Abstract
To investigate the prevalence of, and risk factors for, cervical infection with human papillomavirus (HPV) in the rural province of Shanxi, People's Republic of China, which has relatively high cervical cancer mortality rates, we interviewed and obtained cervical cell samples from 662 women aged 15–59 years. A total of 24 different HPV types were identified using a GP5+/6+-based PCR assay able to detect 44 different HPV types. Human papillomavirus prevalence was 14.8% overall and 9.6% among women without cervical abnormalities (14.2 and 8.9%, respectively, age standardised to the world standard population). Multiple-type infections accounted for 30.6% of all infections. By far the most commonly found type was HPV16 (5.7% of all women and 38.8% of HPV-positive women), followed by HPV 58, 52, 33 and 18. Unlike most previous studies published, HPV prevalence was lower among women younger than 35 years (8.7%) than those older than 35 years (17.8%). High-risk HPV types predominated in all age groups. Although low-risk HPV types were rare in young women, they became more common with increasing age. 92.3% of women with cervical intraepithelial neoplasia grade 3 were infected with high-risk HPV types, but none with low-risk types only. No significant difference in HPV positivity was observed by educational level, sexual habits, reproductive history or use of contraceptive methods in this rural low-income Chinese population.
Keywords: human papillomavirus, cervical neoplasia, China, epidemiology
Following acceptance of human papillomavirus (HPV) as a necessary cause of cervical cancer, primary and secondary cervical cancer prevention strategies have moved towards detection and control of the virus. In order to understand the potential impact of HPV vaccination and HPV-based screening, epidemiological data on HPV type-specific prevalence among women from different populations is important. The International Agency for Research on Cancer (IARC) has therefore carried out surveys in representative samples of women worldwide (Jacobs et al, 2000; Lazcano-Ponce et al, 2001; Molano et al, 2002; Anh et al, 2003; de Sanjosé et al, 2003; Matos et al, 2003; Shin et al, 2003; Sukvirach et al, 2003; Ferreccio et al, 2004; Shin et al, 2004; Thomas et al, 2004; Franceschi et al, 2005; Ronco et al, 2005). These surveys have disclosed wide variations in HPV prevalence and some in the relative frequency of individual HPV types (Clifford et al, 2005).
The population of the People's Republic of China is 1.3 billion, of which 0.9 billion is rural. According to data from a network of 10 Chinese cancer registries, cervical cancer incidence in China is estimated to be below 4/100 000 (Parkin et al, 2002). However, nationwide mortality surveys show a heterogeneous pattern of cervical cancer risk, with particularly high rates in central rural provinces (Yang et al, 2003a). Furthermore, while cervical cancer mortality appears to have declined considerably in urban China between 1987 and 1999, this is less marked in rural areas (Yang et al, 2003b). A recent study in one such high-risk rural area, Shanxi Province, revealed that 23.6% of women aged 35–50 years were infected with high-risk HPV types (Zhao et al, 2006). However, at present no population-based data are available on HPV prevalence across a broader age range of Chinese women, nor on the relative frequency of individual HPV types.
MATERIALS AND METHODS
Study subjects
This study was a collaborative project between the IARC and the Cancer Institute of the Chinese Academy of Medical Sciences (CI-CAMS) and study methods were similar to those used for previous IARC HPV prevalence surveys (Clifford et al, 2005). The study area of Shanxi Province is a rural, mountainous area in central China, with a population mainly employed in agriculture and mining.
A total of 2229 women aged 15–59 years were enumerated in March and April 2004 from the population list of eight randomly chosen villages in Hebei Commune, Yangcheng County, Shanxi Province, China. The study purpose was to enroll approximately 100 women in each 5-year age group between 15–19 and 55–59 years. Women were contacted at their home by village doctors and invited to Yangcheng Tumour Hospital to participate in the study. All mentally and physically competent women aged 15–59 years were eligible for the study. Women were interviewed regardless of their marital status but pelvic examination and cervical cell specimen collection were only possible among married women.
Of the 2229 enumerated women, 157 were not found at the address given on the population list, and 404 were not invited as the required sample size for their age group had been reached. Of the 1668 invited women, 727 (43.6%) did not participate in the study, mainly because they said they did not have time or did not understand the need to undergo gynaecological examination in the absence of symptoms. Of the remaining 941 women who participated between May and June 2004, 197 did not give a cervical cell specimen (159 unmarried, 13 pregnant, nine menstruating, and three hysterectomised women, as well as 13 married women who opted not to undergo pelvic examination).
The interview was administered by one of four research nurses in the Shanxi dialect of Chinese. The structured questionnaires included information on socio-demographic characteristics, reproductive and menstrual factors, sexual habits of women and their husbands, and lifetime use of contraceptive methods.
