Abstract
The antiapoptotic Livin/ML-IAP gene has recently gained much attention as a potential new target for cancer therapy. Reports indicating that livin is expressed almost exclusively in tumours, but not in the corresponding normal tissue, suggested that the targeted inhibition of livin may present a novel tumour-specific therapeutic strategy. Here, we compared the expression of livin in renal cell carcinoma and in non-tumorous adult kidney tissue by quantitative real-time reverse transcription-PCR, immunoblotting, and immunohistochemistry. We found that livin expression was significantly increased in tumours (P=0.0077), but was also clearly detectable in non-tumorous adult kidney. Transcripts encoding Livin isoforms α and β were found in both renal cell carcinoma and normal tissue, without obvious qualitative differences. Livin protein in renal cell carcinoma samples exhibited cytoplasmic and/or nuclear staining. In non-tumorous kidney tissue, Livin protein expression was only detectable in specific cell types and restricted to the cytoplasm. Thus, whereas the relative overexpression of livin in renal cell carcinoma indicates that it may still represent a therapeutic target to increase the apoptotic sensitivity of kidney cancer cells, this strategy is likely to be not tumour-specific.
Keywords: inhibitor of apoptosis, Livin/ML-IAP/KIAP, renal cell carcinoma, tumour therapy
The inhibitor of apoptosis protein (IAP) family encompasses structurally related proteins which may contribute to the development and therapeutic resistance of cancers (Salvesen and Duckett, 2002). Livin, alternatively termed ML-IAP or KIAP, is one of eight known human IAPs (Chang and Schimmer, 2007; Liu et al, 2007). Two splicing variants of Livin, Livin α and Livin β, have been identified which possess an identical amino-acid sequence, except for additional 18 amino acids present only in the α-isoform (Vucic et al, 2000; Ashhab et al, 2001). It has been shown that targeted interference with livin expression can resensitise tumour cells towards apoptosis (Kasof and Gomes, 2001; Crnković-Mertens et al, 2003).
Initially, a role for Livin in tumorigenesis had been proposed for malignant melanoma (Vucic et al, 2000). More recently, however, livin expression was also detected in various additional cancers, including leukaemias (Qiuping et al, 2004; Choi et al, 2007), bladder cancer (Gazzaniga et al, 2003), lung cancer (Tanabe et al, 2004; Hariu et al, 2005; Crnković-Mertens et al, 2006b), neuroblastoma (Kim et al, 2005), nasopharyngeal carcinoma (Xiang et al, 2006), astrocytoma (Liu et al, 2006), malignant pleural mesothelioma (Gordon et al, 2007), pancreatic cancer (Lopes et al, 2007), and renal cell carcinoma (RCC) (Crnković-Mertens et al, 2007). Notably, studies of biopsies from bladder cancer (Gazzaniga et al, 2003), lung cancer (Hariu et al, 2005), nasopharyngeal carcinoma (Xiang et al, 2006), malignant pleural mesothelioma (Gordon et al, 2007), and pancreatic cancer (Lopes et al, 2007) reported that Livin is typically expressed in the tumour samples, but not in the corresponding normal tissues. Thus, it could be envisioned to develop the targeted inhibition of livin as a future tumour-specific therapeutic strategy (Chang and Schimmer, 2007; Liu et al, 2007). It therefore will be of great interest to determine the Livin status of different tumour entities in relation to the corresponding normal tissue.
Renal cell carcinoma (RCC) is estimated to account for more than 51 000 new cases and almost 13 000 cancer-related deaths in the United States in the year 2007, making it the second most lethal of the urological cancers (Jemal et al, 2007). Renal cell carcinomas typically are highly resistant towards chemotherapy and possess a poor prognosis (Flanigan et al, 2003). We recently found that silencing of Livin-positive RCC cell lines was linked to a re-sensitisation of the tumour cells towards proapoptotic agents, including chemotherapeutics (Crnković-Mertens et al, 2007). To evaluate the potential of livin to serve as a tumour-specific therapeutic target, we here compared its expression in primary RCC specimens and in non-tumorous tissue samples from adult kidney. In addition, we analysed the expression of livin isoforms α and β in tumorous and non-tumorous tissue.
