Scholar]
- 22.Nauert J B, Klauck T M, Langeberg L K, Scott J D. Curr Biol. 1997;7:52–62. doi: 10.1016/s0960-9822(06)00027-3. [DOI] [PubMed] [Google Scholar]
- 23.Carr D W, Stofko-Hahn R E, Fraser I D, Bishop S M, Acott T S, Brennan R G, Scott J D. J Biol Chem. 1991;266:14188–14192. [PubMed] [Google Scholar]
- 24.Cummings D E, Brandon E P, Planas J V, Motamed K, Idzerda R L, McKnight G S. Nature (London) 1996;382:622–626. doi: 10.1038/382622a0. [DOI] [PubMed] [Google Scholar]
- 25.Amieux P S, Cummings D E, Motamed K, Brandon E P, Wailes L A, Lee K, Idzerda R L, McKnight G S. J Biol Chem. 1997;272:3993–3998. doi: 10.1074/jbc.272.7.3993. [DOI] [PubMed] [Google Scholar]
- 26.Imaizumi-Scherrer T, Faust D M, Benichou J-C, Hellio R, Weiss M C. J Cell Biol. 1996;134:1241–1254. doi: 10.1083/jcb.134.5.1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huang L J-S, Durick K, Weiner J A, Chun J, Taylor S S. J Biol Chem. 1997;272:8057–8064. doi: 10.1074/jbc.272.12.8057. [DOI] [PubMed] [Google Scholar]
- 28.McCartney S, Little B M, Langeberg L K, Scott J D. J Biol Chem. 1995;270:9327–9333. doi: 10.1074/jbc.270.16.9327. [DOI] [PubMed] [Google Scholar]
- 29.Flucher B E, Morton M E, Froehner S C, Daniels M P. Neuron. 1990;5:339–351. doi: 10.1016/0896-6273(90)90170-k. [DOI] [PubMed] [Google Scholar]
- 30.Carl S L, Felix K, Caswell A H, Brandt N R, Brunschwig J-P, Meissner G, Ferguson D G. Muscle Nerve. 1995;18:1232–1243. doi: 10.1002/mus.880181104. [DOI] [PubMed] [Google Scholar]
- 31.Rubin C S, Erlichmann J, Rosen O M. J Biol Chem. 1972;247:6135–6139. [PubMed] [Google Scholar]
- 32.Skalhegg B S, Tasken K, Hansson V, Huitfeldt H S, Jahnsen T, Lea T. Science. 1994;263:84–87. doi: 10.1126/science.8272870. [DOI] [PubMed] [Google Scholar]