Abstract
In contrast to early breast cancer, the prognostic effect of tumour angiogenesis in tumours with advanced axillary spread has been less studied. We retrospectively analysed the effect of microvessel density (MVD) and vascular endothelial growth factor (VEGF) by immunohistochemistry on the outcome of 215 patients treated uniformly within prospective trials of high-dose chemotherapy for 4–9 and ⩾10 positive nodes, and followed for a median of 9 (range 3–13) years. Microvessel density was associated with epidermal growth factor receptor (EGFR) expression (P<0.001) and tumour size (P=0.001). Vascular endothelial growth factor overexpression (51% of patients) was associated with overexpression of EGFR (P=0.01) and HER2 (P<0.05), but not with MVD (P=0.3). High MVD was associated with worse relapse-free survival (74 vs 44%, P<0.001) and overall survival (76 vs 44%, P<0.001). Vascular endothelial growth factor overexpression had no effect on outcome. Multivariate analyses showed a prognostic effect of MVD independently of other known prognostic factors in this patient population. In conclusion, tumour angiogenesis, expressed as MVD, is a major independent prognostic factor in breast cancer patients with extensive axillary involvement.
Keywords: high-risk breast cancer, angiogenesis, microvessel density, vascular endothelial growth factor, prognostic factor, high-dose chemotherapy
High-risk primary breast cancer (HRPBC) is characterised by extensive axillary involvement, usually defined as four or greater positive nodes. Although still curable, this stage of the disease presents a 50% or greater risk of metastatic relapse despite modern multimodal management. Identification of relevant new biologic targets in this population may allow for improvement of therapeutic outcome.
One such type of target might be those involved in tumour angiogenesis. Angiogenesis promotes tumour growth and metastasis through perfusion of the tumour, facilitation of transport of tumour cells in the bloodstream, and reciprocal paracrine stimulation between endothelial and tumour cells (Folkman, 1990; Fidler and Ellis, 1994). Angiogenesis is part of a complex scenario that also includes tumour suppressor gene and oncogene pathways, adhesion molecules, apoptosis, and the immune system. The angiogenic status of a tumour depends on the balance between proangiogenic and antiangiogenic factors that determines a state of tumour dormancy, which can last for a long time in breast cancer, or a predominance of angiogenesis, which appears to trigger disease progression or relapse.
The vascular endothelial growth factor (VEGF) is one of the most potent inducers of angiogenesis (Ferrara et al, 2003). Although it is produced by many different cells, its mitogenic activity mainly focuses on endothelial cells. Vascular endothelial growth factor and its tyrosine kinase receptors are subjected to regulation by tissue oxygen conditions as well as by oncogenes, such as HER2, or tumour-suppressor genes.
Many studies, but not all, have suggested a prognostic effect of angiogenesis in early breast cancer, defined by no or limited axillary involvement. A meta-analysis detected an overall prognostic adverse effect of microvessel density (MVD) among node-negative patients (Uzzan et al, 2004). The discrepancies among the studies may be the result of methodological differences or heterogeneity of patients and treatments. In consequence, it has been recommended that angiogenesis markers are not to be used as basis for making clinical decisions (Hayes et al, 2001). The College of American Pathologists has stated that further study of quantification of tumour angiogenesis is still required to demonstrate its prognostic value in breast cancer (Fitzgibbons et al, 2000).
Furthermore, less is known about the prognostic effect of angiogenesis in patients with advanced axillary involvement, who present a higher likelihood of micrometastatic spread at the time of diagnosis, and, thus, may constitute a different biological scenario from node-negative tumours. We studied the effect of tumour angiogenesis in HRPBC by immunohistochemical analysis of MVD and VEGF in samples collected retrospectively from patients who were treated uniformly within high-dose chemotherapy (HDC) trials and subjected to long-term follow-up.
MATERIALS AND METHODS
Patient population
This study adhered to the NCI-EORTC guidelines for tumour marker prognostic studies (McShane et al, 2005). We retrospectively analysed tumour samples from patients who were prospectively enrolled in phase II and III trials of HDC for HRPBC at the University of Colorado between 1990 and 2001. A total of 234 patients were accrued in these studies, which were open to patients with 4–9 positive axillary nodes (n=102) (Bearman et al, 1997; Moore et al, 2007) and 10 or greater positive nodes (n=132) (Nieto et al, 2004; Peters et al, 2005). Those trials, as well as the current retrospective review of tumour blocks, were approved by the Cancer Center Protocol Review Committee and the Institutional Review Board. All patients gave written informed consent before study entry. Eleven patients (4.7% of the total enrolment) who died from HDC-related complications were excluded, leaving 223 patients eligible for this analysis. Tumour samples could be obtained from 215 of those 223 patients.