MATERIALS AND METHODS
Tissue samples
Fresh-frozen tissue samples of RCC (n=15), macroscopically and histologically normal tissue derived from kidneys removed because of malignant disease (n=18), and tissue from kidneys removed because of benign disease (n=6) were obtained from the tissue bank of the National Center for Tumor Diseases (NCT) Heidelberg. These tissues included nine paired samples of tumour and adjacent non-tumorous tissue.
Paraffin-embedded tissues encompassed RCC samples (n=13), normal tissue adjacent to tumour (n=14) and kidney removed because of benign disease (n=3).
Tumour stage was classified according to the tumour node metastasis staging system (Greene et al, 2002). The work was covered by a votum of the Ethical Committee of the University of Heidelberg no. 206/2005. Written consent was obtained from each patient.
RNA extraction and quantitative real-time reverse transcription (RT)–PCR
RNA extraction and qRT–PCR analyses were performed as described previously (Crnković-Mertens et al, 2006b). In brief, RNA was isolated from homogenised tissues by phenol–chloroform extraction. We also included a commercially available RNA sample, representing a mixture of 14 different RNAs derived from normal kidney, unrelated to cancer (Clontech, Mountain View, CA, USA). Reverse transcription of 1 μg RNA was performed using the oligo-dT primer and SuperScriptIII First-Strand kit (Invitrogen, Karlsruhe, Germany). Livin, glyceraldehyde-3-phosphate dehydrogenase (GAPDH), and hypoxanthine phosphoribosyl-transferase 1 (HPRT1) expression were determined by real-time PCR. Relative quantification was performed using the comparative Ct (2−ΔΔCt) method (Livak and Schmittgen, 2001). Data are presented as the fold difference in gene expression normalised to a housekeeping gene index (the geometric mean of the expression levels of GAPDH and HPRT1), as an endogenous reference, and relative to a calibrator sample.
Reverse transcription–PCR
Expression of livin α and β mRNAs was analysed by RT–PCR, using primers which distinguish between the two splice variants (Crnković-Mertens et al, 2006a). PCR products (153 bp for livin α, 99 bp for livin β) were analysed by agarose gel electrophoresis.
Protein extraction from fresh-frozen tissue and Western blot analyses
Proteins were extracted from tumorous and non-tumorous adult kidney specimens, as described previously (Crnković-Mertens et al, 2007). Briefly, tissue sections were homogenised in lysis buffer, supplemented with protease inhibitors and Pefabloc (Biomol, Hamburg, Germany). Lysates were centrifuged at 100 000 g for 30 min at 4°C and supernatants collected. Proteins were separated by 12.5% SDS–polyacrylamide gel electrophoresis, transferred on to an Immobilon-P membrane (Millipore, Billerica, MA, USA), and detected with a monoclonal anti-Tubulin antibody (Oncogene, Boston, MA, USA). The hybridoma producing the monoclonal anti-Livin antibody no. 6 was raised in a male Balb/c mouse which was immunised by injection of purified Livin–hexa–His fusion protein. Western blots were performed by employing enhanced chemiluminescence (Millipore).
Immunohistochemistry
Paraffin-embedded tissue samples were cut at 2 μm, placed on slides, and dried for 24 h at 37°C. Sections were dewaxed, rehydrated using xylol and descending series of ethanol, and immersed in 3% H2O2 for 5 min to block endogenic peroxidases. After washing with TBST (50 mM Tris, 300 mM NaCl pH 7.6, 0.1% Tween), unspecific antibody binding sites were blocked using protein block solution provided by the Catalyzed Signal Amplification (CSA) II System (DAKO, Carpinteria, CA, USA). Sections were incubated for 30 min at room temperature with a monoclonal anti-human Livin mouse antibody (Active Motif, San Diego, CA, USA) at a dilution of 1 : 400. Sections were immersed in horseradish peroxidase-conjugated anti-mouse antibody for 15 min at room temperature, followed by an incubation with fluorescyl-tyramide hydrogen peroxide, for 15 min to intensify staining. Thereafter, sections were incubated with anti-fluorescein antibody conjugated to horseradish peroxidase and exposed to DAB solution (3,3′-diaminobenzidine tetrahydrochloride) for 2 min. Counterstaining of cell nuclei was carried out by immersing the section in hemalaun. Sections were thoroughly washed, glass covered, and analysed by light microscopy (Olympus Vanox-T, Hamburg, Germany), using a magnification of up to × 400. Tissue specimens were examined at random order by two independent examiners for the absence or presence of Livin staining in the cytoplasm or nucleus of tumorous and non-tumorous tissues.