All participants signed an informed consent form according to the recommendations of the IARC and CI-CAMS ethical review committees, which approved the study.
Gynaecological examination and cervical specimen collection
A total of 744 married women underwent a pelvic examination by one of four gynaecologists. Firstly, a sample of exfoliated cervical cells for liquid-based cytology and HPV testing was collected. A cytobrush was inserted into the endocervical canal and rotated gently 180o in order to collect cells from the endo- and ectocervix. The brush containing cellular material was then placed in a vial containing CytoRich transport media (Tripath Imaging, Burlington, NC, USA). Two aliquots of cellular material were obtained in the CI-CAMS laboratory using a strict protocol to minimise contamination, and were then sent to IARC for HPV testing. Remaining cellular material was used to perform liquid-based cytology at CI-CAMS. Secondly, the cervix was inspected by visual inspection with acetic acid (VIA) and visual inspection with Lugol's Iodine solution (VILI), followed by a digital colposcopy. Women with suspected abnormalities at VIA, VILI or colposcopy had a colposcopy-directed biopsy taken, and women whose entire squamocolumnar junction could not be visualised underwent endocervical curettage. When liquid-based cytology results became available, all women with cytologically abnormal lesions (atypical squamous cells of undetermined significance (ASCUS) or worse) not detected at VIA, VILI or digital colposcopy, were recalled and had biopsies taken. Treatment of detected lesions was performed according to local protocols using loop electrosurgical excision procedures.
Cytology and histology
Liquid-based cytology was performed at CI-CAMS. Slides were prepared from the CytoRich preserved specimen according to the manufacturer's standard protocol (Belinson et al, 2003), and results classified according to the Bethesda System. At CI-CAMS all abnormal smears, as well as 10% of normal smears chosen at random, were reviewed by an experienced cytologist, and cervical biopsies by a pathologist.
On account of the performance of multiple screening tests and the especially high proportion of histological confirmation of abnormal cervical findings at VIA, VILI, digital colposcopy or liquid-based cytology, cervical abnormalities were defined in the present study as presence of histologically confirmed cervical intraepithelial neoplasia (CIN)1 or worse.
HPV detection
Human papillomavirus testing was performed on exfoliated cervical cells in the Department of Pathology at the Vrije University Medical Center, Amsterdam, The Netherlands. To that end DNA was extracted first from the remainder of the CytoRich sample using a High Pure PCR product Purification Kit according to the manufacturer's recommendations (Roche Applied Science, Mannheim, Germany). β-Globin PCR analysis was performed firstly to confirm the presence of human DNA in all specimens (Roda Husman et al, 1995). The overall presence of HPV DNA was determined by performing a general primer GP5+/6+-mediated PCR, which permits the detection of a broad spectrum of genital HPV types at the subpicogram level (Jacobs et al, 2000). Human papillomavirus positivity was assessed by hybridisation of PCR products in an enzyme immunoassay using two HPV oligoprobe cocktails that, together, detect the following 44 HPV types: HPV 6, 11, 16, 18, 26, 30, 31, 32, 33, 34, 35, 39, 40, 42, 43, 44, 45, 51, 52, 53, 54, 55, 56, 57, 58, 59, 61, 64, 66, 67, 68, 69, 70, 71 (equivalent to CP8061), 72, 73, 81 (equivalent to CP8304), 82 (IS39 and MM4 subtypes), 83 (equivalent to MM7), 84 (equivalent to MM8), cand85, 86, cand89 (equivalent to CP6108) and JC9710. Subsequent HPV typing was performed by reverse line blot hybridisation of PCR products, as described previously (van den Brule et al, 2002).
Human papillomavirus types considered high-risk for this analysis included HPV 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 68, 73 and 82 (Muñoz et al, 2003). All other HPV types were considered low risk.
Statistical analysis
Odds ratios (ORs) for HPV positivity and corresponding 95% confidence intervals (CIs) were calculated by means of unconditional logistic regression equations, adjusted for age (15–24, 25–34, 35–44, 45–54, 55–59 years). The statistical significance of trends for ORs was assessed by considering the categorical variables as a continuous variable in the logistic model.
RESULTS
Of 744 married women who provided cervical cell samples, nine had inadequate cytology results and 73 had β-globin-negative samples, leaving 662 women with valid cytology and HPV results. Among them, 68 (10.3%) had histologically confirmed cervical abnormalities, including 39 CIN1, 16 CIN2, 12 CIN3 and one squamous cell carcinoma.
The prevalence of any HPV type was 14.8% (60.3% and 9.6% among women with and without cervical abnormalities, respectively, Table 1). The corresponding prevalences age-standardised to the world population were 14.2% overall, and 8.9% among those without cervical abnormalities. In total, 68 women had single-type and 30 had multiple-type infections. In all, 24 individual HPV types were identified.
