Abstract
Aldosterone-dependent epithelial sodium transport in the distal nephron is mediated by the absorption of sodium through the highly selective, amiloride-sensitive epithelial sodium channel (ENaC) made of three homologous subunits (α, β, and γ). In human, autosomal recessive mutations of α, β, or γENaC subunits cause pseudohypoaldosteronism type 1 (PHA-1), a renal salt-wasting syndrome characterized by severe hypovolemia, high plasma aldosterone, hyponatremia, life-threatening hyperkaliemia, and metabolic acidosis. In the mouse, inactivation of αENaC results in failure to clear fetal lung liquid at birth and in early neonatal death, preventing the observation of a PHA-1 renal phenotype. Transgenic expression of αENaC driven by a cytomegalovirus promoter in αENaC(−/−) knockout mice [αENaC(−/−)Tg] rescued the perinatal lethal pulmonary phenotype and partially restored Na+ transport in renal, colonic, and pulmonary epithelia. At days 5–9, however, αENaC(−/−)Tg mice showed clinical features of severe PHA-1 with metabolic acidosis, urinary salt-wasting, growth retardation, and 50% mortality. Adult αENaC(−/−)Tg survivors exhibited a compensated PHA-1 with normal acid/base and electrolyte values but 6-fold elevation of plasma aldosterone compared with wild-type littermate controls. We conclude that partial restoration of ENaC-mediated Na+ absorption in this transgenic mouse results in a mouse model for PHA-1.
In mammals, sodium homeostasis is mainly maintained by regulation of electrogenic sodium reabsorption in kidney. Sodium is reabsorbed from urine through highly selective, amiloride-sensitive sodium channels (ENaC) (1). Located in the apical membrane of cortical collecting duct cells in the distal nephron (2), ENaC constitutes the limiting step for transepithelial sodium transport whereas the Na, K-ATPase located in the basolateral membrane provides the driving force (3). Electrogenic sodium transport is controlled by aldosterone, thereby promoting sodium reabsorption and potassium excretion by aldosterone-target cells like in the distal nephron or the distal part of the colon (2). There is a tight relationship among sodium balance, extracellular fluid volume, plasma aldosterone levels, and blood pressure. After a normal or high salt diet, plasma concentrations of aldosterone are low, and ENaC-mediated electrogenic sodium transport is not detectable, as shown in rat (4). By contrast, in rats maintained on a low salt diet, plasma concentration of aldosterone increases rapidly, and electrogenic sodium transport is induced. A similar response is well documented in distal colon (5). Despite large changes in water and salt intake, this precise hormonal regulation maintains the extracellular osmolality and blood volume within narrow margins (6).
Genetic evidence for direct involvement of ENaC in sodium balance came from the observation that mutations in subunits of ENaC cause two human genetic diseases, Liddle syndrome and the autosomal recessive form of pseudohypoaldosteronism (PHA-1). Liddle syndrome is characterized by early onset of hypertension associated with normal plasma aldosterone levels whereas PHA-1 is a salt-wasting syndrome with hypotension and high plasma aldosterone levels and mineralocorticoid resistance in kidney, sweat, and salivary glands and in colon mucosa (7). PHA-1 patients are therefore treated with sodium and bicarbonate supplementation. Expression analysis of ENaC mutations in Xenopus oocytes revealed constitutive activation of ENaC in Liddle syndrome (8) and reduced ENaC activity in PHA-1 patients (9, 10).
In contrast to the kidney and colon, ENaC-mediated sodium transport in the lung is glucocorticoid-sensitive but aldosterone-insensitive (11, 12). Independent of salt diet and plasma aldosterone, a basal constitutive level of sodium transport appears to be required for proper lung fluid clearance from birth to adulthood. In the lung, sodium reabsorption plays an important role at birth, when the pulmonary epithelium switches from a basal net secretory to a basal net reabsorptive mode. In fetal lung, all three subunits (α, β, and γENaC) are developmentally regulated and can be induced by in vivo administration of glucocorticoid hormones (13, 14). The critical role for ENaC in lung liquid clearance was shown in knockout mice in which the gene locus of α subunit of ENaC was inactivated by homologous recombination in embryonic stem cells, thereby abolishing functioning of the channel (15). Newborn mice deficient for αENaC died soon after birth with water-filled lungs. The PHA-1 renal salt-wasting phenotype was not observed in αENaC(−/−) mice, presumably because the neonates died too early from respiratory distress syndrome.