Statistics
Livin mRNA measurements were log transformed to achieve data, which can be assumed to be normally distributed. To compare the distributions of log-livin between tumour and non-tumour tissues, a mixed linear model with the patient as random factor was applied, to account for paired data in nine patients. The test for the difference in log-livin between the different tissues is two-sided with a significance level of P=0.05. Test analysis was carried out with the Statistical Analysis System, Version 9.1 for Windows (SAS Institute Inc., Cary, NC, USA).
RESULTS
Comparative analysis of Livin expression in RCCs and non-tumorous tissue
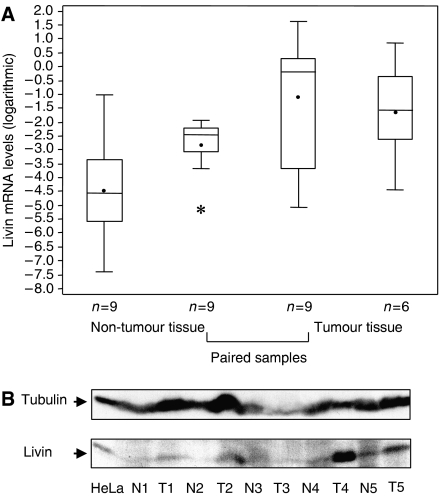
Quantitative real-time RT–PCR analyses were performed, to measure expression of the livin gene in (i) RCC tumour tissues, (ii) macroscopically and histologically normal tissue derived from kidneys removed because of malignant disease, and (iii) tissue from kidneys removed because of benign disease. Livin transcripts were detectable in all specimens examined. We found that livin mRNA levels in tumour tissue were significantly higher (P=0.0077) than in samples from normal adult kidney tissue (Figure 1A). Livin transcript levels in normal tissue and in specimens from benign kidney diseases were in the same range as was a commercially available sample representing a mixture of 14 RNAs derived from normal kidney (data not shown).
Figure 1.
Livin mRNA and protein levels in renal cell carcinoma (RCC) tumour tissue and non-tumorous adult kidney. (A) Livin mRNA expression levels were measured by qRT–PCR in tissue specimens from RCC (n=15) and non-tumorous kidney tissue adjacent to tumour (n=18). This analysis included nine paired samples of tumour and corresponding non-tumorous tissue. Values were log transformed. A mixed linear model with the patient as a random factor was applied to account for paired data in nine patients when comparing the distributions of log-livin between different tissues. Log-livin measurements were visualised in box plots with upper whiskers drawn up to the maximum value below third quartile+1.5* (interquartile range), lower whiskers defined accordingly, asterisk: outlier. (B) Western blot analysis of Livin protein in five paired samples in primary RCCs (T1–5) and non-tumorous tissue adjacent to the tumour (N1–5). HeLa cells served as positive control for Livin expression; Tubulin: loading control.
To investigate whether the difference in Livin expression between tumorous and non-tumorous samples is reflected at the protein level, we analysed five paired samples (tumour tissue and adjacent normal tissue from the same patient) by direct protein extraction from the tissues. As shown in Figure 1B, Livin protein levels were clearly detectable in all tumour samples, but close to the detection limit of this method in adjacent normal tissue. Taken together, these findings demonstrate that both the livin gene and the Livin protein are expressed in tumorous and non-tumorous kidney tissues, with significantly increased expression in RCCs.