Inclusion and exclusion criteria of those trials were similar, except for the required number of axillary nodes involved. The studies required adequate visceral organ function. Pretransplantation staging tests included computed tomographic scans of the head, chest, abdomen, and pelvis, bone scans, and bone marrow biopsies. Patients received HDC within 6 months of their primary surgery (mastectomy or lumpectomy with negative margins). Patients received four cycles of doxorubicin-containing chemotherapy before HDC. Absence of relapse during pretransplantation chemotherapy was required. Following collection of haematopoietic progenitor cells, patients received high-dose cyclophosphamide/cisplatin/carmustine, as described (Peters et al, 2005). Subsequently, unselected haematopoietic progenitor cells were infused, and granulocyte colony-stimulating factor was administered until neutrophil recovery. Post-transplantation treatment included locoregional radiotherapy upon platelet recovery. Tamoxifen was prescribed for 5 years to patients with hormone receptor-positive tumours.
Immunohistochemical analyses
Paraffin-embedded blocks containing whole-tumour sections from the surgical specimens were retrospectively obtained from the referring institutions. All tumour samples were acquired before initiation of any chemotherapy. Intratumoural microvessels were identified by immunostaining of formalin-fixed paraffin-embedded sections with pan-endothelial anti-CD31 monoclonal antihuman mouse antibody (clone JC/70A; Dako, Carpinteria, CA, USA) in a 1 : 40 dilution and overnight incubation. The invasive tumour component was identified on haematoxylin- and eosin-stained sections. Intratumoural MVD was then determined as described previously (Weidner et al, 1991). The area of most intense neovascularisation was selected by scanning on low magnification (× 10–100), preferentially on the peripheral tumour margins. Only the vascularity of tumour areas considered viable, that is, non-necrotic, was taken into account. Subsequently, individual microvessels were counted on a × 400 field. Any brown-staining endothelial cell, containing a visible nucleus, and clearly separate from adjacent microvessels, tumour cells and other connective-tissue elements, was considered a single, countable microvessel, without requirement for a lumen or the presence of erythrocytes. The microvessels were counted in a 0.74-mm2 area (i.e., a × 400 field). Each patient's microvessel count was the average of two separate counts by two different pathologists (SN and JW) who remained blind to patient outcome.
Vascular endothelial growth factor immunostaining was performed on diagnostic sections using mouse monoclonal antibody anti-VEGF (C-1; Santa Cruz Biotechnology, Santa Cruz, CA, USA). Vascular endothelial growth factor immunoreactivity was scored as 0, 1+, 2+, or 3+, according to staining intensity (Toi et al, 1994). A tumour area with any degree of staining was scored as VEGF-positive. Tumours with VEGF-positive and VEGF-negative areas were assigned the score of the area with strongest staining.
Immunohistochemical analyses of HER2 and p53 used the monoclonal antibodies CB11 (Vantana Medical Systems, Tucson, AZ, USA) and DO7 (BioGenex, San Ramon, CA, USA), respectively. The immunohistochemical analysis of epidermal growth factor receptor (EGFR) employed the murine monoclonal antibody 31G7 (Zymed Laboratories, San Francisco, CA, USA) (1 : 100 dilution). Staining intensity was evaluated as 1+ (weak), 2+ (moderate), or 3+ (strong). Cases were graded based on the overall proportion of cells stained with moderate or strong intensity: 0, 0% cells; 1+, 1–33%; 2+, 34–66%; 3+, 67–100%. Cases with ⩾1+ membranous staining for EGFR were considered positive. Cases with any degree of p53 nuclear staining were considered positive. We considered HER2-positive those cases with 2+ (weak complete membrane staining in >10% of cancer cells) or 3+ grading (intense complete membrane staining in >10% of cancer cells). All immunostained slides were reviewed by the same pathologist (SN), who was blinded to patient outcome.
Statistical methods
Associations between categorical and continuous variables were assessed using the χ2 or Fisher's exact test and Student's t-test, respectively. Median follow-up times were estimated among surviving patients. Relapse-free survival (RFS) time was defined as the time from the administration of HDC to documented relapse (local, contralateral, or distant) or to death without relapse. Overall survival (OS) time was defined as the time from HDC to death from any cause. All survival times were analysed using the Kaplan–Meier method (Kaplan and Meier, 1958). The log-rank test was used to study the association of the potential prognostic variables with survival times (Peto and Peto, 1971). The 25th, 50th, and 75th percentile of the microvessel counts were evaluated as potential prognostic cutoffs.