Table 1. Prevalence of various HPV types by presence of cervical abnormalities and overall among 662 women. Shanxi Province, China, 2004.
|
Cervical abnormalities
| |||||||||
|---|---|---|---|---|---|---|---|---|---|
|
Absent
|
Presenta
|
Total
|
|||||||
| HPV types | Single | Multiple | Total (%) | Single | Multiple | Total (%) | Single | Multiple | Total (%) |
| Negative | − | − | 537 (90.4) | − | − | 27 (39.7) | − | − | 564 (85.2) |
| Positive | |||||||||
| Any | 42 | 15 | 57 (9.6) | 26 | 15 | 41 (60.3)b | 68 | 30 | 98 (14.8) |
| High risk | 31 | 13 | 44 (7.4) | 23 | 14 | 37 (54.4) | 54 | 27 | 81 (12.2) |
| Low risk | 10 | 6 | 16 (2.7) | 2 | 7 | 9 (13.2) | 12 | 13 | 25 (3.8) |
| X | 1 | 0 | 1 (0.2) | 1 | 0 | 1 (1.5) | 2 | 0 | 2 (0.3) |
| High risk | |||||||||
| 16 | 10 | 8 | 18 (3.0) | 14 | 6 | 20 (29.4) | 24 | 14 | 38 (5.7) |
| 58 | 7 | 4 | 11 (1.9) | 4 | 6 | 10 (14.7) | 11 | 10 | 21 (3.2) |
| 52 | 3 | 2 | 5 (0.8) | 1 | 2 | 3 (4.4) | 4 | 4 | 8 (1.2) |
| 33 | 1 | 2 | 3 (0.5) | 3 | 2 | 5 (7.4) | 4 | 4 | 8 (1.2) |
| 18 | 3 | 0 | 3 (0.5) | 0 | 2 | 2 (2.9) | 3 | 2 | 5 (0.8) |
| 56 | 1 | 3 | 4 (0.7) | 0 | 1 | 1 (1.5) | 1 | 4 | 5 (0.8) |
| 39 | 1 | 2 | 3 (0.5) | 0 | 1 | 1 (1.5) | 1 | 3 | 4 (0.6) |
| 51 | 1 | 2 | 3 (0.5) | 0 | 1 | 1 (1.5) | 1 | 3 | 4 (0.6) |
| 45 | 1 | 1 | 2 (0.3) | 0 | 2 | 2 (2.9) | 1 | 3 | 4 (0.6) |
| 31 | 3 | 0 | 3 (0.5) | 0 | 0 | 0 | 3 | 0 | 3 (0.5) |
| 59 | 0 | 0 | 0 | 1 | 1 | 2 (2.9) | 1 | 1 | 2 (0.3) |
| 68 | 0 | 0 | 0 | 0 | 1 | 1 (1.5) | 0 | 1 | 1 (0.2) |
| 82 | 0 | 0 | 0 | 0 | 1 | 1 (1.5) | 0 | 1 | 1 (0.2) |
| Low-risk | |||||||||
| 42 | 1 | 0 | 1 (0.2) | 0 | 6 | 6 (8.8) | 1 | 6 | 7 (1.1) |
| 66 | 3 | 1 | 4 (0.7) | 1 | 0 | 1 (1.5) | 4 | 1 | 5 (0.8) |
| 6 | 2 | 1 | 3 (0.5) | 0 | 0 | 0 | 2 | 1 | 3 (0.5) |
| 40 | 0 | 3 | 3 (0.5) | 0 | 0 | 0 | 0 | 3 | 3 (0.5) |
| 67 | 2 | 0 | 2 (0.3) | 0 | 1 | 1 (1.5) | 2 | 1 | 3 (0.5) |
| 43 | 1 | 1 | 2 (0.3) | 0 | 0 | 0 | 1 | 1 | 2 (0.3) |
| 53 | 0 | 1 | 1 (0.2) | 1 | 0 | 1 (1.5) | 1 | 1 | 2 (0.3) |
| 44 | 1 | 0 | 1 (0.2) | 0 | 0 | 0 | 1 | 0 | 1 (0.2) |
| 54 | 0 | 1 | 1 (0.2) | 0 | 0 | 0 | 0 | 1 | 1 (0.2) |
| 55 | 0 | 0 | 0 | 0 | 1 | 1 (1.5) | 0 | 1 | 1 (0.2) |
| 64 | 0 | 1 | 1 (0.2) | 0 | 0 | 0 | 0 | 1 | 1 (0.2) |
Includes all histologically confirmed cervical intraepithelial neoplasia (CIN)1 and worse;
Among 12 women with CIN3, six were positive for HPV16, one for HPV58, one each for HPV16 and 45, HPV16 and 68, HPV45 and 52, HPV51 and 59; one woman with squamous cell carcinoma was HPV16 positive.