To distinguish the physiological importance of constitutive vs. aldosterone-dependent sodium transport by ENaC in lung, kidney, and colon, we generated transgenic mice carrying the α subunit of ENaC under the control of an ubiquitously and constitutively expressed cytomegalovirus promoter on an αENaC knockout background. The resulting mice [αENaC(−/−)Tg] had sufficient basal Na+ absorptive capacity to clear lung liquid and survive the early neonatal period but developed a phenotype similar to PHA-1 with salt-wasting, metabolic acidosis, high aldosterone levels, growth retardation, and increased early mortality.
MATERIALS AND METHODS
Generation and Analysis of Transgenic Mice.
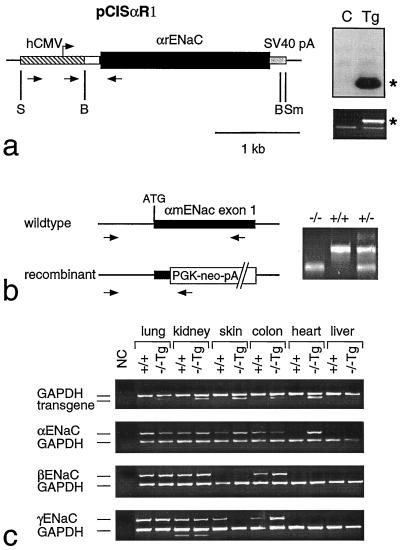
The transgene vector pCisαR1 contains the cytomegalovirus promoter (16), an IgE intron (100 mer), the SalI–EcoRV αrENaC fragment (position −78 to +2149; ref. 17), and simian virus 40 large T antigen polyadenylation sequences. The insert was excised as a 3.3-kb SpeI–SmaI fragment and microinjected into fertilized oocytes (NMR1 mice). Founder animals were identified by Southern blotting and/or PCR-based analysis of tail DNA as described previously (refs. 15 and 18; Fig. 1a). Gender of newborn mice was determined by using Y-specific primers (19). To generate transgenic knockout mice [αENaC(−/−)Tg], αENaC heterozygous mutant mice (+/−) were bred to transgenic pCisαR1 mice and genotyped (see Fig. 1b). Detailed sequences for the primers are available on request. Reverse transcription-PCR was performed as described (18) and was controlled by detection of glyceraldehyde-3-phosphate dehydrogenase message (Fig. 1c). In situ analysis of αENaC mRNA was performed as described (20) using a 35S-labeled antisense riboprobe of rat αENaC subunit (position +90–542; ref. 17). Control hybridization was performed with a sense αENaC riboprobe.
Figure 1.
(a) Generation of αENaC(−/−)Tg mice. (Left) pCisαR1 transgene vector containing human cytomegalovirus promoter (hatched box) with an artificial intron (open box), rat αENaC cDNA (black box), and simian virus 40 large T poly(A) (stippled box). S = SpeI; B = BamHI; Sm = SmaI. (Right, Upper) Southern blot of BamHI-digested DNA from transgenic mouse (Tg; line 1352) and nontransgenic control (C) littermate probed with αrENaC cDNA. ∗, transgene-specific fragment. (Right, Lower) PCR-based genotyping for the transgene (651 bp*) using a set of specific primers as indicated in a. (b) PCR-based genotyping for the gene targeting status (−/−, +/+, +/−) using specific primers (arrows). (c) Detection of mRNA transcripts (reverse transcription-PCR) for transgene (507 bp; primers indicated in a), αENaC, βmENaC, and γmENaC in transgenic homozygous mutant [αENaC(−/−)Tg] mice and wild-type αENaC(+/+) mice using specific intron-spanning primers (15). γENaC mRNA transcript in skin of αENaC(−/−)Tg mice is visible on the original.
Analytical Procedures.
Blood values were determined from mixed blood after decapitation (newborns, 5- to 9-day-old pups) or from arterial blood (adult mice) under anesthesia. To measure plasma aldosterone and corticosterone concentrations in adult αENaC(+/+) and (−/−)Tg mice, blood was collected into ice-cold microcentrifuge tubes (containing EDTA), which were centrifuged to isolate plasma. Aldosterone and corticosterone assays were run in duplicate on plasma samples. Corticosterone was determined using an 125I RIA (detection limit 10 ng/ml). Aldosterone was determined by 125I RIA (detection limit 6 pg/ml; Biodata, Cortland Manor, NY). To measure urinary Na+/K+ from newborns and 5- to 9-day-old pups, samples of urine were diluted into 0.1 N of nitric acid and analyzed for Na+ and K+ by flame emission photometry. Urinary electrolytes in adult αENaC(+/+) and (−/−)Tg mice were measured and normalized with creatinine.
Electrophysiological Studies.