Multivariate analyses of RFS and OS used proportional hazards Cox regression models (Cox, 1972). These models included the variables previously identified as independent prognostic factors in this population: nodal ratio (i.e., no. of involved axillary nodes/no. of dissected nodes), combined oestrogen (ER) and progesterone receptor (PR) status, the pathologic tumour size and HER2 (Nieto et al, 1999, 2000). The remaining variables studied, such as age, menopausal status, family history of breast cancer, histologic grade, tumour ploidy, S-phase fraction, multifocality, vascular invasion, lymphatic invasion, extensive intraductal component, or p53 status, which lacked an independent prognostic effect, were excluded from the multivariate analyses. Likewise, of all the axillary node-related variables previously analysed (nodal ratio, absolute number of involved nodes, presence of involved nodes greater than 2 cm, and extracapsular extension), only the nodal ratio was independently associated with outcome, and included in the multivariate models.
The probability of relapse after HDC for HRPBC, based on a linear regression model with the independent variables, can be expressed by the following equation (Nieto et al, 1999):
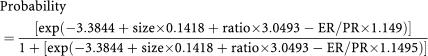 |
A simpler scoring system was subsequently derived from the previous formula:
Score=(nodal ratio × 3.05)+(tumour size × 0.15)–(ER/PR × 1.19).
In both formulae, tumour size is entered in centimetres, and ER/PR status is assigned ‘1’ if positive (i.e., if ER and/or PR are positive) and ‘0’ if negative (i.e., if both ER and PR are negative). The patient is assigned the high or low risk of relapse category if the score is ⩾2.41 or <2.41, respectively, with highly significant differences in RFS (P<10−5) and OS (P<10−5) between both groups. The optimal cutoff score of 2.41, as identified in the receiver operating characteristic curves, conferred on the model a sensitivity of 0.6, a specificity of 0.88, a positive predictive value of 0.65, a negative predictive value of 0.86, and an accuracy of 83%. The prognostic value of this score was confirmed externally (Nieto et al, 1999) and prospectively (Nieto et al, 2004).
The significance of the current multivariate models was evaluated with the likelihood ratio test. Individual coefficients were tested using Wald's statistic. The proportionality assumption for the variables was assessed with Kaplan–Meier curves. All P-values presented are two-tailed. All statistical analyses were carried out using Statview 5.01 software (SAS Institute, Cary, NC, USA).
RESULTS
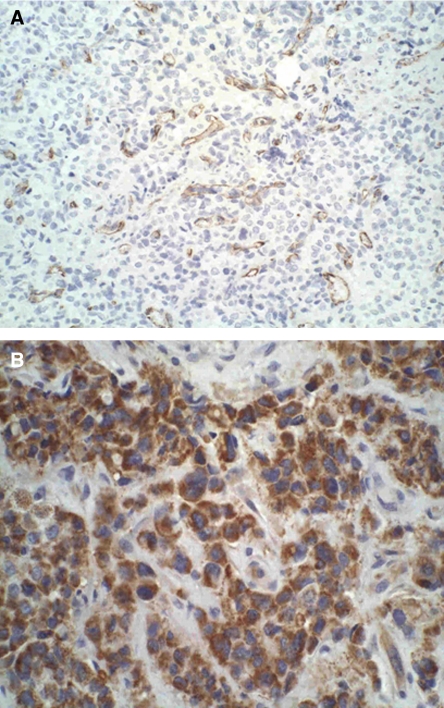
We studied specimens from 215 patients (96% of the eligible population of 223 patients) (Table 1). CD31 staining of tumour vessels is shown in Figure 1A. The median microvessel count was 8 per field (range, 2–27), with low (6%) interobserver variation. The microvessel count was higher in tumours overexpressing EGFR than in EGFR-negative tumours (11.5 vs 6; P<0.001), and in tumours >2 cm than in those ⩽2 cm (10 vs 6.5; P=0.001). In contrast, we did not observe an association between MVD and HER2 status (median microvessel counts for HER2-positive and HER2-negative tumours of 8 and 7, respectively, P=0.5), nodal status (expressed as either the nodal ratio (P=0.8), or the absolute number of positive nodes (P=0.4)), p53 status (P=0.9), or histologic grade (P=0.8). Finally, there was a nonsignificant trend for a higher microvessel count in ER/PR-negative than in ER/PR-positive tumours (8.5 vs 7; P=0.09).