High-risk HPV types were substantially more frequent (12.2% of all women) than low-risk types (3.8%). The most common types in either single- or multiple-type infections were HPV16 (5.7%), 58 (3.2%), 52 (1.2%), 33 (1.2%), 42 (a low-risk type, 1.1%), but HPV type distribution varied by the presence of cervical abnormalities. High-risk HPV types were found in 54.4% of women with cervical abnormalities and in 11 out of 12 (91.7%) women with CIN3. The only woman identified with squamous cell carcinoma was HPV16 positive.
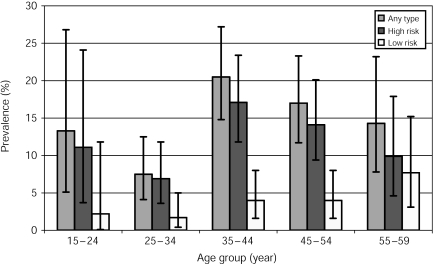
Figure 1 and Table 2 show the prevalence of HPV (any type, high- and low-risk types, separately) by age group. No significant trend in prevalence by age group emerged for any HPV type, nor for high-risk HPV types. Low-risk HPV types were found very rarely among women younger than 35 years, but their frequency increased significantly with age (P for trend=0.03). For both high-risk and low-risk types, lowest prevalence was found in the 25–34 year age group. Table 2 also shows the association between the presence of cervical abnormalities and different HPV types. Positivity for high-risk HPV types was strongly associated with increasing cervical lesion severity (OR for CIN3 vs normal=136; 95% CI: 17.2–1082). Positivity for low-risk HPV types was associated with CIN1 and CIN2, but not CIN3 (Table 2).
Figure 1.
Age-specific prevalence of cervical HPV DNA and corresponding 95% CI. Shanxi Province, China, 2004.
Table 2. Detection of cervical HPV any type, high-risk types and low-risk types according to age and the presence of cervical abnormalities among 662 women. Shanxi Province, China, 2004.
|
HPV positive
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
Any
|
High riska
|
Low riskb
|
||||||||
| Total no. | No. | % | OR (95% CI)c | No. | % | OR (95% CI)c | No. | % | OR (95% CI)c | |
| Age (years) | ||||||||||
| 15–24 | 45 | 6 | 13.3 | 1.9 (0.7–5.3) | 5 | 11.1 | 1.7 (0.6–5.0) | 1 | 2.2 | 1.3 (0.1–12.7) |
| 25–34d | 173 | 13 | 7.5 | 1 | 12 | 6.9 | 1 | 3 | 1.7 | 1 |
| 35–44 | 176 | 36 | 20.5 | 3.2 (1.6–6.2) | 30 | 17.1 | 2.8 (1.4–5.6) | 7 | 4.0 | 2.3 (0.6–9.2) |
| 45–54 | 177 | 30 | 17.0 | 2.5 (1.3–5.0) | 25 | 14.1 | 2.2 (1.1–4.5) | 7 | 4.0 | 2.3 (0.6–9.2) |
| 55–59 | 91 | 13 | 14.3 | 2.1 (0.9–4.6) | 9 | 9.9 | 1.5 (0.6–3.6) | 7 | 7.7 | 4.7 (1.2–18.7) |
| p for trend | =0.11 | =0.34 | =0.03 | |||||||
| Presence of cervical abnormalities | ||||||||||
| Normald | 594 | 57 | 9.6 | 1 | 44 | 7.4 | 1 | 16 | 2.7 | 1 |
| CIN1 | 39 | 18 | 46.2 | 7.6 (3.7–15.4) | 16 | 41.0 | 8.6 (4.1–18.0) | 6 | 15.4 | 5.5 (2.0–15.5) |
| CIN2 | 16 | 11 | 68.8 | 22.0 (7.2–67.4) | 9 | 56.3 | 16.8 (5.8–48.4) | 3 | 18.8 | 8.8 (2.2–35.1) |
| CIN3 and worse | 13 | 12 | 92.3 | 102 (12.8–803) | 12 | 92.3 | 136 (17.2–1082) | 0 | 0 | 0 (0–11.4) |
| p for trend | <0.001 | <0.001 | =0.007 | |||||||
Includes that in multiple infections with low-risk types.
Includes that in multiple infections with high-risk types.
Adjusted for age (10-year groups);
Reference category. OR=odds ratio; CI=confidence interval; CIN=cervical intraepithelial neoplasia.
Table 3 shows the relationship between HPV positivity and various characteristics of study women after adjustment for age. No significant association was found between HPV positivity and education level, age at first sexual intercourse, lifetime number of sexual partners, husband's extramarital sexual relationships, age at menarche or menopause, number of births, or the use of the two most widespread contraceptive methods in the study area (i.e. intrauterine device and tubal ligation). Only 17.7% of study women reported two or more sexual partners, and 24.2% reported sexual debut before age 19 years. No significant associations arose when we evaluated correlates of high-risk HPV positivity only (data not shown).
