Abstract
In Scotland, since 1975–1979 (world) age-standardised incidence of squamous cell carcinoma of the anus has more than doubled, reaching 0.37 per 100 000 in males and 0.55 in females during 1998–2002, being somewhat higher in socioeconomically deprived areas.
Keywords: anus neoplasms, epidemiology, incidence, Scotland, squamous cell carcinoma
Cancers of the anus and anal canal are relatively rare, accounting for approximately 2% of all cancers of the large bowel (Parkin et al, 2002). However, in recent decades the incidence of anal cancer has been reported to be increasing in a number of countries, including the United States (Johnson et al, 2004; Chiao et al, 2005), Denmark (Frisch et al, 1993), and Sweden (Goldman et al, 1989). In the United States, particular increases have been observed in never married men, and in men living in the San Francisco Bay area, even before the HIV/AIDS era (Melbye et al, 1994a; Biggar and Melbye, 1996; Cress and Holly, 2003).
In the present study, we describe secular trends in incidence of squamous cell carcinoma of the anus in Scotland before and during the HIV epidemic, and describe geographical and socioeconomic variations in incidence within Scotland.
MATERIALS AND METHODS
Incident cases of ‘malignant neoplasms of the anus and anal canal’ (ICD-9 154.2, 154.3, and 154.8; ICD-10 C21) for the years 1975–2002 were extracted from the Scottish Cancer Registry. This registry covers the whole of Scotland (population approximately 5.1 million); data quality is believed to be high, both in terms of accuracy and completeness (Harris et al, 1998; Brewster et al, 2002). A range of patient and tumour-related information is collected, including demographic and diagnostic details. For the present analysis, tumours were categorised according to the histological classification developed by the International Agency for Research on Cancer (Parkin et al, 2002), in which squamous cell carcinomas are defined by ICD-O morphology codes in the range M-8050/3–M-8076/3.
Mid-year population estimates, derived from the Annual Reports of the Registrar General for Scotland (Registrar General for Scotland, 1976–2003), were used to calculate sex-specific, 5-year moving average age-adjusted incidence rates by direct standardisation to the World Population (Waterhouse et al., 1976). Age- and sex-specific incidence rates were calculated for three diagnosis periods: a pre-HIV period (1975–1981); the HIV period (1982–1995); and the highly active antiretroviral therapy (HAART) period (1996–2002) (Chiao et al, 2005).
The incidence of squamous cell carcinoma of the anus by sex in each health board area of residence was compared to the incidence in Scotland as a whole for the period 1975–2002 by indirect standardisation to produce standardised incidence ratios (SIR); exact 95% confidence intervals were calculated (Breslow and Day, 1987). To investigate the influence of socioeconomic status on risk during 1975–2002, sex-specific age-standardised incidence rates were calculated by Carstairs deprivation quintiles (Morris and Carstairs, 1991), based on the 1981 census for the period 1975–1985 and the 1991 census thereafter. The statistical significance of trends in incidence by deprivation quintile was tested by Poisson regression.
RESULTS
For the total period, 1975–2002, the percentage distribution of histological categories of anal cancer in Scotland was as follows: squamous cell carcinoma (56.3%); basaloid and cloacogenic carcinoma (12.0%); adenocarcinoma (21.2%); other specified carcinomas (1.9%); unspecified carcinomas (4.8%); melanoma (2.3%); other specified cancers (0.0%); and unspecified cancers (1.6%). Compared to females, males had a lower percentage of squamous cell carcinomas (52.1 vs 59.1%), and of basaloid and cloacogenic carcinomas (9.9 vs 13.3%), but a higher percentage of adenocarcinomas (27.2 vs 17.3%). Hereafter, the results relate to squamous cell carcinomas only.