Bioelectric measurements were performed on fetal tracheal explants, freshly excised adult tracheal and nasal epithelia, freshly excised colon epithelium, and rectal epithelium in vivo. Fetal tracheal explants were cultured for 6–8 days in F12 and 10% fetal bovine serum (21). Transepithelial bioelectric potential difference (PD) was measured by microelectrode puncture. Na+ transport was determined by inhibition of basal PD after amiloride microinjection (10−4 M) as described (21). The short circuit current (ISC) generated by freshly excised adult tracheal and nasal tissues and colon epithelium was measured in Ussing chambers as described (22).
Sodium transport across the rectal epithelium was estimated in vivo, under anesthesia, as the amiloride-sensitive rectal PD. Rectal PD was measured between a reference electrode placed in the s.c. tissue of the abdominal wall and a double-barreled glass pipette placed in the rectal lumen. The first barrel of the rectal pipette was filled with saline (isotonic NaCl buffered with 10 mM Na+ Hepes, pH 7.2) and was connected to Ag/AgCl electrodes via plastic tubing filled with 3 M KCl in 2% agar (Q.W., E.H., M.B., J.-D.H., unpublished work); the second barrel was filled with the same solution containing 25 μM amiloride. Rectal PD was measured after injection of 50 μl of control saline through the first barrel and after injection of the same volume of the amiloride-containing solution through the second barrel.
RESULTS
Rescue of Lung Phenotype.
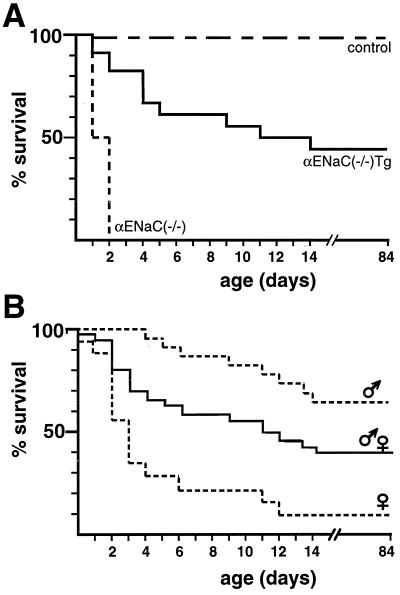
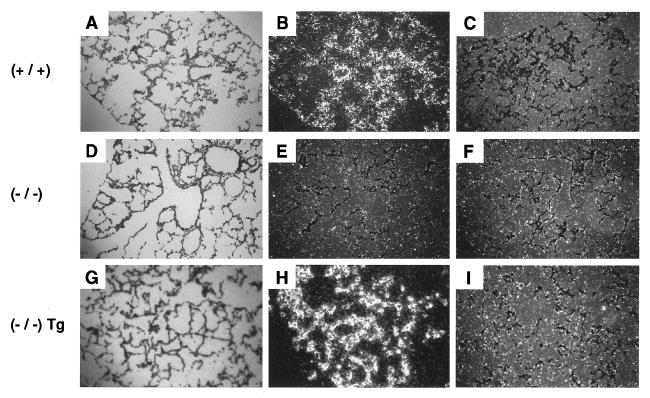
Transgenic αrENaC mRNA expression in αmENaC(−/−)Tg mice was identified in lung, kidney, skin, colon, and heart. Transcripts of β and γmENaC subunits were present in αENaC(−/−)Tg and wild-type (+/+) animals (Fig. 1c). Transgenic αrENaC subunit expression in αENaC(−/−)Tg mice resulted in prolonged survival (Fig. 2). The survival curve was biphasic because 8/18 (44%) of αENaC(−/−)Tg mice (of 16 complete litters) died within the first 2 weeks of life (Fig. 2a). The overall survival rate among female αENaC(−/−)Tg mice was lower (11%) compared with male mice (64%; Fig. 2b), which might be explained by the X chromosomal localization of the transgene in that line leading to X inactivation of the transgene (pCisαR1) in female tissues (23). In situ hybridization analysis of neonatal lung (Fig. 3) showed transgenic αrENaC mRNA expression in lung airways and alveolar epithelium with a distribution and intensity of expression similar to that of wild-type mice.
Figure 2.
Survival of αENaC(−/−)Tg mice (A) Survival rate of αENaC(−/−)mice, αENaC(−/−)Tg mice, and control mice [αENaC(+/+) and αENaC(+/−) mice, transgenic and nontransgenic]. Sixteen complete litters from heterozygous breeding (in total 181 mice) were analyzed [αENaC(+/+), n = 29; αENaC(+/+) Tg, n = 26; αENaC(+/−), n = 59; αENaC(+/−) Tg, n = 39; αENaC(−/−), n = 10; and αENaC(−/−)Tg, n = 18]. Dead animals were collected and recorded daily. (B) Overall survival rate of male [14/22 (64%); n = 22] vs. female [2/18 (11%); n = 18] αENaC(−/−)Tg mice.