Table 3. Detection of cervical HPV DNA according to selected characteristics among 662 women. Shanxi, China, 2004.
|
HPV DNA positive
|
||||
|---|---|---|---|---|
| Characteristics | No. of women | No. | % | Age-adjusted OR (95% CI)a |
| Education | ||||
| Illiterate or primaryb | 247 | 37 | 15.0 | 1 |
| Junior high school | 336 | 51 | 15.2 | 1.0 (0.6–1.7) |
| Senior high school and above | 79 | 10 | 12.7 | 0.8 (0.3–1.7) |
| p for trend=0.64 | ||||
| Age at first sexual intercourse (years) | ||||
| ⩾21b | 209 | 34 | 16.3 | 1 |
| 19–20 | 293 | 47 | 16.0 | 0.9 (0.6–1.5) |
| ⩽18 | 160 | 17 | 10.6 | 0.5 (0.2–1.0) |
| p for trend=0.08 | ||||
| Lifetime number of sexual partners | ||||
| 1b | 545 | 85 | 15.6 | 1 |
| ⩾2 | 117 | 13 | 11.1 | 0.7 (0.3–1.2) |
| Husband's extramarital sexual relationships | ||||
| Nob | 583 | 88 | 15.1 | 1 |
| Yes | 79 | 10 | 12.7 | 0.8 (0.4–1.7) |
| Age at menarche (years) | ||||
| ⩽14b | 235 | 36 | 15.3 | 1 |
| 15–16 | 225 | 32 | 14.2 | 0.7 (0.4–1.2) |
| ⩾17 | 202 | 30 | 14.9 | 0.7 (0.4–1.2) |
| p for trend=0.18 | ||||
| Menopausal statusc | ||||
| Premenopauseb | 90 | 18 | 20.0 | 1 |
| Menopause <50 years old | 106 | 17 | 16.0 | 0.7 (0.3–1.7) |
| Menopause ⩾50 years old | 72 | 8 | 11.1 | 0.5 (0.2–1.3) |
| No. of births | ||||
| 0 | 15 | 1 | 6.7 | 0.7 (0.1–5.7) |
| 1b | 154 | 16 | 10.4 | 1 |
| 2 | 336 | 54 | 16.1 | 1.3 (0.6–2.7) |
| ⩾3 | 157 | 27 | 17.2 | 1.5 (0.6–3.7) |
| p for trend=0.31 | ||||
| Intrauterine device use | ||||
| Neverb | 308 | 44 | 14.3 | 1 |
| Ever | 354 | 54 | 15.3 | 1.1 (0.7–1.7) |
| Tubal ligation | ||||
| Nob | 284 | 33 | 11.6 | 1 |
| Yes | 378 | 65 | 17.2 | 1.4 (0.8–2.3) |
Adjusted for age (10-year groups);
Reference category;
Women ⩾45 years only (n=268). OR=odds ratio; CI=confidence interval.
Other characteristics that were examined and found not to be associated with HPV positivity included multiple marriages (50 women, OR=0.4; 95% CI: 0.2–1.3), being a widow (24 women, OR=1.1; 95% CI: 0.3–3.2), history of spontaneous abortion (98 women, OR=0.9; 95% CI: 0.5–1.7), and induced abortion (241 women, OR=0.8; 95% CI: 0.5–1.2). No study woman reported divorce or cigarette smoking. Use of oral contraceptives and condom was rare (21 and 14 women, respectively) and was associated with ORs of 2.0 (95% CI: 0.7–5.4) and 0.5 (95% CI: 0.1–3.7), respectively. Inflammation and/or infection upon colposcopy was found in 55% of women, but was not significantly associated with HPV infection (OR 1.3, 95% CI: 0.8–2.0). In all, 64 women reported to have had at least one previous cytological smear (OR for HPV positivity=0.5; 95% CI: 0.2–1.2, data not shown).
DISCUSSION
Relative to previous population-based surveys of HPV worldwide, noteworthy findings from Shanxi Province, China, include a high prevalence of HPV infection, a marked predominance of HPV16 among HPV-positive women, and the lack of an observable decline in HPV prevalence with increasing age.
Age-standardised HPV prevalence in Shanxi Province was found to be similar to that found in other high-risk areas for cervical cancer studied by IARC using the same HPV testing protocol in Latin America (Molano et al, 2002; Matos et al, 2003; Ferreccio et al, 2004) and India (Franceschi et al, 2005), although lower than in some areas of sub-Saharan Africa (Thomas et al, 2004). In contrast to previous studies in high-resource countries (Peto et al, 2004), however, no decline of HPV prevalence was seen with increasing age, and highest HPV prevalence was found among middle-aged women. This age pattern is similar to the flat age curve seen in rural India (Franceschi et al, 2005) and Africa (Thomas et al, 2004; Clifford & Franceschi, 2005).