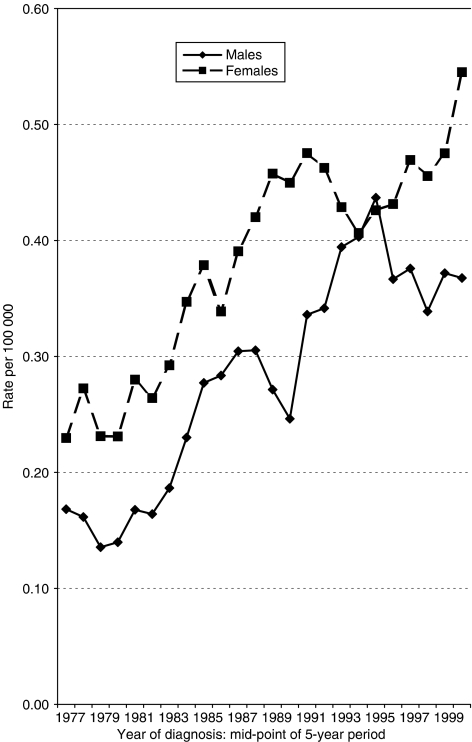
In total, 757 cases of squamous cell carcinoma (278 males, 479 females) were registered with an incidence date falling within the 28-year period of study (1975–2002). Secular trends in age-standardised incidence rates by sex are shown in Figure 1. In males, the rates per 100 000 increased from 0.14 to 0.17 in the late 1970s to around 0.37 in the late 1990s, but with a peak of 0.44 in the period 1993–1997. In females, the incidence rate has generally been higher, increasing from 0.23 to 0.27 in the late 1970s to a peak of 0.55 in the period 1998–2002.
Figure 1.
Age-standardised incidence rates of squamous cell carcinoma of the anus by year of diagnosis (5-year moving averages) and sex, Scotland, 1975–2002.
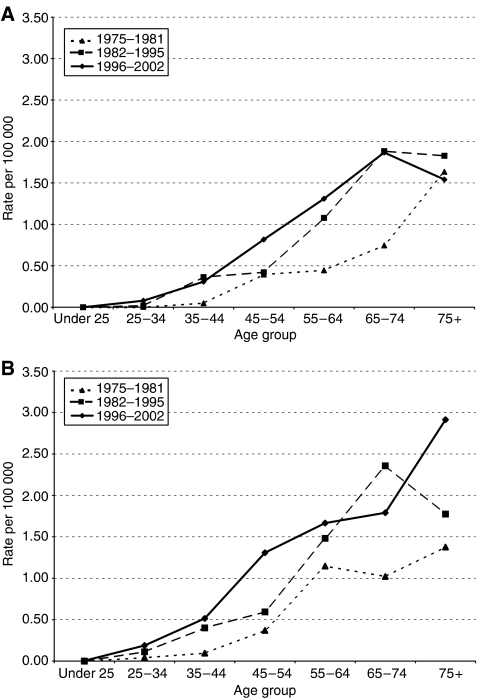
Figure 2A and B showing for each sex age-specific rates for three consecutive periods of diagnosis (1975–1981, 1982–1995, and 1996–2002), demonstrate an increase in incidence over time across most of the age groups, particularly in middle age.
Figure 2.
(A) Age-specific incidence rates of squamous cell carcinoma of the anus in males, by period of diagnosis, Scotland, 1975–2002. (B): Age-specific incidence rates of squamous cell carcinoma of the anus in females, by period of diagnosis, Scotland, 1975–2002.
Comparing incidence in each health board area to Scotland as a whole during the whole period 1975–2002, there were no statistically significantly increased risks, with the exception of males in Lothian Health Board area (which includes the city of Edinburgh), for whom the SIR was 165.3 (95% confidence intervals 129.9–210.4).
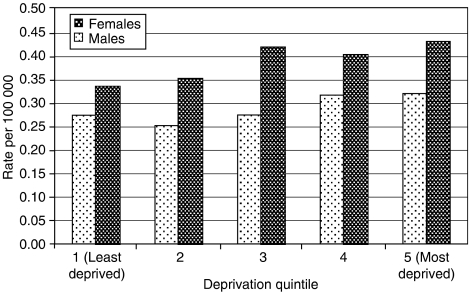
Figure 3 shows the age-standardised incidence rates of squamous cell carcinoma of the anus by Carstairs deprivation quintile and sex, for patients diagnosed during the period 1975–2002. Rates were somewhat higher in deprived areas, but this trend is only significant for females (P-values for trend: 0.173 and 0.027, for males and females, respectively).
Figure 3.
Age-standardised incidence rates of squamous cell carcinoma of the anus by deprivation quintile and sex, Scotland, 1975–2002.
DISCUSSION
Although rare in absolute terms, over nearly three decades, the risk of squamous cell carcinoma of the anus in Scotland has more than doubled in both sexes. Available data published routinely by the Office for National Statistics in London indicate that, age standardised to the European standard population, incidence rates per 100 000 of anal cancer (ICD-10 C21, all histologies combined) in England have increased between 1986 and 2003 from 0.7 to 1.1 in males, and from 0.6 to 1.3 in females (Office for National Statistics, 2001, 2005).