Figure 3.
In situ hybridization analysis in lung. Localization of αENaC mRNA and αrENaC transgene mRNA in lung of 12-h-old pups. Bright-field (A, D, and G) and dark-field (B, C, E, F, H, and I) photographs of sections from wild-type (+/+; A, B, and C), αENaC(−/−) (D, E, and F), and αENaC(−/−)Tg mice (G, H, and I) after hybridization with α antisense (B, E, and H) or α sense (C, F, and I) rENaC 35S-labeled probes.
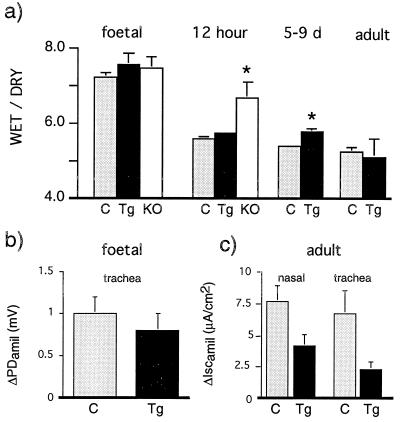
αENaC(−/−)Tg mice were able to clear fetal liquid (as determined by lung water content) from the air spaces at 12 h of age, when the lung has normally cleared most of the residual fetal liquid (Fig. 4a). ENaC-mediated Na+ transport measured in fetal trachea as amiloride-sensitive inhibition of PD (ΔPDamil) and in excised adult airway epithelia measured as amiloride-sensitive short circuit current (ΔIscamil) were slightly, but not significantly, decreased in αENaC(−/−)Tg mice compared with epithelia from control mice (Fig. 4 b and c). We therefore conclude that there was sufficient transgenic expression of αrENaC in αENaC(−/−)Tg mice to clear newborn lungs of fetal lung liquid and to maintain liquid balance in adult lung.
Figure 4.
Physiological measurements in lung. Control littermates (C, light gray column), αENaC(−/−)Tg mice (Tg, black column), and αENaC(−/−) mice (KO, white column) of different ages. (a) Mean whole lung water content (wet/dry ratio); 12 h: αENaC(−/−) vs. αENaC(−/−)Tg and control littermates, ∗, P < 0.05; 5–9 days: αENaC(−/−)Tg vs. control littermates, ∗, P < 0.05. (b) Amiloride-sensitive inhibition of potential difference (ΔPDamil) in tracheal explants from 19-day-old fetuses. αENaC(−/−)Tg, n = 7 vs. control littermates, n = 22. (c) Amiloride-sensitive short circuit current (ΔIscamil) in excised adult nasal and tracheal epithelium. αENaC(−/−)Tg, n = 4 vs. control littermates, n = 4.
After Birth, αENaC(−/−)Tg Mice Exhibit Clinical Features of Severe PHA-1.
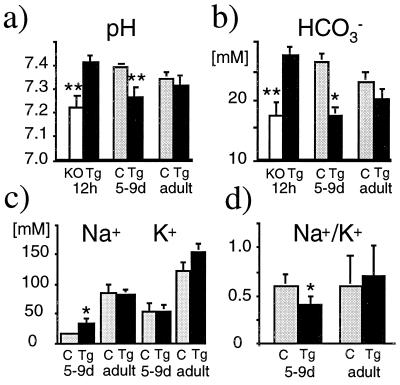
The decreased survival rate suggested metabolic imbalances in the transgenic knockout mice. Acid/base studies of mixed venous blood and measurements of urinary sodium and potassium in neonatal mice evaluated the effect of αENaC on renal function. From shortly after birth, αENaC(−/−)Tg mice were generally smaller (mean weight at 5 days: 1.55 ± 0.3 g, n = 4) than littermate controls (2.0 ± 0.2 g, n = 23). Twelve hours after birth, αENaC(−/−) mice showed significant metabolic acidosis whereas pH and bicarbonate concentrations in αENaC(−/−)Tg mice were normal (Fig. 5 a and b). However, by 5–9 days, αENaC(−/−)Tg mice showed significant urinary salt-wasting [Na+] with no significant decrease in urinary [K+] (Fig. 5c; P < 0.05). The urinary [Na+]/[K+] ratio was higher in the transgenic αENaC(−/−) mice as in controls (Fig. 5d; P < 0.05). In addition to the urinary salt wasting, 5- to 9-day-old αENaC(−/−)Tg mice showed metabolic acidosis (Fig. 5 a and b). Because the clinical signs of a salt-wasting syndrome were not obvious in newborn αENaC(−/−)Tg mice, we suggest that the metabolic consequences of abnormal renal Na+ absorption in αENaC(−/−)Tg mice take ≈5 days to become apparent.