Large cohort studies in Colombia (Muñoz et al, 2004) and Costa Rica (Castle et al, 2005) have shown that new HPV infection in middle-aged women is not rare, but, Castle et al (2005) suggested a stronger role for HPV persistence than acquisition of new infection in determining HPV prevalence in women over 45 years of age. Thus, high HPV prevalence in middle-aged women in Shanxi Province may indicate a relative lack of clearance or high frequency of reactivation of HPV infection. Furthermore, in conservative societies like rural China, it is likely that young women are not more frequently exposed to HPV than middle-aged women. Indeed, we observed no differences in indicators of sexual behaviour (e.g. age at first sexual intercourse (19.4 and 19.0 years, respectively) or frequency of husband's extramarital sexual relationships (15.2 and 14.0%, respectively)) among women aged under 25 years and over 45 years.
Our survey is the first population-based study in China to assess HPV type-specific prevalence across a broad age-range of women. It showed that HPV16 is the most frequently found type in Shanxi Province, affecting 38.8% of HPV-positive women. The corresponding percentages in other IARC HPV prevalence surveys in different continents were 32.6% in Europe, 22.5% in Asia, 22.0% in Latin America, and 12.2% in Africa (Clifford et al, 2005), making the proportion of HPV16-positive women in our present population-based study among the highest recorded worldwide. In a study of 809 cervical cancer cases from four areas in China, a relatively high proportion (66.9%) were positive for HPV16 (Lo et al, 2002).
HPV58 was the second most common HPV type in Shanxi Province, and was found in one quarter of all HPV-positive cervical abnormalities, confirming the importance of HPV58 infection in Asia (Chan, 2005). Studies of cervical cancer cases from China have reported varying HPV58 prevalence between 2.9 and 27.5% (Clifford et al, 2003). The distribution of other commonly identified HPV types in Shanxi Province (e.g. HPV18, 33 and 42), largely mimics those reported in a previous pooled analysis of IARC HPV prevalence surveys (Clifford et al, 2005).
No questionnaire-based information, including sexual habit indicators, reproductive and menstrual characteristics, oral contraceptive or condom use, showed any significant associations with HPV positivity. This may be partly related to the fact that this study took place in a rather homogenous, low-income rural population.
Our present study has strengths and limitations. Among the former, the use of highly sensitive PCR assays and enrolment of a representative sample of the married female population. A high proportion of histological confirmation was also an asset, allowing a clear distinction between the similar potential of high-risk and low-risk types to induce CIN1 and 2 but not CIN3. The main limitation of the present study was that only approximately half of the initially invited population had cells collected for HPV testing. Of the nonparticipants that were interviewed, a large proportion was unmarried. Indeed HPV testing was offered only to married women, who were expected to be a reasonably good proxy of sexually active women in this conservative society. Furthermore, as HPV infection is asymptomatic and does not show, contrary to cervical cancer (Rajkumar et al, 2003), any strong socio-economic gradient, it is unlikely that participation was related to women's HPV infection status.
In conclusion, our study allowed the disclosure of a high prevalence of HPV and an age-specific prevalence curve that is in sharp contrast with the peak in HPV prevalence below age 25 years observed in Europe (de Sanjosé et al, 2003; Peto et al, 2004; Ronco et al, 2005) and North America (Bauer et al, 1993).
Acknowledgments
Financial support was received from the Bill & Melinda Gates Foundation (Grant number 35537). We thank Annie Arslan for data management, statistical analysis and useful comments on the draft, and Trudy Pedrix-Thoma for technical assistance.