Analysis of Livin isoforms α and β in RCCs and non-tumorous tissue
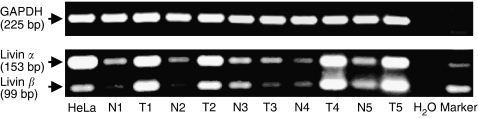
We analysed expression of Livin isoforms α and β by isoform-specific RT–PCR (Crnković-Mertens et al, 2006a). Both livin α and β mRNA were consistently detected. Overall, livin α and β mRNAs expression levels were similar in 3 out of 9 tumour tissues and in 6 out of 13 non-tumorous adult kidney samples whereas 6 out of 9 tumours and 7 out of 13 non-tumorous adult kidney samples exhibited higher livin α mRNA levels. Thus, whereas the relative ratio between livin α and β varied for individual sample pairs, it did not show an obvious link to tumorigenicity. Exemplary results for five corresponding samples from non-tumorous and tumorous tissue are shown in Figure 2.
Figure 2.
Analysis of primary renal cell carcinomas (RCCs) and non-tumorous adult kidney for the expression of mRNAs encoding Livin isoforms α and β. Five paired samples of tumour (T1–5) and adjacent non-tumorous tissue (N1–5) were analysed by isoform-specific RT–PCR. HeLa cells, which express both isoforms (Crnković-Mertens et al, 2006a), served as a positive control. GAPDH, internal standard.
Immunohistochemical analysis of Livin protein expression in RCCs and non-tumorous tissue
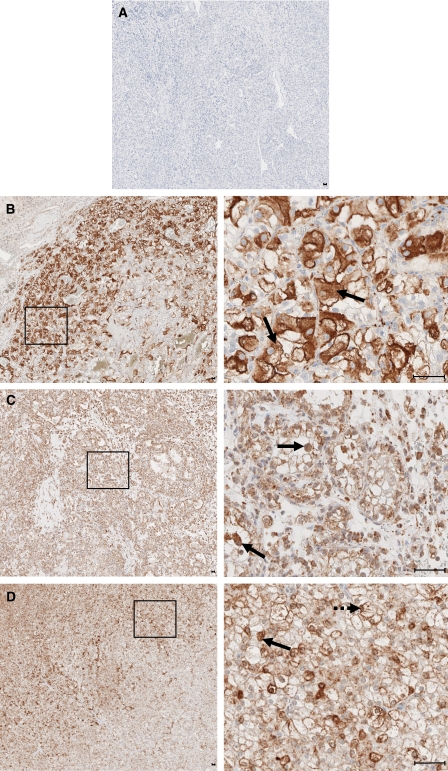
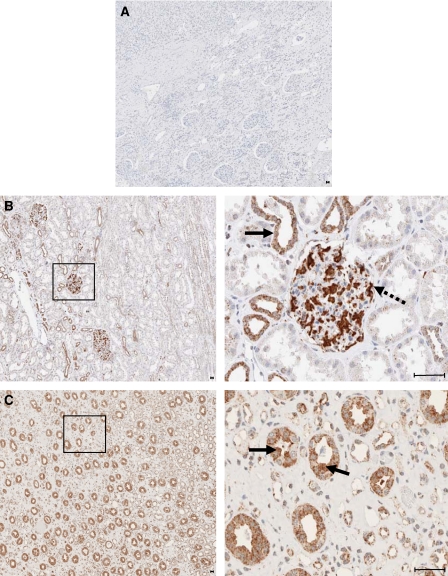
Next, we investigated the expression of Livin protein in paraffin-embedded tissues from RCCs and non-tumorous tissue, by immunohistochemistry. Livin expression was detectable in 10 out of 13 RCC tissue samples (Figure 3B–D). Moreover, Livin could be clearly visualised in all 17 specimens from non-tumorous adult kidney (Figure 4B and C). Livin expression was restricted to specific cells in non-tumorous kidney, namely to glomerular mesangial cells and podocytes (Figure 4B) as well as to distal and collect tubule epithelial cells (Figure 4B and C). Proximal tubular cells, which are thought to be the origin of most RCCs (Campbell et al, 2007), did not express detectable levels of Livin.
Figure 3.
Immunohistochemical analysis of Livin protein expression in primary renal cell carcinomas (RCCs). (A) Negative control: RCC tissue specimen, incubated with mouse IgG instead of the anti-Livin antibody. (B) Overview (left panel) and higher resolution (right panel) of an RCC sample showing cytoplasmic localisation of Livin (arrows), sparing the nucleus. (C) Overview (left panel) and higher resolution (right panel) of an RCC sample showing nuclear localisation of Livin (arrows). (D) Overview (left panel) and higher resolution of an RCC sample exhibiting both nuclear (interrupted arrow) and cytoplasmic (arrow) staining. Higher resolved areas in the right panels correspond to the framed regions in the left panels, bars, 5 μm.