Figure 5.
Physiological parameters in αENaC(−/−)Tg. pH (a) and bicarbonate measurements (b) and urinary Na+ and K+ measurements (c and d) in control littermates (C, light gray column), αENaC(−/−)Tg mice (Tg, black column), and αENaC(−/−) mice (KO, white column) of different ages. ∗, P < 0.05; ∗∗, P < 0.01.
Adult αENaC(−/−)Tg Exhibit a Compensated PHA-1.
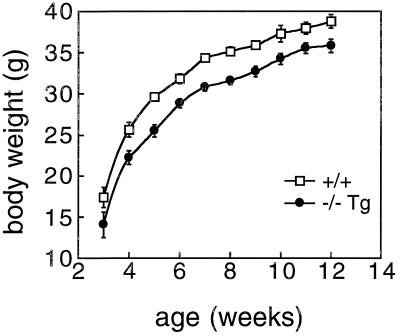
The αENaC(−/−)Tg adult survivors showed compensation of the acid/base imbalances and electrolyte disturbances that were seen in the neonatal group. Adult αENaC(−/−)Tg mice exhibited normal values in blood (Fig. 5 a and b), serum Na+ [αENaC(−/−)Tg; n = 8: 152.8 ± 2.0 mM vs. 152.3 ± 1.1 mM; n = 8 in wild-type mice] and serum K+ [αENaC(−/−)Tg; n = 8: 5.8 ± 0.4 mM vs. 5.3 ± 0.3 mM; n = 8 in wild-type mice], and of urine electrolytes [Na+ and K+] (Fig. 5 c and d), suggesting that there was a spontaneous improvement in renal Na+ absorption over time. Adult αENaC(−/−)Tg mice were smaller (mouse weight at 12 weeks: 35.8 ± 0.8 g, n = 15; wild-type mice: 38.8 ± 0.9 g, n = 16; P < 0.05) although the rate of growth in the two groups was similar after the 2nd week (Fig. 6).
Figure 6.
Growth curve of αENaC(−/−)Tg mice. Growth curve of male αENaC(−/−)Tg mice (closed circles) and male littermate controls (+/+; open squares). Mice were weighed once a week on the same day and time.
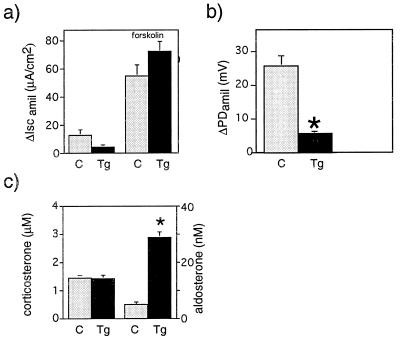
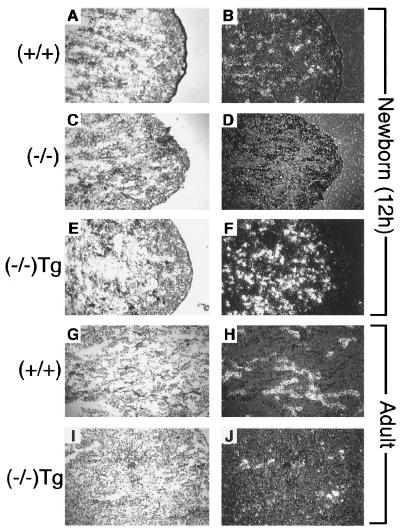
In colon, αENaC(−/−)Tg fetuses (19 days old) exhibited lower, but not significantly different, levels of amiloride-sensitive short circuit current (ΔIscamil; Fig. 7a). Responses to intracellular cAMP agonist, forskolin, were similar in both groups. In adults, ENaC-mediated sodium transport across the rectal epithelium was significantly decreased. In vivo measurements of colon PD in adults revealed a significant lower amiloride-sensitive PD compared with wild-type littermates [αENaC(+/+); Fig. 7b; P < 0.001]. This decrease in ENaC-mediated sodium transport occurred in the context of a significant 6-fold elevation of aldosterone levels in αENaC(−/−)Tg mice compared with the wild-type littermates (P < 0.001; Fig. 7c) whereas serum corticosterone levels were not different in both groups (Fig. 7c). In neonates, mRNA levels of the transgenic αrENaC seem to be in the same range (or even higher) as the endogenous αENaC (Fig. 8 B and F), whereas they are lower in the adult (Fig. 8 H and J). Northern blot analysis of adult lung, kidney, and colon revealed lower mRNA levels of the transgene compared with the endogene (data not shown). We suggest that, in surviving αENaC(−/−)Tg, transgene expression is sufficient to restore ENaC-mediated transport in lung but not in kidney or colon.
