Abstract
In a cohort study, ovarian cancer (280 cases) showed no significant association with tea or coffee, the multivariable rate ratios being 0.94 (95% confidence interval (CI): 0.89, 1.00) and 1.04 (95% CI: 0.97, 1.12) per cup per day, respectively. A meta-analysis also produced no significant findings overall, though the cohort studies showed a significant inverse association for tea.
Keywords: tea, coffee, ovarian neoplasms, meta-analysis, aetiology, cohort studies
Tea and coffee are widely consumed around the world and may affect human health. Several case-control and cohort studies have analysed the relationship with ovarian cancer risk, yielding inconclusive results. We analysed the association between tea, coffee and ovarian cancer in a prospective cohort study and summarised results of previous studies in a meta-analysis.
MATERIALS AND METHODS
The cohort
The Netherlands Cohort Study on Diet and Cancer is a prospective cohort study (n=120 852) that started in September 1986 with the enrolment of participants aged 55–69, among whom 62 573 were women (van den Brandt et al, 1990). Data processing and analysis were based on the case-cohort approach. A subcohort of 2589 women was randomly drawn from the cohort at baseline. After exclusion of prevalent cancer cases (other than skin cancer) (n=151), women who reported at baseline to have undergone an oophorectomy (n=32) and subcohort members with incomplete or inconsistent dietary questionnaire data (n=190) (Goldbohm et al, 1994), incomplete information on tea or coffee consumption (n=80) or confounders (n=53), 2083 subcohort members were available for analysis.
Follow-up
The subcohort has been followed up for emigration and vital status. No female subcohort members were lost to follow-up. Follow-up for epithelial ovarian cancer incidence was performed by record linkage to the Netherlands Cancer Registry. During 13.3 years of follow-up, 362 incident, microscopically confirmed ovarian carcinomas (ICD-O-3 code C56.9) were identified. After exclusion of borderline invasive (n=14) and non-epithelial tumours (n=12) and women with incomplete or inconsistent dietary questionnaire data (n=36), missing information on tea or coffee consumption (n=11) or confounders (n=9), 280 cases remained available for analyses.
Questionnaire
A self-administered questionnaire on risk factors for cancer, including a food frequency questionnaire (FFQ), was completed by all cohort members at baseline. The respondents were asked whether they drank tea or coffee and, if so, how many cups per day. The type of tea was not specified but this population rarely drank any tea other than black tea in 1986 (Goldbohm et al, 1996). We validated the FFQ against a 9-day diet record (Goldbohm et al, 1994) and established the 5-year reproducibility of the FFQ (Goldbohm et al, 1995).
Data analysis
Person-years of follow-up were calculated for the subcohort members from the start of the study until the date of ovarian cancer diagnosis, death, emigration or end of follow-up. Incidence rate ratios (RR) and corresponding 95% confidence intervals (CI) were estimated in age-adjusted and multivariable-adjusted analyses using Cox proportional hazards model with Stata 9.0.
Meta-analysis
We performed a meta-analysis of case-control and cohort studies on tea, coffee and ovarian cancer using the Stata procedure ‘metan’. A random effects model was used because of significant heterogeneity. On tea drinking and ovarian cancer, eight case-control studies (Byers et al, 1983; Miller et al, 1987; La Vecchia et al, 1992; Kuper et al, 2000; Tavani et al, 2001; Zhang et al, 2002; Jordan et al, 2004; Baker et al, 2007) and, including this study, five cohort studies (Zheng et al, 1996; Larsson and Wolk, 2005b; Gates et al, 2007; Silvera et al, 2007) have been conducted. Coffee drinking and ovarian cancer risk was investigated in 16 case-control studies (Trichopoulos et al, 1981; Hartge et al, 1982; Byers et al, 1983; Cramer et al, 1984; La Vecchia et al, 1984; Tzonou et al, 1984; Miller et al, 1987; Whittemore et al, 1988; Polychronopoulou et al, 1993; Kuper et al, 2000; Tavani et al, 2001; Jordan et al, 2004; Riman et al, 2004; Baker et al, 2007) and, including this study, five cohort studies (Snowdon and Phillips, 1984; Stensvold and Jacobsen, 1994; Larsson and Wolk, 2005a; Silvera et al, 2007). We had to exclude from the meta-analysis studies that did not report 95% CIs (Trichopoulos et al, 1981; Byers et al, 1983; Cramer et al, 1984; Tzonou et al, 1984; Stensvold and Jacobsen, 1994).