Figure 4.
Immunohistochemical analysis of Livin protein expression in non-tumorous kidney. (A) Negative control: non-tumorous kidney, incubated with mouse IgG. (B) Overview (left panel) and higher resolution (right panel) of normal kidney, showing cytoplasmic localisation of Livin in distal tubule epithelial cells (arrow) and in glomerular mesangial cells and podocytes (interrupted arrow). (C) Overview (left panel) and higher resolution (right panel) of normal kidney, showing cytoplasmic localisation of Livin in distal tubule epithelial cells (arrows). Higher resolved areas in the right panels correspond to the framed regions in the left panels, bars, 5 μm.
At the cellular level, Livin protein expression was detected in the cytoplasm of renal cancer cells in 7 out of 10 positive tumour specimens (Figure 3B). In addition, 6 out of 10 positive tumour specimens exhibited nuclear staining for Livin, to varying degrees (Figure 3C), which included three specimens staining positive both in the cytoplasm and nucleus (Figure 3D). In these latter cases, the ratio of cells with nuclear or cytoplasmic staining was approximately 30–70%. On the contrary, Livin expression in non-tumorous adult kidney cells was always limited to the cytoplasm (Figure 4B and C).
Taken together, these findings demonstrate that Livin is not only expressed in a large proportion of RCCs but also to a significant degree in specific cells of the normal kidney.
DISCUSSION
In contrast to many other ubiquitously expressed IAPs, Livin is commonly considered to exhibit a largely tumour-specific expression pattern. This, together with the documented antiapoptotic activity of Livin, has raised considerable interest in developing strategies for the therapeutic inhibition of Livin in cancers (Chang and Schimmer, 2007; Liu et al, 2007). Conceptionally, these approaches would aim at correcting the increased apoptotic resistance of Livin-expressing cancer cells, thereby specifically increasing the susceptibility of tumour cells towards proapoptotic anticancer agents. Indeed, previous studies have shown that the targeted inhibition of livin by antisense oligonucleotides or RNA interference reduces the growth of Livin-expressing tumour cells in clonogenic survival assays and can resensitise them towards proapoptotic agents, including chemotherapeutics (Kasof and Gomes, 2001; Crnković-Mertens et al, 2003, 2006a, 2006b, 2007). Currently followed strategies aiming at the targeted inhibition of Livin include inhibitory peptides and small molecules (Franklin et al, 2003) and nucleic acid-based approaches (Crnković-Mertens et al, 2003). Since the specificity of these approaches would greatly profit from a tumour-specific expression of their target, we here investigated the expression of Livin in RCC and non-tumorous adult kidney.
Here, we demonstrate that Livin is not only expressed in RCCs, but also clearly detectable in specific cells of normal kidney. These findings contradict the common view that Livin expression is largely tumour-specific. Notably, previous studies did not detect livin expression in adult kidney by Northern blot analysis (Lin et al, 2000; Vucic et al, 2000; Kasof and Gomes, 2001), possibly due to the detection limit of this method. Only one RT–PCR analysis reported weak expression of livin β, but not livin α mRNA, in a single sample from adult kidney present in a commercially available multiple tissue cDNA panel (Ashhab et al, 2001). This finding was not followed further and is in contrast to our findings which indicate expression of both isoforms in non-tumorous tissue.
In non-tumorous kidney, highest levels of Livin protein were detected in glomerular mesangial cells and podocytes as well in distal and collecting tubule epithelial cells. These cells are in direct contact with urine during the filtration process. Although at present purely speculative, one could envision that antiapoptotic factors play a role for the protection of normal kidney cells from toxic substances in the urine.