Figure 7.
Bioelectric measurements (a and b) and hormone levels (c) of control (C, light gray column) and αENaC(−/−) Tg mice (Tg, black column); mean ± SEM. (a) Amiloride-sensitive short circuit current (ΔIscamil) in fetal colon epithelia (19 days old). Responses to intracellular cAMP stimulant, forskolin were similar in both groups. (b) In vivo measurements of amiloride-sensitive rectal PD (ΔPDamil) in adult male αENaC(−/−)Tg and αENaC(+/+) mice. ∗, P < 0.001. (c) Plasma corticosterone levels (μM) (left columns) and plasma aldosterone levels (nM) (right columns) in adult αENaC(−/−)Tg vs. control littermates [αENaC(+/+); ∗, P < 0.001].
Figure 8.
In situ hybridization analysis in kidney. Bright-field (Left) and dark-field (Right) photographs of kidney sections from 12-h-old pups. A and B, (+/+), wild-type; C and D, (−/−), αENaC(−/−); E and F, (−/−)Tg, αENaC(−/−)Tg from adult; G and H, (+/+) wild-type; and I and J, (−/−)Tg, αENaC(−/−)Tg after hybridization with αENaC antisense probe (right columns).
DISCUSSION
PHA-1 is a life-threatening disease characterized by severe neonatal salt-wasting, hyperkaliemia, metabolic acidosis, and unresponsiveness to exogenous mineralocorticoid hormones (24). This disease is caused by diminished aldosterone-responsive Na+ absorption in the renal distal tubule. Mutations in all three subunits of the epithelial sodium channel (α, β, and γENaC) have been identified in patients suffering from this disease (9). The metabolic findings of surviving αENaC(−/−)Tg mice provide further insight into the role of the sodium channel (ENaC) in a defective Na+ absorption in kidney. The clinical course observed in the αENaC(−/−)Tg mice was similar to that seen in infants with PHA-1. These infants have no apparent problem with clearance of fetal lung liquid in the perinatal period and generally present with clinical symptoms related to metabolic dysfunction only after the first 48 h of life (24).
Rescue of Perinatal Lethality in αENaC(−/−)Tg Mice.
αENaC(−/−)Tg mice were able to clear fetal liquid from the air spaces during the perinatal period. This near-normal status might be due to a higher level of transgene expression in lung (Fig. 3). Surviving adult αENaC(−/−)Tg mice had normal or near-normal liquid absorption as determined by water content. Likewise, the liquid absorptive needs of the adult lung in the resting state were met in αENaC(−/−)Tg lungs although amiloride-sensitive Na+ transport tended to be lower than in littermate controls (Fig. 4 b and c). It might well be that non-ENaC-mediated pathways of Na+ or liquid absorption may contribute to liquid absorption in the postnatal lung. In lung, ENaC activity is regulated and highly dependent on glucocorticoid stimulation (12). As a consequence, αENaC mRNA levels in lung were decreased markedly in mice deficient for the glucocorticoid receptor. These knockout mice die soon after birth because of respiratory failure (25).
αENaC(−/−)Tg Mice Exhibit Severe PHA-1.
After birth, at ≈5–9 days, metabolic factors appeared to be most determining of survival. αENaC(−/−)Tg mice showed significant metabolic acidosis and urinary Na+ loss compared with littermate controls whereas at 12 h after birth this group exhibited no metabolic derangements (Fig. 5 a and b). The significantly diminished activity of ENaC occurred in the context of a 6-fold increase in aldosterone levels in adult αENaC(−/−)Tg mice (Fig. 7c). In the rectum, the aldosterone-induced sodium transport creates an amiloride-sensitive transepithelial PD that may serve as an indicator of aldosterone activity. Under normal salt-diet, wild-type mice (+/+) show circadian variations of amiloride-sensitive rectal PD. Our αENaC transgenic mice demonstrated a lower Na+ transport in distal colon (Fig. 7b) and a loss of this cyclic variation despite elevated aldosterone levels (Q.W., E.H., M.B., and J.-D.H., unpublished work). We conclude that, in αENaC(−/−)Tg, the α subunit is limiting for the sodium channel and suggest that ENaC-mediated sodium transport in kidney and colon became aldosterone-insensitive. This insensitivity might be explained by low expression of the transgene and, consequently, any aldosterone-mediated effect on the protein itself might not be visible (26). Alternatively, the lack of aldosterone effects might be due to the absence of corresponding regulatory elements in the cytomegalovirus promoter.