RESULTS
After 13.3 years of follow-up, there were 280 epithelial ovarian carcinomas, 47.1% of which were serous carcinomas, 10.0% mucinous carcinomas, 9.3% endometrioid carcinomas and 4.3% clear cell carcinomas. Tea was drunk by >89% of the subjects, coffee by >96% and only 0.2% drank neither. Mean daily tea consumption (s.d.) was 3.1 (2.1) cups for subcohort members and 2.9 (1.8) cups for cases. Older women, never smokers, more highly educated respondents and women with a normal body mass index (BMI) drank more tea. Mean daily coffee consumption (s.d.) was 4.0 (2.0) cups for subcohort members and 4.1 (2.0) cups for cases. A lower age, current smoking, a lower level of education and a higher BMI were associated with higher coffee consumption.
Table 1 shows RRs for ovarian cancer for tea and coffee consumption. Tea consumption was inversely associated with ovarian cancer risk, although not statistically significant and coffee consumption showed no association. The proportional hazards assumption was not violated and there was no effect modification. The category 1–<3 cups per day was chosen as a reference because there were too few subjects in the lowest category. We excluded subjects with <2 years of follow-up to check for protopathic bias, analysed stable coffee drinkers separately and corrected for acrylamide in coffee, but none of these analyses affected the results. Subtype analyses showed that tea was inversely associated with serous tumours, whereas it was related to increased risk of mucinous and endometrioid tumours, giving some indication for heterogeneity.
Table 1. Rate ratios of ovarian cancer according to coffee and tea consumptiona.
|
Age-adjusted
|
Multivariable adjustedb
|
||||||
|---|---|---|---|---|---|---|---|
| Mean (cups per day) | Cases (n) | Person- years in subcohort | RR | 95% CI | RR | 95% CI | |
| Coffee (cups per day) | |||||||
| 0–<1 | 0.5 | 15 | 1913 | 0.70 | 0.39, 1.25 | 0.73 | 0.41, 1.31 |
| 1–<3 | 2.5 | 87 | 7647 | 1.00 | Reference | 1.00 | Reference |
| 3–<5 | 4.3 | 119 | 11,243 | 0.96 | 0.71, 1.29 | 1.00 | 0.74, 1.35 |
| ⩾5 | 6.9 | 59 | 5124 | 1.07 | 0.75, 1.53 | 1.08 | 0.75, 1.57 |
| P-trend=0.33 | P-trend=0.35 | ||||||
| Coffee increment (1 cup per day) | 1.04 | 0.98, 1.11 | 1.04 | 0.97, 1.12 | |||
| Tea (cups per day) | |||||||
| 0–<1 | 0.5 | 66 | 5455 | 1.13 | 0.81, 1.57 | 1.10 | 0.78, 1.54 |
| 1–<3 | 2.4 | 107 | 9856 | 1.00 | Reference | 1.00 | Reference |
| 3–<5 | 4.2 | 83 | 7262 | 1.03 | 0.76, 1.40 | 1.04 | 0.76, 1.42 |
| ⩾5 | 6.8 | 24 | 3354 | 0.64 | 0.40, 1.02 | 0.65 | 0.41, 1.03 |
| P-trend=0.07 | P-trend=0.12 | ||||||
| Tea increment (1 cup per day) | 0.94 | 0.88, 1.00 | 0.94 | 0.89, 1.00 | |||
Abbreviations: RR=rate ratio; CI=confidence interval.
The Netherlands Cohort Study (1986–1999).
Adjusted for age (years), use of oral contraceptives (ever/never), parity (number of children), cigarette smoking (current, ex-smoker, never smoker). Coffee and tea were mutually adjusted.
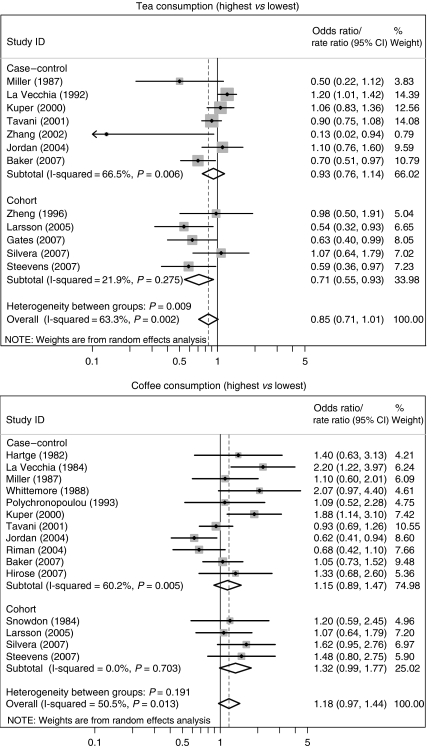
In the meta-analysis (Figure 1), tea was found to be inversely associated with ovarian cancer when study results were pooled. The association was strongest, and statistically significant, in cohort studies. Coffee consumption was related to increased ovarian cancer risk when study results were pooled. A stronger, borderline significant association was seen in cohort studies.