Table 1. Patient demographics (n=215).
| Characteristic | No. of patients (%) |
|---|---|
| Age (years): median, range | 49, 28–67 |
| Menopausal status | |
| Pre/perimenopausal | 123 (57) |
| Postmenopausal | 92 (43) |
| Tumour size (cm) | |
| ⩽2 | 59 (28) |
| >2–5 | 91 (42) |
| >5 | 65 (31) |
| ER/PR status a | |
| Negative | 90 (42) |
| Positive | 125 (58) |
| No. of involved nodes | |
| 4–9 | 90 (42) |
| 10–20 | 83 (39) |
| >20 | 42 (19) |
| No. of dissected nodes: median, range | 19 (10–46) |
| Nodal ratio (no. of involved nodes/no. of dissected nodes): median, range | 0.55, 0–1 |
| Predictive score b | |
| Low (<2.41) | 151 (70) |
| High (⩾2.41) | 64 (30) |
| Histologic grade | |
| 1–2 | 78 (36) |
| 3 | 137 (54) |
| HER2 status | |
| Negative | 124 (58) |
| Positive | 91 (42) |
| p53 status | |
| Negative | 96 (66) |
| Positive | 50 (34) |
| Undetermined | 69 |
| EGFR status | |
| Negative | 124 (58) |
| Positive | 91 (42) |
ER=oestrogen receptors; nodal ratio=number of involved nodes/number of dissected nodes; PR=progesterone receptors.
ER/PR=‘1’ if positive and =‘0’ if negative.
Predictive score=(nodal ratio × 3.05)+(tumour size × 0.15)−ER/PR × 1.15.
Figure 1.
Immunohistochemical staining. (A) Intratumour CD31-stained microvessels. (B) VEGF staining.
Intratumoural VEGF expression was detected in 51% of the patients (Figure 1B). Its staining intensity was graded as 1+ in 29 (26%) patients, as 2+ in 44 (40%) patients, and as 3+ in 37 (34%) patients. No correlation was seen between MVD and VEGF expression (P=0.3). Vascular endothelial growth factor overexpression was detected in 56% of the patients with HER2-positive tumours, compared with 39% of those with HER2-negative tumours (P=0.04). Likewise, 61% of patients with EGFR-positive tumours coexpressed VEGF, compared with 39% of those with EFGR-negative tumours (P=0.01). Vascular endothelial growth factor was not associated with the nodal ratio (P=0.8), the absolute number of positive nodes (P=0.6), tumour size (P=0.6), ER/PR status (P=0.6), p53 status (P=0.9), or histologic grade (P=0.7).
Prognostic analyses
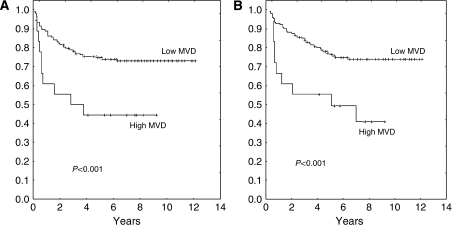
We identified a prognostic cutoff count of 14 microvessels (75th percentile). The RFS rates in patients with low and high MVD were 74% (95% confidence interval (CI), 67–80%) and 44% (95% CI, 36–51%), respectively (P<0.001) (Figure 2A). Their respective OS rates were 76% (95% CI, 69–82%) and 44% (95% CI, 36–51%) (P<0.001) (Figure 2B).
Figure 2.
Outcome according to MVD, according to a cutoff 14 microvessels (75th percentile). (A) Relapse-free survival; (B) overall survival.
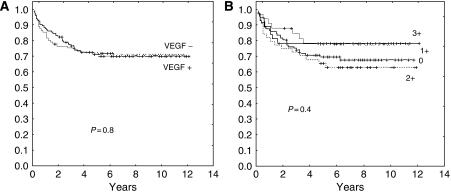
In contrast, the groups with VEGF-positive and VEGF-negative tumours did not have significantly different RFS (71.5 vs 70%, P=0.8, Figure 3A) or OS (75 vs 70%, P=0.4). Analysing VEGF expression according to its semiquantitated grading found no significant outcome differences between the groups with 0, 1+, 2+, or 3+ staining, with RFS rates of 69, 79, 64, and 78%, respectively (P=0.4) (Figure 3B).
Figure 3.
Lack of effect of VEGF expression on RFS. (A) Expressors vs non-expressors (P=0.8). (B) According to semiquantitated grading (P=0.4).
Multivariate analyses
Multivariate models included MVD, HER2, and the three independent clinical variables, nodal ratio, tumour size, and ER/PR status. Model 1 included the clinical variables combined as the score. Model 2 analysed all variables separately. Both models showed the independent prognostic effect of MVD on RFS and OS (Table 2).
