Abstract
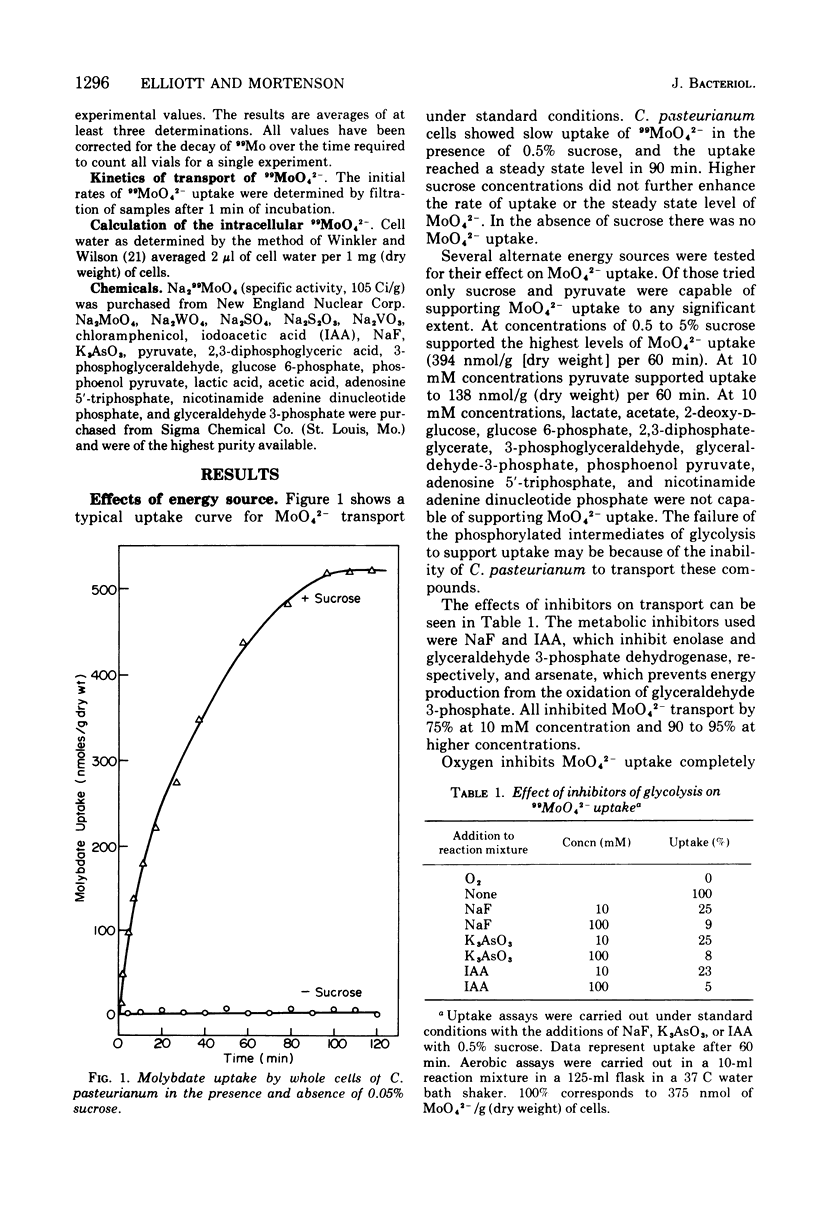
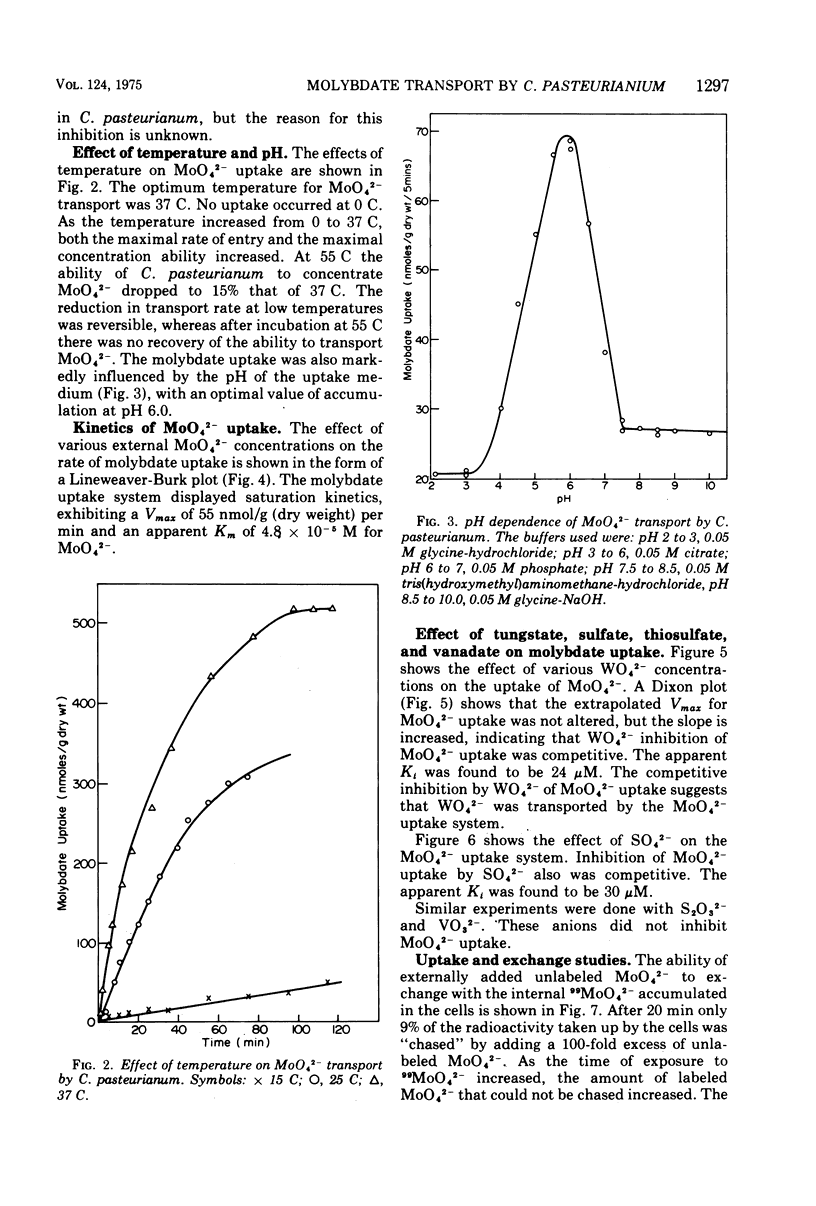
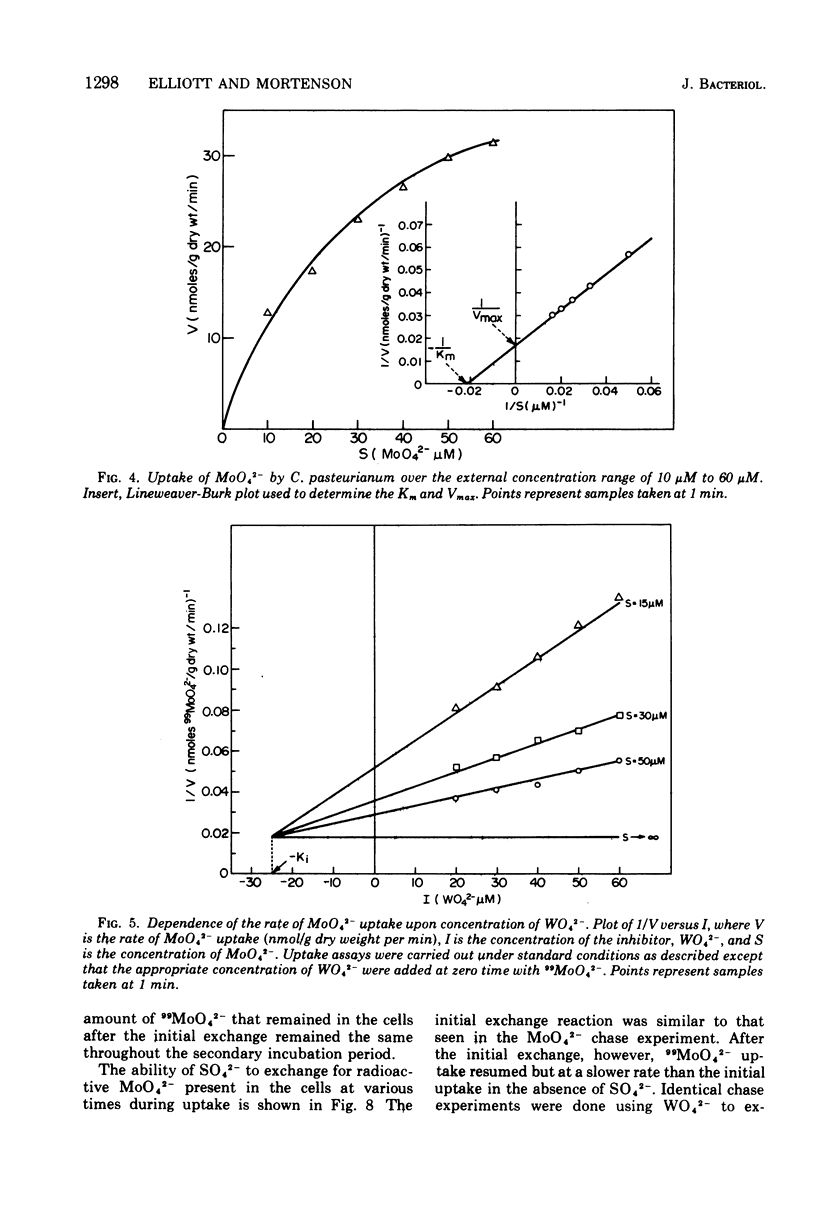
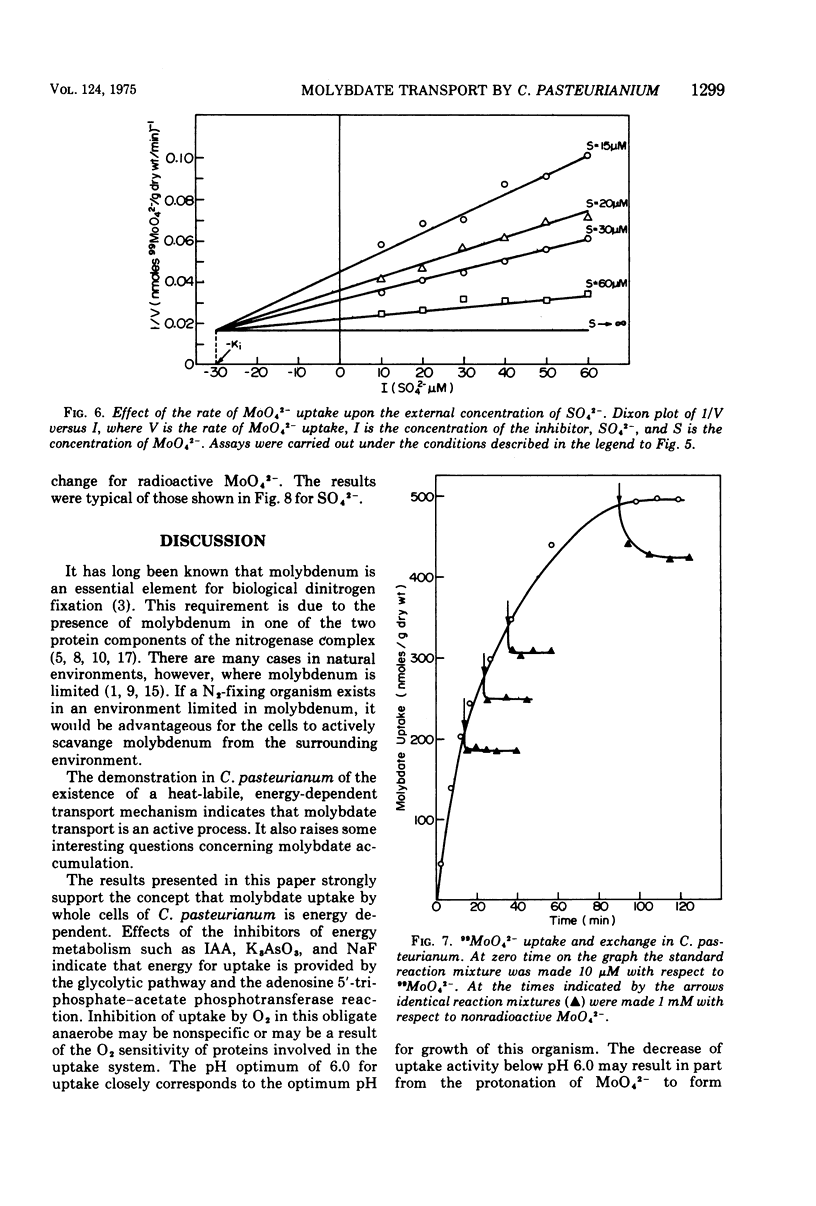
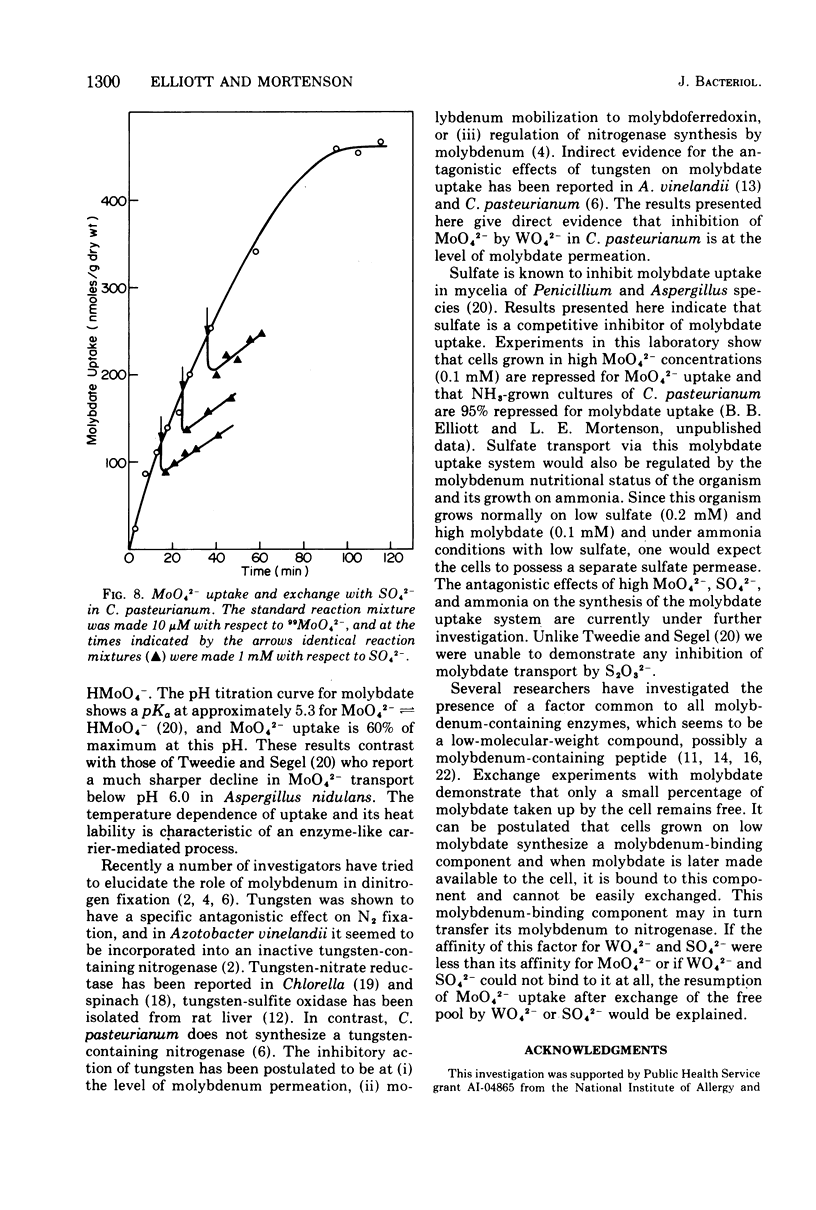
The transport of 99MoO42- into dinitrogen-fixing cells of Clostridium pasteurianum was investigated. Transport of molybdate in this organism is energy dependent; sucrose is required in the minimal media, and the system is inhibited by the glycolysis inhibitors, NaF, iodoacetic acid, and arsenate. The cells accumulate molybdate against a concentration gradient, and the uptake shows a marked dependence on temperature (optimum 37 C) and pH (optimum 6.0). The rate of molybdate uptake with increasing molybdate concentrations shows saturation kinetics with an apparent Km and Vmax of 4.8 X 10(-5) M and 55 nmol/g of dry cells per min, respectively. Inhibition studies with the anions SO42-, S2O32-, WO42-, and VO32- show that SO42- and WO42- competitively