Figure 1.
Meta-analysis of case-control and cohort studies investigating tea and coffee consumption (highest vs lowest) in relation to ovarian cancer risk. Note: In this meta-analysis, we used the lowest category as a reference in all studies, for reasons of comparability.
DISCUSSION
In this cohort study, coffee consumption was not associated with the risk of epithelial ovarian cancer in postmenopausal women. Tea drinking was inversely, but not statistically significantly, associated with ovarian cancer risk. An important strength of this study is the prospective character, which makes recall bias unlikely.
The heterogeneity between the outcomes observed in different studies may be attributable to effect modification by genetic factors, for example polymorphisms of the CYP1A2 genotype, an enzyme involved in the metabolism of caffeine (Goodman et al, 2003). As tea and coffee are very common beverages, even a modest decrease or increase of ovarian cancer risk found in the meta-analysis is of importance.
Acknowledgments
This study was financially supported by grant 2003/30 from the World Cancer Research Fund-UK. We are indebted to the participants of this study and further thank the cancer registries (IKA, IKL, IKMN, IKN, IKO, IKR, IKST, IKW, IKZ and VIKC), and the Netherlands nationwide registry of pathology (PALGA). We also thank Dr A Volovics and Dr A Kester for statistical advice; S van de Crommert, H Brants, J Nelissen, C de Zwart, W van Dijk, M Jansen and A Pisters for assistance and H van Montfort, J Berben, L van den Bosch and R Schmeitz for programming assistance.
References
- Baker JA, Boakye K, McCann SE, Beehler GP, Rodabaugh KJ, Villella JA, Moysich KB (2007) Consumption of black tea or coffee and risk of ovarian cancer. Int J Gynecol Cancer 17: 50–54, doi: 10.1111/j.1525-1438.2006.00773.x [DOI] [PubMed] [Google Scholar]
- Byers T, Marshall J, Graham S, Mettlin C, Swanson M (1983) A case-control study of dietary and nondietary factors in ovarian cancer. J Natl Cancer Inst 71: 681–686 [PubMed] [Google Scholar]
- Cramer DW, Welch WR, Hutchison GB, Willett W, Scully RE (1984) Dietary animal fat in relation to ovarian cancer risk. Obstet Gynecol 63: 833–838 [PubMed] [Google Scholar]
- Gates MA, Tworoger SS, Hecht JL, De Vivo I, Rosner B, Hankinson SE (2007) A prospective study of dietary flavonoid intake and incidence of epithelial ovarian cancer. Int J Cancer doi:10.1002/ijc.22790 [DOI] [PubMed]
- Goldbohm RA, Hertog MG, Brants HA, van Poppel G, van den Brandt PA (1996) Consumption of black tea and cancer risk: a prospective cohort study. J Natl Cancer Inst 88: 93–100 [DOI] [PubMed] [Google Scholar]
- Goldbohm RA, van den Brandt PA, Brants HA, van't Veer P, Al M, Sturmans F, Hermus RJ (1994) Validation of a dietary questionnaire used in a large-scale prospective cohort study on diet and cancer. Eur J Clin Nutr 48: 253–265 [PubMed] [Google Scholar]
- Goldbohm RA, van't Veer P, van den Brandt PA, van't Hof MA, Brants HA, Sturmans F, Hermus RJ (1995) Reproducibility of a food frequency questionnaire and stability of dietary habits determined from five annually repeated measurements. Eur J Clin Nutr 49: 420–429 [PubMed] [Google Scholar]
- Goodman MT, Tung KH, McDuffie K, Wilkens LR, Donlon TA (2003) Association of caffeine intake and CYP1A2 genotype with ovarian cancer. Nutr Cancer 46: 23–29 [DOI] [PubMed] [Google Scholar]
- Hartge P, Lesher LP, McGowan L, Hoover R (1982) Coffee and ovarian cancer. Int J Cancer 30: 531–532 [DOI] [PubMed] [Google Scholar]
- Jordan SJ, Purdie DM, Green AC, Webb PM (2004) Coffee, tea and caffeine and risk of epithelial ovarian cancer. Cancer Causes Control 15: 359–365 [DOI] [PubMed] [Google Scholar]