In the first 2 weeks after birth, we lost 50% of our αENaC(−/−)Tg mice that exhibited severe PHA-1 symptoms including metabolic acidosis, urinary salt-wasting, and growth retardation (Fig. 6). It seems that this postnatal period is quite sensitive to electrolyte disturbances as observed in mice deficient for the mineralocorticoid receptor. These mice die around day 9 due to electrolyte disturbances despite elevated plasma aldosterone levels (27).
Adult αENaC(−/−)Tg Mice Escape from a Severe PHA-1.
The metabolic profiles of our αENaC(−/−)Tg mice changed with age. In contrast to the metabolic acidosis observed in newborn αENaC knockout mice (at 12 h), αENaC(−/−)Tg mice exhibited no metabolic derangement (Fig. 5a). However, by 5–9 days, the αENaC(−/−)Tg group showed significant metabolic acidosis and urinary Na+ loss compared with littermate controls. When studied in adulthood, metabolic problems were no longer evident in αENaC(−/−)Tg survivors (Fig. 5); they had normal blood gases and normal serum and urinary electrolyte concentrations despite elevated aldosterone levels (Fig. 7c). In patients with PHA-1, supplemental Na+ requirements diminish over time, but the mechanism of this apparent change in renal Na+ handling is not yet well understood (28). Salt and water homeostasis in adult life may be mediated by alternative (i.e., non-ENaC-mediated) paths of sodium and water absorption in the kidney, colon, and lung. Alternatively, salt-intake might change as well with age.
Partial restoration of Na+ transport in αENaC(−/−)Tg mice resulted in a model that mimics several clinical features of PHA-1. This model may provide opportunities for further study of aldosterone-sensitive Na+ absorption and paths of Na+ and water transport that are not mediated by ENaC. Our αENaC(−/−)Tg mice allow the study of physiologic consequences of this altered expression and the molecular analysis of the regulatory cascade controlling the activity of this sodium channel. ENaC channel activity derived from transgene expression in the αENaC(−/−)Tg mice provides a similar amount of Na+ absorptive function in the critical Na+-absorbing organs, like lung, kidney, and colon, as seen in PHA-1 patients.
It will be interesting to study whether PHA-1 in our αENaC(−/−)Tg mice confers salt-resistance. Following breeding with hypertensive mouse strains should allow analysis of the effect of salt resistance (at a genetic level) on the control of blood pressure.
Acknowledgments
We thank F. Verrey for suggestions on the manuscript, H.-P. Gäggeler for help with photographic work, and L. and Y. Guibert for animal caretaking. This work was supported by grants from the Swiss National Science Foundation (31–43384.95 and 32–42543.94), the National Institutes of Health, and the Cystic Fibrosis Foundation.
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
Abbreviations: ENaC, highly selective, amiloride-sensitive epithelial sodium channel; PHA-1, pseudohypoaldosteronism type 1; PD, potential difference.
References
- 1.Canessa C M, Schild L, Buell G, Thorens B, Gautschi I, Horisberger J D, Rossier B C. Nature (London) 1994;367:463–467. doi: 10.1038/367463a0. [DOI] [PubMed] [Google Scholar]
- 2.Duc C, Farman N, Canessa C M, Bonvalet J-P. J Cell Biol. 1994;127:1907–1921. doi: 10.1083/jcb.127.6.1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rossier B C. Adv Nephrol. 1996;25:275–286. [PubMed] [Google Scholar]
- 4.Pacha J, Frindt G, Antonian L, Silver R B, Palmer L G. J Gen Physiol. 1993;102:25–42. doi: 10.1085/jgp.102.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rajendran V M, Kashgarian M, Binder H J. J Biol Chem. 1989;264:18638–18644. [PubMed] [Google Scholar]