Table 2. Multivariate models.
|
Relapse-free survival
|
Overall survival
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
Model 1
|
Model 2
|
Model 1
|
Model 2
|
||||||||
| Variable | Odds ratio (95% CI) | P-values | Variable | Odds ratio (95% CI) | P-values | Variable | Odds ratio (95% CI) | P-values | Variable | Odds ratio (95% CI) | P-values |
| MVDa | 2.7 (2–3.4) | 0.005 | MVDa | 2.4 (1.7–3.1) | 0.01 | MVDa | 3.0 (2.3–3.7) | 0.002 | MVDa | 2.6 (1.9–3.3) | 0.008 |
| HER2b | 2 (1.5–2.6) | 0.001 | HER2b | 1.7 (1.2–2.3) | <0.05 | HER2b | 2.0 (1.4–2.5) | 0.02 | HER2b | 1.66 (1.2–2.25) | <0.05 |
| Scorec | 2.1 (1.6–2.7) | <0.05 | Nodal ratiod | 2.2 (1.7–2.8) | <0.01 | Scorec | 2.2 (1.7–2.8) | 0.005 | Nodal ratiod | 2.2 (1.6–2.8) | 0.01 |
| Tumour sizee | 1.4 (0.95–2) | 0.08 | Tumour sizee | 1.5 (0.9–2.15) | 0.1 | ||||||
| ER/PRf | 0.29 (0.1–0.9) | <0.05 | ER/PRf | 0.3 (0.04–0.9) | <0.05 | ||||||
CI=confidence interval; ER=oestrogen receptors; MVD=microvessel density; PR=progesterone receptors.
MVD: >14 vs ⩽14.
HER2: positive vs negative.
Predictive score (high vs low)=(nodal ratio × 3.05)+(tumour size × 0.15)−(ER/PR × 1.15). ER/PR=‘1’ if positive and=‘0’ if negative. Scores <2.41 are low, scores ⩾2.41 are high.
Nodal ratio (⩾0.8 vs <0.8)=number of involved nodes/number of dissected nodes.
Tumour size: ⩾5 vs <5 cm.
ER/PR status: positive vs negative.
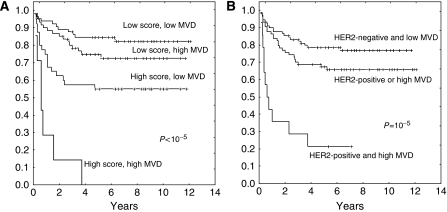
The combined analysis of MVD and the clinical score offered a highly significant separation of four prognostic categories (P<10−5) (Figure 4A). Patients with a high score and a low MVD had significantly better RFS than those with a high score and a high MVD (55 vs 0%, P=0.005). Within the low-score subgroup, however, the differences between patients with low and high MVD approached but did not reach the level of statistical significance (83 vs 74%, P=0.09).
Figure 4.
Relapse-free survival combining MVD and other independent prognostic variables. (A) Analysis of MVD (cutoff, 14 microvessels – 75th percentile) and the predictive score (cutoff score, 2.41). (B) Analysis of MVD and HER2 status.
Combining MVD and HER2 resulted in the following four prognostic groups (P<0.0001): (1) HER2 negativity and low MVD (78% RFS), (2) HER2 negativity and high MVD (67% RFS), (3) HER2 positivity and low MVD (66% RFS), and (4) HER2 positivity and high MVD (21% RFS). Merging groups 2 and 3 results in a three-group classification based on the presence of 0, 1, or 2 of those poor-risk features (P=0.00001) (Figure 4B).
DISCUSSION
In this study, we found that a highly angiogenic phenotype, determined by MVD, was a powerful adverse prognostic factor in our group of 215 patients with HRPBC treated with uniform multimodal treatment including HDC. The effect of MVD was independent of other known prognostic factors in this patient population. Tumour microvessels were counted separately by two different pathologists following the international consensus guidelines on angiogenesis quantification (Vermeulen et al, 1996), with low interobserver variability.
Although HER2 signaling is believed to promote tumour angiogenesis (Kumar and Yarmand-Bagheri, 2001), we found no association between HER2 overexpression and MVD, in keeping with previous results (Vogl et al, 2006). In contrast, others observed an association of MVD and HER2 overexpression in small series of patients with node-negative disease (Koukourakis et al, 2003). We noted a significant association between HER2 and VEGF, as did previous studies of mixed populations with node-negative and node-positive tumours (Konecny et al, 2004; Linderholm et al, 2004). We considered it important that the prognostic effects of tumour angiogenesis and HER2 in our study were mutually independent, suggesting the potential benefit of their concurrent targeting in this population. The feasibility of dual VEGF and HER2 targeting with bevacizumab and trastuzumab has been recently shown (Pegram et al, 2004), and could be tested in HRPBC patients.
Another interesting observation was the association between MVD or VEGF and EGFR. Activation of EGFR signaling upregulates VEGF and activates angiogenesis in human brain cancer cells (Goldman et al, 1993). An association of EGFR with MVD in breast cancer was previously suggested in a small study of 45 patients (De Jong et al, 1998).