The main risk factors for anal cancer, identified through recent case–control studies, are a history of multiple sexual partners, men having sex with men, receptive anal intercourse (especially in men), a history of other anogenital cancers in women, a history of sexually transmitted infections, and current smoking (Frisch et al, 1997, 1999; Tseng et al, 2003; Daling et al, 2004), though a study in Denmark and Sweden found the risk from smoking only in premenopausal women (Frisch et al, 1999). The risk of anogenital cancers is also recognised to be increased among renal transplant recipients (Penn, 1986; Sillman et al, 1997). Although high risks of anal cancer have been found in homosexual men with AIDS, the contribution of HIV infection is unclear because homosexual men were at increased risk even before the AIDS epidemic (Melbye et al, 1994b). Irrespective of HIV status, a high proportion of anal cancers have detectable human papillomavirus (HPV), notably HPV type 16, suggesting that infection with HPV is a necessary cause of anal cancer (Frisch et al, 1997; Daling et al, 2004). An association between HPV seropositivity and risk of subsequent anal and perianal skin cancer has also been demonstrated in a prospective study (Bjorge et al, 2002).
Unpublished data from the National Survey of Sexual Attitudes and Lifestyles (Natsal) 2000 suggests that, in Scotland, the reported prevalence of receptive anal intercourse within the previous year among 16–44-year olds was substantially higher in women (11.16%, 95% confidence intervals 7.71–15.87%) than in men (0.71%, 0.25–1.97%) (Dr Catherine Mercer, personal communication). Although a lower prevalence was reported in the same age group in the equivalent survey in 1990, this was again much higher in women. It seems reasonable to assume that this sex difference has existed for some years, and may explain, at least in part, the higher incidence of squamous cell carcinoma of the anus among women in our study.
While the risk of cancer in the pre-HAART era has been shown to be high among a cohort of HIV-positive persons in Scotland compared to national rates, no case of anal cancer has been reported in the HIV-positive cohort up to the end of 1996 (Allardice et al, 2003). Follow-up of this cohort is currently being extended to the end of 2003, and based on experience in other countries, it seems not unlikely that cases of anal cancer will be recorded.
Although our data suggest that the age-standardised squamous cell anal cancer incidence in Scotland began to plateau or even decrease in males in the HAART period, rates have continued to increase overall in females, and in middle age groups of both sexes. In the US, during the same period, incidence has continued to increase in all age groups over 35 years in males, and in middle age groups in females (Chiao et al, 2005). The risk of anal cancer has also been found to be higher in the HAART compared to the pre-HAART period among an HIV-seropositive cohort in London (Bower et al, 2004), and among a cohort of patients with AIDS in San Diego (Diamond et al, 2005). This is consistent with the observation that among HIV-infected men who have sex with men, the prevalence of anal squamous intraepithelial lesions (SIL), including high-grade SIL, and anal HPV infection remains high, despite immune restoration under HAART (Piketty et al, 2004). As there is some evidence that duration of HIV infection is more important than immune status in invasive anal carcinoma risk (Frisch et al, 2000; Fagan et al, 2005), this cancer may become an increasing problem among the HIV-positive population as HAART improves long-term survival (Frisch, 2002; Diamond et al, 2005; Fagan et al, 2005). This may be compounded by the apparent increase in high-risk sexual behaviour among homosexual men in Scotland between 1996 and 2002 (Hart and Williamson, 2005).
Given the risk factor profile of squamous cell carcinoma of the anus, the excess risk of this disease in males resident in Lothian Health Board during the period 1975–2002 is consistent with the higher rates of rectal gonococcal infection in Lothians than Scotland as a whole (suggesting higher rates of unprotected anal intercourse) (Young et al, 1997).
A Los Angeles study suggested that the risk of anal carcinoma increased with decreasing social class (Peters et al, 1984). In our study, the absence of a significant relationship with social deprivation in males may simply be due to our relatively small numbers of cases. Additionally, it may reflect misclassification of socioeconomic status since the Carstairs deprivation index is based on place of residence at diagnosis, the characteristics being measured at a single point in time (here, at the 1981 and 1991 censuses).
Since currently available data do not support the screening of high-risk groups for anal cancer or its probable precursor, anal intraepithelial neoplasia (Anderson et al, 2004), health education should aim at reducing high-risk behaviour and encouraging early presentation with symptomatic disease. In the longer term, newly emerging HPV vaccines may enable risk reductions (Geipert, 2005).
Acknowledgments
We are grateful to Jim Chalmers for his helpful comments on an earlier draft of the manuscript.