- 6.Rossier B C, Palmer L G. In: The Kidney: Physiology and Pathophysiology. Seldin D W, Giebisch G, editors; Seldin D W, Giebisch G, editors. New York: Raven; 1992. pp. 1373–1409. [Google Scholar]
- 7.Hanukoglu A, Bistrizer T, Rakover V, Mandelberg A. J Pediatr. 1994;125:752–755. doi: 10.1016/s0022-3476(94)70071-0. [DOI] [PubMed] [Google Scholar]
- 8.Schild L, Canessa C M, Shimkets R A, Warnock D G, Lifton R P, Rossier B C. Proc Natl Acad Sci USA. 1995;92:5699–5703. doi: 10.1073/pnas.92.12.5699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chang S S, Gründer S, Hanukoglu A, Rösler A, Mathew P M, Hanukoglu I, Schild L, Lu Y, Shimkets R A, Nelson-Williams C, Rossier B C, Lifton R P. Nat Genet. 1996;12:248–253. doi: 10.1038/ng0396-248. [DOI] [PubMed] [Google Scholar]
- 10.Gründer S, Firsov D, Chang S S, Fowler-Jaeger N, Gautschi I, Schild L, Lifton R L, Rossier B C. EMBO J. 1997;16:899–907. doi: 10.1093/emboj/16.5.899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Renard S, Voilley N, Bassilana F, Lazdunski M, Barbry P. Eur J Physiol. 1995;430:299–307. doi: 10.1007/BF00373903. [DOI] [PubMed] [Google Scholar]
- 12.Champigny G, Voilley N, Lingueglia E, Friend V, Barbry P, Lazdunski M. EMBO J. 1994;13:2177–2181. doi: 10.1002/j.1460-2075.1994.tb06494.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.O’Brodovich H, Canessa C M, Ueda J, Rafii B, Rossier B C, Edelson J. Am J Physiol. 1993;265:C491–C496. doi: 10.1152/ajpcell.1993.265.2.C491. [DOI] [PubMed] [Google Scholar]
- 14.Tchepichev S, Ueda J, Canessa C, Rossier B C, O’Brodovich H. Am J Physiol. 1995;269:C805–C812. doi: 10.1152/ajpcell.1995.269.3.C805. [DOI] [PubMed] [Google Scholar]
- 15.Hummler E, Barker P, Gatzy J, Beermann F, Verdumo C, Schmidt A, Boucher R, Rossier B C. Nat Genet. 1996;12:325–328. doi: 10.1038/ng0396-325. [DOI] [PubMed] [Google Scholar]
- 16.Boshart M, Weber F, Jahn G, Dorsch H K, Fleckenstein B, Schaffner W. Cell. 1985;41:521–30. doi: 10.1016/s0092-8674(85)80025-8. [DOI] [PubMed] [Google Scholar]
- 17.Canessa C M, Horisberger J D, Rossier B C. Nature (London) 1993;361:467–470. doi: 10.1038/361467a0. [DOI] [PubMed] [Google Scholar]
- 18.Beermann F, Hummler E, Schmid E, Schütz G. Mech Dev. 1993;42:49–65. doi: 10.1016/0925-4773(93)90098-i. [DOI] [PubMed] [Google Scholar]
- 19.Koopman P, Gubbay J, Vivian N, Goodfellow P, Lovell-Badge R. Nature (London) 1991;351:117–120. doi: 10.1038/351117a0. [DOI] [PubMed] [Google Scholar]
- 20.Burch L H, Talbot C, Knowles M R, Canessa C, Rossier B, Boucher R C. Am J Physiol. 1995;269:C511–C518. doi: 10.1152/ajpcell.1995.269.2.C511. [DOI] [PubMed] [Google Scholar]
- 21.Barker P M, Gatzy J T. Am J Physiol. 1993;265:L512–L517. doi: 10.1152/ajplung.1993.265.5.L512. [DOI] [PubMed] [Google Scholar]
- 22.Grubb B R. Am J Physiol. 1995;268:G505–G513. doi: 10.1152/ajpgi.1995.268.3.G505. [DOI] [PubMed] [Google Scholar]
- 23.Krumlauf R, Chapman V M, Hammer R E, Brinster R, Tilghmam S M. Nature (London) 1986;319:224–226. doi: 10.1038/319224a0. [DOI] [PubMed] [Google Scholar]
- 24.Cheek D B, Perry J W. Arch Dis Child. 1957;33:252–256. doi: 10.1136/adc.33.169.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cole T J, Blendy J A, Monaghan A P, Krieglstein K, Schmid W, Aguzzi A, Fantuzzi G, Hummler E, Unsicker K, Schütz G. Genes Dev. 1995;9:1608–1621. doi: 10.1101/gad.9.13.1608. [DOI] [PubMed] [Google Scholar]
- 26.May, A., Puoti, A., Gaeggeler, H.-P., Horisberger, J.-D. & Rossier, B. C. (1997) J. Am. Soc. Nephrol., in press. [DOI] [PubMed]
- 27.Berger S, Cole T J, Schmid W, Schütz G. Endocr Res. 1996;22:641–652. doi: 10.1080/07435809609043758. [DOI] [PubMed] [Google Scholar]
- 28.Rösler A. J Clin Endocrinol Metabol. 1984;59:689–700. doi: 10.1210/jcem-59-4-689. [DOI] [PubMed] [Google Scholar]