In contrast to MVD, we did not observe any significant prognostic effect of VEGF, one of the most potent inducers of angiogenesis. As such, it constitutes a major target for strategies aiming at disrupting angiogenesis. Indeed, the most successful antiangiogenic intervention to date has been the use of bevacizumab in metastatic breast cancer (Miller et al, 2005), colorectal cancer (Hurwitz et al, 2004), and non-small-cell lung cancer (Sandler et al, 2006). However, its usefulness as a potentially informative prognostic marker is a separate issue from its value as a therapeutic target. Similar to previous observations in breast cancer (Lantzsch et al, 2002; Chhieng et al, 2003), we observed no significant association between VEGF expression and MVD, supporting the notion that multiple angiogenic factors, besides VEGF, play a role in the angiogenic process. Redundancy is a major characteristic of tumours, and different angiogenesis markers may be relevant in different phases of development.
Our study has several potential limitations. One is the inherent bias in all retrospective studies of archival tissue which may be lessened in this study by the high sample collection rate. Another limitation is the fact that all patients analysed received HDC, which is not currently part of the standard management of HRPBC. Although significant superiority of HDC over standard-dose chemotherapy has been seen in some randomised trials (Roché et al, 2001; Nitz et al, 2005), several other studies have not shown any appreciable outcome differences (Tallman et al, 2003; Leonard et al, 2004; Coombes et al, 2005; Peters et al, 2005; Moore et al, 2007). In addition, our analysis excluded those patients who relapsed during pretransplantation chemotherapy and never received HDC, which could represent a selection bias. However, this very poor prognosis subgroup represented only 2% of all patients enrolled in those trials (Peters et al, 2005).
In conclusion, tumour angiogenesis, expressed as MVD but not as VEGF, was found to be a major independent prognostic factor in patients with HRPBC treated with HDC. These results support the evaluation of antiangiogenic interventions in this high-risk population.
Acknowledgments
We thank the referring institutions for their willingness to submit to us the patients' tumour blocks and their support of our research. This work was supported by Grant 1 R21 CA095762-01 (Yago Nieto) from the National Cancer Institute (Bethesda, MD).
References
- Bearman SI, Overmoyer BA, Bolwell BJ, Taylor CW, Shpall EJ, Cagnoni PJ, Mechling BE, Ronk B, Baron AE, Purdy MH, Ross M, Jones RB (1997) High-dose chemotherapy with autologous peripheral blood progenitor cell support for primary breast cancer in patients with 4–9 involved axillary lymph nodes. Bone Marrow Transplant 20: 931–937 [DOI] [PubMed] [Google Scholar]
- Chhieng DC, Tabbara SO, Marley EF, Talley LI, Frost AR (2003) Microvessel density and vascular endothelial growth factor expression in infiltrating lobular mammary carcinoma. Breast J 9: 200–207 [DOI] [PubMed] [Google Scholar]
- Coombes RC, Howell A, Emson M, Peckitt C, Gallagher C, Bengala C, Tres A, Welch R, Lawton P, Rubens R, Woods E, Haviland J, Vigushin D, Kanfer E, Bliss JM (2005) High dose chemotherapy and autologous stem cell transplantation as adjuvant therapy for primary breast cancer patients with four or more lymph nodes involved: long-term results of an international randomised trial. Ann Oncol 16: 726–734 [DOI] [PubMed] [Google Scholar]
- Cox DR (1972) Regression models and life tables. J R Stat Soc B 34: 187–202 [Google Scholar]
- De Jong JS, van Diest PJ, van der valk P, Baak JP (1998) Expression of growth factors, growth-inhibiting factors, and their receptors in invasive breast cancer. II: correlations with proliferation and angiogenesis. J Pathol 184: 53–57 [DOI] [PubMed] [Google Scholar]
- Ferrara N, Gerber HP, LeCouter J (2003) The biology of VEGF and its receptors. Nat Med 9: 669–676 [DOI] [PubMed] [Google Scholar]
- Fidler IJ, Ellis LM (1994) The implications of angiogenesis for the biology and therapy of cancer metastasis. Cell 79: 185–188 [DOI] [PubMed] [Google Scholar]