inhibit MoO42- uptake (apparent Ki [SO42-] is 3.0 X 10(-5) M; apparent Ki [WO42-] is 2.4 X 10(-5), whereas S2O32- and VO32- have no inhibitory effect. Exchange experiments with MoO42- show that only a small percentage of the 99MoO42- taken up by the cells is exchangeable. Exchange experiments with WO42- and SO42- indicate that once inside the cells WO42- and SO42- cannot substitute for MoO42-.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benemann J. R., Smith G. M., Kostel P. J., McKenna C. E. Tungsten incorporation into Azotobacter vinelandii nitrogenase. FEBS Lett. 1973 Feb 1;29(3):219–221. doi: 10.1016/0014-5793(73)80023-7. [DOI] [PubMed] [Google Scholar]
- Brill W. J., Steiner A. L., Shah V. K. Effect of molybdenum starvation and tungsten on the synthesis of nitrogenase components in Klebsiella pneumonia. J Bacteriol. 1974 Jun;118(3):986–989. doi: 10.1128/jb.118.3.986-989.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulen W. A., LeComte J. R. The nitrogenase system from Azotobacter: two-enzyme requirement for N2 reduction, ATP-dependent H2 evolution, and ATP hydrolysis. Proc Natl Acad Sci U S A. 1966 Sep;56(3):979–986. doi: 10.1073/pnas.56.3.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardenas J., Mortenson L. E. Role of molybdenum in dinitrogen fixation by Clostridium pasteurianum. J Bacteriol. 1975 Sep;123(3):978–984. doi: 10.1128/jb.123.3.978-984.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daesch G., Mortenson L. E. Effect of ammonia on the synthesis and function of the N 2 -fixing enzyme system in Clostridium pasteurianum. J Bacteriol. 1972 Apr;110(1):103–109. doi: 10.1128/jb.110.1.103-109.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eady R. R., Smith B. E., Cook K. A., Postgate J. R. Nitrogenase of Klebsiella pneumoniae. Purification and properties of the component proteins. Biochem J. 1972 Jul;128(3):655–675. doi: 10.1042/bj1280655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson J. L., Cohen H. J., Rajagopalan K. V. Molecular basis of the biological function of molybdenum. Molybdenum-free sulfite oxidase from livers of tungsten-treated rats. J Biol Chem. 1974 Aug 25;249(16):5046–5055. [PubMed] [Google Scholar]
- KEELER R. F., VARNER J. E. Tungstate as an antagonist of molybdate in Azotobacter vinelandii. Arch Biochem Biophys. 1957 Aug;70(2):585–590. doi: 10.1016/0003-9861(57)90146-7. [DOI] [PubMed] [Google Scholar]
- Ketchum P. A., Cambier H. Y., Frazier W. A., 3rd, Madansky C. H., Nason A. In vitro assembly of Neurospora assimilatory nitrate reductase from protein subunits of a Neurospora mutant and the xanthine oxidizing or aldehyde oxidase systems of higher animals. Proc Natl Acad Sci U S A. 1970 Jul;66(3):1016–1023. doi: 10.1073/pnas.66.3.1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenna C. E., L'vov N. P., Ganelin V. L., Sergeev N. S., Kretovich V. L. O sushchestvovanii nizkomolekuliarnogo faktora, obshchego dlia razlichnykh molibdensoderzhashchikh fermentov. Dokl Akad Nauk SSSR. 1974;217(1):228–231. [PubMed] [Google Scholar]
- Mortenson L. E., Morris J. A., Jeng D. Y. Purification, metal composition and properties of molybdoferredoxin and azoferredoxin, two of the components of the nitrogen-fixing system of Clostridium pasteurianum. Biochim Biophys Acta. 1967 Aug 29;141(3):516–522. doi: 10.1016/0304-4165(67)90180-8. [DOI] [PubMed] [Google Scholar]
- Notton B. A., Hewitt E. J. The role of tungsten in the inhibition of nitrate reductase activity in spinach (spinacea oleracea L.) leaves. Biochem Biophys Res Commun. 1971 Aug 6;44(3):702–710. doi: 10.1016/s0006-291x(71)80140-7. [DOI] [PubMed] [Google Scholar]
- Tweedie J. W., Segel I. H. Specificity of transport processes for sulfur, selenium, and molybdenum anions by filamentous fungi. Biochim Biophys Acta. 1970 Jan 6;196(1):95–106. doi: 10.1016/0005-2736(70)90170-7. [DOI] [PubMed] [Google Scholar]