References
- Anh PTH, Hieu NT, Herrero R, Vaccarella S, Smith JS, Thuy NT, Nga NH, Duc NB, Ashley R, Snijders PJF, Meijer CJLM, Muñoz N, Parkin DM, Franceschi S (2003) Human papillomavirus infection among women in South and North Vietnam. Int J Cancer 104: 213–220 [DOI] [PubMed] [Google Scholar]
- Bauer HM, Hildesheim A, Schiffman MH, Glass AG, Rush BB, Scott DR, Cadell DM, Kurman RJ, Manos MM (1993) Determinants of genital human papillomavirus infection in low-risk women in Portland, Oregon. Sex Transm Dis 20: 274–278 [DOI] [PubMed] [Google Scholar]
- Belinson JL, Qiao YL, Pretorius RG, Zhang WH, Rong SD, Huang MN, Zhao FH, Wu LY, Ren SD, Huang RD, Washington MF, Pan QJ, Li L, Fife D (2003) Shanxi Province cervical cancer screening study II: self-sampling for high-risk human papillomavirus compared to direct sampling for human papillomavirus and liquid based cervical cytology. Int J Gynecol Cancer 13: 819–826 [DOI] [PubMed] [Google Scholar]
- Castle PE, Schiffman M, Herrero R, Hildesheim A, Rodriguez AC, Bratti MC, Sherman ME, Wacholder S, Tarone R, Burk RD (2005) A prospective study of age trends in cervical human papillomavirus acquisition and persistence in Guanacaste, Costa Rica. J Infect Dis 191: 1808–1816 [DOI] [PubMed] [Google Scholar]
- Chan PKS (2005) Epidemiology of human papillomavirus in Asia: do HPV-52 and HPV-58 play a special role? Papillomavirus Rep 16: 265–271 [Google Scholar]
- Clifford GM, Smith JS, Plummer M, Muñoz N, Franceschi S (2003) Human papillomavirus types in invasive cervical cancer worldwide: a meta-analysis. Br J Cancer 88: 63–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clifford GM, Franceschi S (2005) HPV in sub-Saharan Africa (editorial). Papillomavirus Rep 16: 322–326 [Google Scholar]
- Clifford GM, Gallus S, Herrero R, Muñoz N, Snijders PJF, Vaccarella S, Anh PTH, Ferreccio C, Hieu NT, Matos E, Molano M, Rajkumar R, Ronco G, de Sanjosé S, Shin HR, Sukvirach S, Thomas JO, Tunsakul S, Meijer CJLM, Franceschi S (2005) Worldwide distribution of human papillomavirus types in cytologically normal women in the International Agency for Research on Cancer HPV prevalence surveys: a pooled analysis. Lancet 366: 991–998 [DOI] [PubMed] [Google Scholar]
- de Sanjosé S, Almirall R, Lloveras B, Font R, Diaz M, Muñoz N, Catala I, Meijer CJLM, Snijders PJF, Herrero R, Bosch FX (2003) Cervical human papillomavirus infection in the female population in Barcelona, Spain. Sex Transm Dis 30: 788–793 [DOI] [PubMed] [Google Scholar]
- Ferreccio C, Prado RB, Luzoro AV, Ampuero SL, Snijders PJF, Meijer CJLM, Vaccarella S, Jara AT, Puschel KI, Robles SC, Herrero R, Franceschi S, Ojeda JM (2004) Population-based prevalence and age distribution of human papillomavirus among women in Santiago, Chile. Cancer Epidemiol Biomarkers Prev 13: 2271–2276 [PubMed] [Google Scholar]
- Franceschi S, Rajkumar R, Snijders PJF, Arslan A, Mahé C, Plummer M, Sankaranarayanan R, Cherian J, Meijer CJLM, Weiderpass E (2005) Papillomavirus infection in rural women in southern India. Br J Cancer 92: 601–606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs MV, Walboomers JM, Snijders PJF, Voorhorst FJ, Verheijen RH, Fransen-Daalmeijer N, Meijer CJLM (2000) Distribution of 37 mucosotropic HPV types in women with cytologically normal cervical smears: the age-related patterns for high-risk and low-risk types. Int J Cancer 87: 221–227 [PubMed] [Google Scholar]
- Lazcano-Ponce E, Herrero R, Muñoz N, Cruz A, Shah KV, Alonso P, Hernández P, Salmeron J, Hernández M (2001) Epidemiology of HPV infection among Mexican women with normal cervical cytology. Int J Cancer 91: 412–420 [DOI] [PubMed] [Google Scholar]
- Lo KW, Wong YF, Chan MK, Li JC, Poon JS, Wang VW, Zhu SN, Zhang TM, He ZG, Wu QL, Li GD, Tam JS, Kahn T, Lam P, Cheung TH, Chung TK (2002) Prevalence of human papillomavirus in cervical cancer: a multicenter study in China. Int J Cancer 100: 327–331 [DOI] [PubMed] [Google Scholar]
- Matos E, Loria D, Amestoy G, Herrera L, Prince MA, Moreno J, Krunfly C, van den Brule AJ, Meijer CJLM, Muñoz N, Herrero R, Proyecto Concordia Collaborative Group (2003) Prevalence of human papillomavirus infection among women in Concordia, Argentina: a population-based study. Sex Transm Dis 30: 593–599 [DOI] [PubMed] [Google Scholar]