- Fitzgibbons PL, Page DL, Weaver D, Thor AD, Allred DC, Clark GM, Ruby SG, O'Malley F, Simpson JF, Connolly JL, Hayes DF, Edge SB, Lichter A, Schnitt SJ (2000) Prognostic factors in breast cancer. College of American pathologists consensus statement. Arch Pathol Lab Med 124: 966–978 [DOI] [PubMed] [Google Scholar]
- Folkman J (1990) What is the evidence that tumors are angiogenesis dependent? J Natl Cancer Inst 82: 4–6 [DOI] [PubMed] [Google Scholar]
- Goldman CK, Kim J, Wong WL, King V, Brock T, Gillespie GY (1993) Epidermal growth factor stimulates vascular endothelial growth factor production by human malignant glioma cells: a model of glioblastoma multiforme pathophysiology. Mol Biol Cell 4: 121–133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes DF, Isaacs C, Stearns V (2001) Prognostic factors in breast cancer: current and new predictors of metastasis. J Mamm Gland Biol Neo 6: 375–392 [DOI] [PubMed] [Google Scholar]
- Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, Berlin J, Baron A, Griffing S, Holmgren E, Ferrara N, Fyfe G, Rogers B, Ross R, Kabbinavar F (2004) Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med 350: 2335–2342 [DOI] [PubMed] [Google Scholar]
- Kaplan EL, Meier P (1958) Nonparametric estimation from incomplete observations. JAMA 53: 457–481 [Google Scholar]
- Konecny GE, Meng YG, Untch M, Wang HJ, Bauerfeind I, Epstein M, Stieber P, Vernes JM, Gutierrez J, Hong K, Beryt M, Hepp H, Slamon DJ, Pegram MD (2004) Association between HER-2/neu and vascular endothelial growth factor expression predicts clinical outcome in primary breast cancer patients. Clin Cancer Res 10: 1706–1716 [DOI] [PubMed] [Google Scholar]
- Koukourakis MI, Manolas C, Minopoulos G, Giatromanolaki A, Sivridis E (2003) Angiogenesis relates to estrogen receptor negativity, c-erbB-2 overexpression and early relapse in node-negative ductal carcinoma of the breast. Int J Surg Pathol 11: 29–34 [DOI] [PubMed] [Google Scholar]
- Kumar R, Yarmand-Bagheri R (2001) The role of HER2 in angiogenesis. Semin Oncol 28: 27–32 [DOI] [PubMed] [Google Scholar]
- Lantzsch T, Hefler L, Krause U, Kehl A, Goepel C, Koelbl H, Dunst J, Lampe D (2002) The correlation between immunohistochemically-detected markers of angiogenesis and serum vascular endothelial growth factor in patients with breast cancer. Anticancer Res 22: 1925–1928 [PubMed] [Google Scholar]
- Leonard RC, Lind M, Twelves C, Coleman R, van Belle S, Wilson C, Ledermann J, Kennedy I, Barrett-Lee P, Perren T, Verrill M, Cameron D, Foster E, Yellowlees A, Crown J (2004) Conventional adjuvant chemotherapy versus single-cycle, autograft-supported, high-dose, late-intensification chemotherapy in high-risk breast cancer patients: a randomized trial. J Natl Cancer Inst 96: 1076–1083 [DOI] [PubMed] [Google Scholar]
- Linderholm B, Andersson J, Lindh B, Beckman L, Erlanson M, Edin K, Tavelin B, Grankvist B, Henriksson R (2004) Overexpression of c-erbB-2 is related to a higher expression of vascular endothelial growth factor (VEGF) and constitutes an independent prognostic factor in primary node-positive breast cancer after adjuvant systemic treatment. Eur J Cancer 40: 33–42 [DOI] [PubMed] [Google Scholar]
- McShane LM, Altman DG, Sauerbrei W, Taube SE, Gion M, Clark GM (2005) REporting recommendations for tumor MARKer prognostic studies (REMARK). Nat Clin Pract Oncol 2: 416–422 [PubMed] [Google Scholar]
- Miller KD, Wang W, Gralow J, Dickler M, Cobleigh MA, Perez EA, Shenkier TN, Davidson NE (2005) A randomized phase III trial of paclitaxel versus paclitaxel plus bevacizumab as first-line therapy for locally recurrent or metastatic breast cancer: a trial coordinated by the Eastern Cooperative Oncology Group (E2100). Breast Cancer Res Treat 94(Suppl 1): S6 [Google Scholar]
- Moore HC, Green SJ, Gralow JR, Bearman SI, Lew D, Barlow WE, Hudis C, Wolff AC, Ingle JN, Chew HK, Elias AD, Livingston RB, Martino S (2007) Intensive dose-dense compared with high-dose adjuvant chemotherapy for high-risk operable breast cancer: Southwest Oncology group/Intergroup study 9623. J Clin Oncol 25: 1677–1682 [DOI] [PubMed] [Google Scholar]
- Nieto Y, Cagnoni PJ, Nawaz S, Shpall EJ, Yerushalmi R, Cook B, Russell P, McDermit J, Murphy J, Bearman SI, Jones RB (2000) Prognostic value of HER2 overexpression and mutations in p53 in high-risk primary breast cancer treated with high-dose chemotherapy and autologous stem cell transplant. J Clin Oncol 18: 2070–2080 [DOI] [PubMed] [Google Scholar]