It is remarkable that proximal tubular cells, from which most RCCs are believed to originate (Campbell et al, 2007), did not stain positive for Livin. Although we cannot exclude that these cells express low levels of Livin which are below the detection threshold of immunohistochemistry, the strong signals obtained in RCC cells indicate that an increase in Livin expression is acquired during RCC tumorigenesis. Furthermore, and in contrast to non-tumorous kidney tissue where Livin protein expression was restricted to the cytoplasm, we observed in 6 out of 10 positive tumour tissues nuclear staining. This suggests that Livin may also play a nuclear role during the transformation process. Nuclear activities which could be relevant for cell transformation have been demonstrated for other IAPs, such as survivin (Altieri, 2006) or cIAP1 (Samuel et al, 2005). Moreover, nuclear staining for IAPs has been related to the clinical prognosis of some tumours (Imoto et al, 2002; Li et al, 2005; Gordon et al, 2007). It therefore will be interesting to relate the subcellular localisation of Livin to the clinical prognosis of RCC patients in future studies, which will require higher patient numbers for statistical validation.
The significant levels of Livin expression in specific cells of normal kidney suggest that its targeted inhibition will not be a strictly tumour-specific therapeutic strategy. This should be taken into account for the development of livin inhibitors, to avoid unwanted side effects, such as nephrotoxicity. Furthermore, Livin has been reported to be a suitable target for the immunotherapy of lung cancer and of malignant melanoma where it can serve as a tumour rejection antigen (Schmollinger et al, 2003; Andersen et al, 2004; Schmollinger and Dranoff, 2004; Hariu et al, 2005). These immunotherapeutic strategies may also be complicated by expression of the targeted antigen in normal cells. Yet, we also would like to stress the point that our findings do not disqualify Livin as a therapeutic target. The observed overexpression of livin in RCCs may still allow a preferential, rather than a specific attack on tumour cells, depending on the therapeutic index of livin inhibitors. Accordingly, other IAPs overexpressed in cancers, such as XIAP or survivin, are considered as potential therapeutic targets, despite they are also expressed in normal tissues (Fukuda and Pelus, 2006; LaCasse et al, 2006).
Acknowledgments
NW is financed by a ‘Gerok’ scholarship from the German Cancer Research Center. This work was sponsored in part by a grant from the Deutsche Krebshilfe (106213). This study was supported by the tissue bank of the National Center for Tumor Diseases (NCT) Heidelberg.
References
- Altieri DC (2006) The case for survivin as a regulator of microtubule dynamics and cell-death decisions. Curr Opin Cell Biol 18: 609–615 [DOI] [PubMed] [Google Scholar]
- Andersen MH, Reker S, Becker JC, thor Straten P (2004) The melanoma inhibitor of apoptosis protein: a target for spontaneous cytotoxic T cell responses. J Invest Dermatol 122: 392–399 [DOI] [PubMed] [Google Scholar]
- Ashhab Y, Alian A, Polliack A, Panet A, Yehuda D (2001) Two splicing variants of a new inhibitor of apoptosis gene with different biological properties and tissue distribution pattern. FEBS Lett 495: 56–60 [DOI] [PubMed] [Google Scholar]
- Campbell SC, Novick AC, Bukowski RM (2007) Renal tumors. In Campbell-Walsh Urology Wein AJ, Kavoussi LR, Novick AC, Partin AW, Peters CA (eds) pp 1567–1637. Oxford: WB Saunders [Google Scholar]