- Kuper H, Titus Ernstoff L, Harlow BL, Cramer DW (2000) Population based study of coffee, alcohol and tobacco use and risk of ovarian cancer. Int J Cancer 88: 313–318 [DOI] [PubMed] [Google Scholar]
- La Vecchia C, Franceschi S, Decarli A, Gentile A, Liati P, Regallo M, Tognoni G (1984) Coffee drinking and the risk of epithelial ovarian cancer. Int J Cancer 33: 559–562 [DOI] [PubMed] [Google Scholar]
- La Vecchia C, Negri E, Franceschi S, D'Avanzo B, Boyle P (1992) Tea consumption and cancer risk. Nutr Cancer 17: 27–31 [DOI] [PubMed] [Google Scholar]
- Larsson SC, Wolk A (2005a) Coffee consumption is not associated with ovarian cancer incidence. Cancer Epidemiol Biomarkers Prev 14: 2273–2274, doi:10.1158/1055-9965.EPI-05-0280 [DOI] [PubMed] [Google Scholar]
- Larsson SC, Wolk A (2005b) Tea consumption and ovarian cancer risk in a population-based cohort. Arch Intern Med 165: 2683–2686 [DOI] [PubMed] [Google Scholar]
- Miller DR, Rosenberg L, Kaufman DW, Helmrich SP, Schottenfeld D, Lewis J, Stolley PD, Rosenshein N, Shapiro S (1987) Epithelial ovarian cancer and coffee drinking. Int J Epidemiol 16: 13–17 [DOI] [PubMed] [Google Scholar]
- Polychronopoulou A, Tzonou A, Hsieh CC, Kaprinis G, Rebelakos A, Toupadaki N, Trichopoulos D (1993) Reproductive variables, tobacco, ethanol, coffee and somatometry as risk factors for ovarian cancer. Int J Cancer 55: 402–407 [DOI] [PubMed] [Google Scholar]
- Riman T, Dickman PW, Nilsson S, Nordlinder H, Magnusson CM, Persson IR (2004) Some life-style factors and the risk of invasive epithelial ovarian cancer in Swedish women. Eur J Epidemiol 19: 1011–1019 [DOI] [PubMed] [Google Scholar]
- Silvera SA, Jain M, Howe GR, Miller AB, Rohan TE (2007) Intake of coffee and tea and risk of ovarian cancer: a prospective cohort study. Nutr Cancer 58: 22–27 [DOI] [PubMed] [Google Scholar]
- Snowdon DA, Phillips RL (1984) Coffee consumption and risk of fatal cancers. Am J Public Health 74: 820–823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stensvold I, Jacobsen BK (1994) Coffee and cancer: a prospective study of 43 000 Norwegian men and women. Cancer Causes Control 5: 401–408 [DOI] [PubMed] [Google Scholar]
- Tavani A, Gallus S, Dal Maso L, Franceschi S, Montella M, Conti E, La Vecchia C (2001) Coffee and alcohol intake and risk of ovarian cancer: an Italian case-control study. Nutr Cancer 39: 29–34 [DOI] [PubMed] [Google Scholar]
- Trichopoulos D, Papapostolou M, Polychronopoulou A (1981) Coffee and ovarian cancer. Int J Cancer 28: 691–693 [DOI] [PubMed] [Google Scholar]
- Tzonou A, Day NE, Trichopoulos D, Walker A, Saliaraki M, Papapostolou M, Polychronopoulou A (1984) The epidemiology of ovarian cancer in Greece: a case-control study. Eur J Cancer Clin Oncol 20: 1045–1052 [DOI] [PubMed] [Google Scholar]
- van den Brandt PA, Goldbohm RA, van't Veer P, Volovics A, Hermus RJ, Sturmans F (1990) A large-scale prospective cohort study on diet and cancer in The Netherlands. J Clin Epidemiol 43: 285–295 [DOI] [PubMed] [Google Scholar]
- Whittemore AS, Wu ML, Paffenbarger Jr RS, Sarles DL, Kampert JB, Grosser S, Jung DL, Ballon S, Hendrickson M (1988) Personal and environmental characteristics related to epithelial ovarian cancer. II. Exposures to talcum powder, tobacco, alcohol, and coffee. Am J Epidemiol 128: 1228–1240 [DOI] [PubMed] [Google Scholar]
- Zhang M, Binns CW, Lee AH (2002) Tea consumption and ovarian cancer risk: a case-control study in China. Cancer Epidemiol Biomarkers Prev 11: 713–718 [PubMed] [Google Scholar]
- Zheng W, Doyle TJ, Kushi LH, Sellers TA, Hong CP, Folsom AR (1996) Tea consumption and cancer incidence in a prospective cohort study of postmenopausal women. Am J Epidemiol 144: 175–182 [DOI] [PubMed] [Google Scholar]