- Molano M, Posso H, Weiderpass E, van den Brule AJ, Ronderos M, Franceschi S, Meijer CJLM, Arslan A, Muñoz N (2002) Prevalence and determinants of HPV infection among Colombian women with normal cytology. Br J Cancer 87: 324–333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muñoz N, Bosch FX, de Sanjosé S, Herrero R, Castellsagué X, Shah KV, Snijders PJF, Meijer CJLM (2003) Epidemiologic classification of human papillomavirus types associated with cervical cancer. N Engl J Med 348: 518–527 [DOI] [PubMed] [Google Scholar]
- Muñoz N, Mendez F, Posso H, Molano M, van den Brule AJ, Ronderos M, Meijer CJLM, Muñoz A, for the Instituto Nacional de Cancerología HPV Study Group (2004) Incidence, duration, and determinants of cervical human papillomavirus infection in a cohort of Colombian women with normal cytological results. J Infect Dis 190: 2077–2087 [DOI] [PubMed] [Google Scholar]
- Parkin DM, Whelan SL, Ferlay J, Thomas DB, Teppo L (2002) Cancer Incidence in Five Continents, Vol. VIII. IARC Scientific Publications No. 155 IARC Press: Lyon [Google Scholar]
- Peto J, Gilham C, Deacon J, Taylor C, Evans C, Binns W, Haywood M, Elanko N, Coleman D, Yule R, Desai M (2004) Cervical HPV infection and neoplasia in a large population-based prospective study: the Manchester cohort. Br J Cancer 91: 942–953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajkumar T, Franceschi S, Vaccarella S, Gajalakshmi V, Sharmila A, Snijders PJF, Muñoz N, Meijer CJLM, Herrero R (2003) Role of paan chewing and dietary habits in cervical carcinoma in Chennai, India. Br J Cancer 88: 1388–1393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roda Husman AM, Snijders PJF, Stel HV, van den Brule AJ, Meijer CJLM, Walboomers JM (1995) Processing of long-stored archival cervical smears for human papillomavirus detection by the polymerase chain reaction. Br J Cancer 72: 412–417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronco G, Ghisetti V, Segnan N, Snijders PJF, Gillio-Tos A, Meijer CJ, Merletti F, Franceschi S (2005) Prevalence of human papillomavirus infection in women in Turin, Italy. Eur J Cancer 41: 297–305 [DOI] [PubMed] [Google Scholar]
- Shin HR, Franceschi S, Vaccarella S, Roh JW, Ju YH, Oh JK, Kong HJ, Rha SH, Jung SI, Kim JI, Jung KY, van Doorn LJ, Quint W (2004) Prevalence and determinants of genital infection with papillomavirus, in female and male university students in Busan, South Korea. J Infect Dis 190: 468–476 [DOI] [PubMed] [Google Scholar]
- Shin HR, Lee DH, Herrero R, Smith JS, Vaccarella S, Hong SH, Jung KY, Kim HH, Park UD, Cha HS, Park S, Touze A, Muñoz N, Snijders PJF, Meijer CJLM, Coursaget P, Franceschi S (2003) Prevalence of human papillomavirus infection in women in Busan, South Korea. Int J Cancer 103: 413–421 [DOI] [PubMed] [Google Scholar]
- Sukvirach S, Smith JS, Tunsakul S, Muñoz N, Kesararat V, Opasatian O, Chichareon S, Kaenploy V, Ashley R, Meijer CJLM, Snijders PJF, Coursaget P, Franceschi S, Herrero R (2003) Population-based human papillomavirus prevalence in Lampang and Songkla, Thailand. J Infect Dis 187: 1246–1256 [DOI] [PubMed] [Google Scholar]
- Thomas JO, Herrero R, Omigbodun AA, Ojemakinde K, Ajayi IO, Fawole A, Oladepo O, Smith JS, Arslan A, Muñoz N, Snijders PJF, Meijer CJLM, Franceschi S (2004) Prevalence of papillomavirus infection in women in Ibadan, Nigeria: a population-based study. Br J Cancer 90: 638–645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Brule AJ, Pol R, Fransen-Daalmeijer N, Schouls LM, Meijer CJLM, Snijders PJF (2002) GP5+/6+ PCR followed by reverse line blot analysis enables rapid and high-throughput identification of human papillomavirus genotypes. J Clin Microbiol 40: 779–787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Huangpu XM, Zhang SW, Lu FZ, Sun XD, Sun J, Mu R, Li LD, Qiao YL (2003a) Changes of mortality rate for cervical cancer during 1970's and 1990's periods in China. Acta Acad Med Sin 25: 386–390 [PubMed] [Google Scholar]
- Yang L, Parkin DM, Li L, Chen Y (2003b) Time trends in cancer mortality in China: 1987–1999. Int J Cancer 106: 771–783 [DOI] [PubMed] [Google Scholar]
- Zhao FH, Forman MR, Belinson J, Shen YH, Graubard BI, Patel AC, Rong SD, Pretorius RG, Qiao YL (2006) Risk factors for HPV infection and cervical cancer among unscreened women in a high-risk rural area of China. Int J Cancer 118: 442–448 [DOI] [PubMed] [Google Scholar]