- Chang H, Schimmer AD (2007) Livin/melanoma inhibitor of apoptosis protein as a potential therapeutic target for the treatment of malignancy. Mol Cancer Ther 6: 24–30 [DOI] [PubMed] [Google Scholar]
- Choi J, Hwang YK, Sung KW, Lee SH, Yoo KH, Jung HL, Koo HH, Kim HJ, Kang HJ, Shin HY, Ahn HS (2007) Expression of Livin, an antiapoptotic protein, is an independent favorable prognostic factor in childhood acute lymphoblastic leukemia. Blood 109: 471–477 [DOI] [PubMed] [Google Scholar]
- Crnković-Mertens I, Hoppe-Seyler F, Butz K (2003) Induction of apoptosis in tumor cells by siRNA-mediated silencing of the livin/ML-IAP/KIAP gene. Oncogene 22: 8330–8336 [DOI] [PubMed] [Google Scholar]
- Crnković-Mertens I, Muley T, Meister M, Hartenstein B, Semzow J, Butz K, Hoppe-Seyler F (2006b) The anti-apoptotic livin gene is an important determinant for the apoptotic resistance of non-small cell lung cancer cells. Lung Cancer 54: 135–142 [DOI] [PubMed] [Google Scholar]
- Crnković-Mertens I, Semzow J, Hoppe-Seyler F, Butz K (2006a) Isoform-specific silencing of the Livin gene by RNA interference defines Livin beta as key mediator of apoptosis inhibition in HeLa cells. J Mol Med 84: 232–240 [DOI] [PubMed] [Google Scholar]
- Crnković-Mertens I, Wagener N, Semzow J, Gröne EF, Haferkamp A, Hohenfellner M, Butz K, Hoppe-Seyler F (2007) Targeted inhibition of Livin resensitizes renal cancer cells towards apoptosis. Cell Mol Life Sci 64: 1137–1144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flanigan RC, Campbell SC, Clark JI, Picken MM (2003) Metastatic renal cell carcinoma. Curr Treat Options Oncol 4: 385–390 [DOI] [PubMed] [Google Scholar]
- Franklin MC, Kadkhodayan S, Ackerly H, Alexandru D, Distefano MD, Elliot LO, Flygare JA, Mausisa G, Okawa DC, Ong D, Vucic D, Deshayes K, Fairbrother WJ (2003) Structure and function analysis of peptide antagonists of melanoma inhibitor of apoptosis (ML-IAP). Biochemistry 42: 8223–8231 [DOI] [PubMed] [Google Scholar]
- Fukuda S, Pelus LM (2006) Survivin, a cancer target with an emerging role in normal adult tissues. Mol Cancer Ther 5: 1087–1098 [DOI] [PubMed] [Google Scholar]
- Gazzaniga P, Gradilone A, Giuliani L, Gandini O, Silvestri I, Nofroni I, Saccani G, Frati L, Agliano AM (2003) Expression and prognostic significance of LIVIN, SURVIVIN and other apoptosis-related genes in the progression of superficial bladder cancer. Ann Oncol 14: 85–90 [DOI] [PubMed] [Google Scholar]
- Gordon GJ, Mani M, Mukhopadhyay L, Dong L, Edenfield HR, Glickman JN, Yeap BY, Sugarbaker DJ, Bueno R (2007) Expression patterns of inhibitor of apoptosis proteins in malignant mesothelioma. J Pathol 211: 447–454 [DOI] [PubMed] [Google Scholar]
- Greene FL, Page DL, Fleming ID, Fritz A, Balch CM, Haller DG, Morrow M (eds) (2002) In AJCC Cancer Staging Manual pp 323–328. Springer: Berlin [Google Scholar]
- Hariu H, Hirohashi Y, Torigoe T, Asanuma H, Hariu M, Tamura Y, Aketa K, Nabeta C, Nakanishi K, Kamiguchi K, Mano Y, Kitamura H, Kobayashi J, Tsukahara T, Shijubo N, Sato N (2005) Aberrant expression and potency as a cancer immunotherapy target of inhibitor of apoptosis protein family, Livin/ML-IAP in lung cancer. Clin Cancer Res 11: 1000–1009 [PubMed] [Google Scholar]
- Imoto I, Tsuda H, Hirasawa A, Miura M, Sakamoto M, Hirohashi S, Inazawa J (2002) Expression of cIAP1, a target for 11q22 amplification, correlates with resistance of cervical cancers to radiotherapy. Cancer Res 62: 4860–4866 [PubMed] [Google Scholar]
- Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ (2007) Cancer statistics, 2007. CA Cancer J Clin 57: 43–66 [DOI] [PubMed] [Google Scholar]
- Kasof GM, Gomes BC (2001) Livin, a novel inhibitor of apoptosis protein family member. J Biol Chem 276: 3238–3246 [DOI] [PubMed] [Google Scholar]