- Nieto Y, Cagnoni PJ, Shpall EJ, Xu X, Murphy J, Vredenburgh J, Chao NJ, Bearman SI, Jones RB (1999) A predictive model for relapse in high-risk primary breast cancer patients treated with high-dose chemotherapy and autologous stem cell transplant. Clin Cancer Res 5: 3425–3431 [PubMed] [Google Scholar]
- Nieto Y, Nawaz S, Shpall EJ, Bearman SI, Murphy J, Jones RB (2004) Long-term analysis and prospective validation of a prognostic model for patients with high-risk primary breast cancer receiving high-dose chemotherapy. Clin Cancer Res 10: 2609–2617 [DOI] [PubMed] [Google Scholar]
- Nitz UA, Mohrmann S, Fischer J, Lindemann W, Berdel WE, Jackisch C, Werner C, Ziske C, Kirchner H, Metzner B, Souchon R, Ruffert U, Schutt G, Pollmanns A, Schmoll HJ, Middecke C, Baltzer J, Schrader I, Wiebringhaus H, Ko Y, Rosel S, Schwenzer T, Wernet P, Hinke A, Bender HG, Frick M (2005) Comparison of rapidly cycled tandem high-dose chemotherapy plus peripheral-blood stem-cell support versus dose-dense conventional chemotherapy for adjuvant treatment of high-risk breast cancer: results of a multicentre phase III trial. Lancet 366: 1935–1944 [DOI] [PubMed] [Google Scholar]
- Pegram MD, Yeon C, Kun NC, Gaudreault J, Slamon DJ (2004) Phase I combined biological therapy of breast cancer using two humanized monoclonal antibodies directed against HER2 proto-oncogene and vascular endothelial growth factor (VEGF). Breast Cancer Res Treat 88: S124 [Google Scholar]
- Peters WP, Rosner G, Vredenburgh J, Shpall EJ, Crump M, Richardson PG, Schuster MW, Marks LB, Cirrincione C, Norton L, Henderson IC, Schilsky RL, Hurd DD (2005) Prospective, randomized comparison of high-dose chemotherapy with stem-cell support versus intermediate-dose chemotherapy after surgery and adjuvant chemotherapy in women with high-risk primary breast cancer: a report of CALGB 9082, SWOG 9114, and NCIC MA-13. J Clin Oncol 23: 2191–2200 [DOI] [PubMed] [Google Scholar]
- Peto R, Peto J (1971) Asymptomatically efficient rank invariant test procedures. J R Stat Soc A 135: 185–198 [Google Scholar]
- Roché HH, Pouillart P, Meyer N, Biron P, Spielmann M, Janvier M, Spaeth D, Fabbro M, Linessier C, Peny A, Asselain B (2001) Adjuvant high dose chemotherapy (HDC) improves early outcome for high risk (N>7) breast cancer patients: The PEGASE 01 trial. Proc Am Soc Clin Oncol 20: 26a [Google Scholar]
- Sandler A, Gray R, Perry MC, Brahmer J, Schiller JH, Dowlati A, Lilenbaum R, Johnson DH (2006) Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med 355: 2542–2550 [DOI] [PubMed] [Google Scholar]
- Tallman M, Gray R, Robert N, LeMaistre CF, Osborne CK, Vaughan WP, Gradishar WJ, Pisansky TM, Fetting J, Paietta E, Lazarus HM (2003) Conventional adjuvant chemotherapy with or without high-dose chemotherapy and autologous stem-cell transplantation in high-risk breast cancer. N Engl J Med 349: 17–26 [DOI] [PubMed] [Google Scholar]
- Toi M, Hoshina S, Takayanagi T, Tominaga T (1994) Association of vascular endothelial growth factor expression with tumor angiogenesis and with early relapse in primary breast cancer. Jpn J Cancer Res 85: 1045–1049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uzzan B, Nicolas P, Cucherat M, Perret G-Y (2004) Microvessel density as a prognostic factor in women with breast cancer: a systematic review of the literature and meta-analysis. Cancer Res 64: 2941–2955 [DOI] [PubMed] [Google Scholar]
- Vermeulen P, Gasparini G, Fox SB, Toi M, Martin L, McCulloch P, Pezzella F, Viale G, Weidner N, Harris AL, Dirix LY (1996) Quantification of angiogenesis in solid human tumours: an international consensus on the methodology and criteria of evaluation. Eur J Cancer 32: 2474–2484 [DOI] [PubMed] [Google Scholar]
- Vogl G, Bartel H, Dietze O, Hauser-Kronenberg C (2006) HER2 is unlikely to be involved in directly regulating angiogenesis in human breast cancer. Appl Immunohistochem Mol Morphol 14: 138–145 [DOI] [PubMed] [Google Scholar]
- Weidner N, Semple JP, Welch WR, Folkman J (1991) Tumor angiogenesis and metastases – correlation in invasive breast carcinoma. N Engl J Med 324: 1–8 [DOI] [PubMed] [Google Scholar]