- Kim DK, Alvarado CS, Abramowsky CR, Gu L, Zhou M, Soe MM, Sullivan K, George B, Schemankewitz E, Findley HW (2005) Expression of inhibitor-of-apoptosis protein (IAP) livin by neuroblastoma cells: correlation with prognostic factors and outcome. Pediatr Dev Pathol 8: 621–629 [DOI] [PubMed] [Google Scholar]
- LaCasse EC, Cherton-Horvat CG, Hewitt KE, Jerome LJ, Morris SJ, Kandimalla ER, Yu D, Wang H, Wang W, Zhang R, Agrawal S, Gillard JW, Durkin JP (2006) Preclinical characterization of AEG35156/GEM 640, a second generation antisense oligonucleotide targeting X-linked inhibitor of apoptosis. Clin Cancer Res 12: 5231–5241 [DOI] [PubMed] [Google Scholar]
- Li F, Yang J, Ramnath N, Javle MM, Tan D (2005) Nuclear or cytoplasmic expression of survivin: what is the significance? Int J Cancer 114: 509–512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin JH, Deng G, Huang Q, Morser J (2000) KIAP, a novel member of the inhibitor of apoptosis protein family. Biochem Biophys Res Commun 279: 820–831 [DOI] [PubMed] [Google Scholar]
- Liu B, Han M, Wen JK, Wang L (2007) Livin/ML-IAP as a new target for cancer treatment. Cancer Lett 250: 168–176 [DOI] [PubMed] [Google Scholar]
- Liu X, Chen N, Wang X, He Y, Chen X, Huang Y, Yin W, Zhou Q (2006) Apoptosis and proliferation markers in diffusely infiltrating astrocytomas: profiling of 17 molecules. J Neuropathol Exp Neurol 65: 905–913 [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCt method. Methods 25: 402–408 [DOI] [PubMed] [Google Scholar]
- Lopes RB, Gangeswaran R, McNeish IA, Wang Y, Lemoine NR (2007) Expression of the IAP protein family is dysregulated in pancreatic cancer cells and is important for resistance to chemotherapy. Int J Cancer 120: 2344–2352 [DOI] [PubMed] [Google Scholar]
- Qiuping Z, Jei X, Youxin J, Wei J, Chun L, Jin W, Qun W, Yan L, Chunsong H, Mingzhen Y, Qingping G, Kejian Z, Zhimin S, Qun L, Junyan L, Jinquan T (2004) CC chemokine ligand 25 enhances resistance to apoptosis in CD4+ T cells from patients with T-cell lineage acute and chronic lymphocytic leukaemia by means of livin activation. Cancer Res 64: 7579–7587 [DOI] [PubMed] [Google Scholar]
- Salvesen GS, Duckett CS (2002) IAP proteins: blocking the road to death's door. Nat Rev Mol Cell Biol 3: 401–410 [DOI] [PubMed] [Google Scholar]
- Samuel T, Okada K, Hyer M, Welsh K, Zapata JM, Reed JC (2005) cIAP1 localizes to the nuclear compartment and modulates the cell cycle. Cancer Res 65: 210–218 [PubMed] [Google Scholar]
- Schmollinger JC, Dranoff G (2004) Targeting melanoma inhibitor of apoptosis protein with cancer immunotherapy. Apoptosis 9: 309–313 [DOI] [PubMed] [Google Scholar]
- Schmollinger JC, Vonderheide RH, Hoar KM, Maecker B, Schultze JL, Hodi FS, Soiffer RJ, Jung K, Kuroda MJ, Letvin NL, Greenfield EA, Mihm M, Kutok JL, Dranoff G (2003) Melanoma inhibitor of apoptosis protein (ML-IAP) is a target for immune-mediated tumor destruction. Proc Natl Acad Sci USA 100: 3398–3403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanabe H, Yagihashi A, Tsuji N, Shijubo Y, Abe S, Watanabe N (2004) Expression of survivin mRNA and livin mRNA in non-small-cell lung cancer. Lung Cancer 46: 299–304 [DOI] [PubMed] [Google Scholar]
- Vucic D, Stennicke HR, Pisabarro MT, Salvesen GS, Dixit VM (2000) ML-IAP, a novel inhibitor of apoptosis that is preferentially expressed in human melanomas. Curr Biol 10: 1359–1366 [DOI] [PubMed] [Google Scholar]
- Xiang Y, Yao H, Wang S, Hong M, He J, Cao S, Min H, Song E, Guo X (2006) Prognostic value of Survivin and Livin in nasopharyngeal carcinoma. Laryngoscope 116: 126–130 [DOI] [PubMed] [Google Scholar]