References
- Allardice GM, Hole DJ, Brewster DH, Boyd J, Goldberg DJ (2003) Incidence of malignant neoplasms among HIV infected persons in Scotland. Br J Cancer 89: 505–507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson JS, Vajdic C, Grulich AE (2004) Is screening for anal cancer warranted in homosexual men? Sex Health 1: 137–140 [DOI] [PubMed] [Google Scholar]
- Biggar RJ, Melbye M (1996) Marital status in relation to Kaposi's sarcoma, non-Hodgkin's lymphoma, and anal cancer in the pre-AIDS era. J Acquir Immune Defic Syndr Hum Retrovirol 11: 178–182 [DOI] [PubMed] [Google Scholar]
- Bjorge T, Engeland A, Luostarinen T, Mork J, Gislefoss RE, Jellum E, Koskela P, Lehtinen M, Pukkala E, Thoresen SO, Dillner J (2002) Human papillomavirus as a risk factor for anal and perianal skin cancer in a prospective study. Br J Cancer 87: 61–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bower M, Powles T, Newsom-Davis T, Thirlwell C, Stebbing J, Mandalia S, Nelson M, Gazzard B (2004) HIV-associated anal cancer: has highly active antiretroviral therapy reduced the incidence or improved the outcome? J Acquir Immune Defic Syndr 37: 1563–1565 [DOI] [PubMed] [Google Scholar]
- Breslow NE, Day NE (1987) Statistical Methods in Cancer Research. Vol II - The Design and Analysis of Cohort Studies. IARC Scientific Publication no. 82 Lyon: International Agency for Research on Cancer [PubMed] [Google Scholar]
- Brewster DH, Stockton D, Harvey J, Mackay M (2002) Reliability of cancer registration data in Scotland, 1997. Eur J Cancer 38: 414–417 [DOI] [PubMed] [Google Scholar]
- Chiao EY, Krown SE, Stier EA, Schrag D (2005) A population-based analysis of temporal trends in the incidence of squamous anal canal cancer in relation to the HIV epidemic. J Acquir Immune Defic Syndr 40: 451–455 [DOI] [PubMed] [Google Scholar]
- Cress RD, Holly EA (2003) Incidence of anal cancer in California: increased incidence among men in San Francisco, 1973–1999. Prev Med 36: 555–560 [DOI] [PubMed] [Google Scholar]
- Daling JR, Madeleine MM, Johnson LG, Schwartz SM, Shera KA, Wurscher MA, Carter JJ, Porter PL, Galloway DA, McDougall JK (2004) Human papillomavirus, smoking, and sexual practices in the etiology of anal cancer. Cancer 101: 270–280 [DOI] [PubMed] [Google Scholar]
- Diamond C, Taylor TH, Aboumrad T, Bringman D, Anton-Culver H (2005) Increased incidence of squamous cell anal cancer among men with AIDS in the era of highly active antiretroviral therapy. Sex Transm Dis 32: 314–320 [DOI] [PubMed] [Google Scholar]
- Fagan SP, Bellows III CF, Albo D, Rodriquez-Barradas M, Feanny M, Awad SS, Berger DH (2005) Length of human immunodeficiency virus disease and not immune status is a risk factor for development of anal carcinoma. Am J Surg 190: 732–735 [DOI] [PubMed] [Google Scholar]
- Frisch M, Melbye M, Møller H (1993) Trends in incidence of anal cancer in Denmark. BMJ 306: 419–422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frisch M, Glimelius B, van den Brule AJ, Wohlfahrt J, Meijer CJ, Walboomers JM, Goldman S, Svensson C, Adami HO, Melbye M (1997) Sexually transmitted infection as a cause of anal cancer. N Engl J Med 337: 1350–1358 [DOI] [PubMed] [Google Scholar]
- Frisch M, Glimelius B, Wohlfahrt J, Adami HO, Melbye M (1999) Tobacco smoking as a risk factor in anal carcinoma: an antiestrogenic mechanism? J Natl Cancer Inst 91: 708–715 [DOI] [PubMed] [Google Scholar]
- Frisch M, Biggar RJ, Goedert JJ (2000) Human papillomavirus-associated cancers in patients with human immunodeficiency virus infection and acquired immunodeficiency syndrome. J Natl Cancer Inst 92: 1500–1510 [DOI] [PubMed] [Google Scholar]
- Frisch M (2002) On the etiology of anal squamous carcinoma. Dan Med Bull 49: 194–209 [PubMed] [Google Scholar]
- Geipert N (2005) Vaccinating men for HPV: new strategy for preventing cervical cancer in women. J Natl Cancer Inst 97: 630–631 [DOI] [PubMed] [Google Scholar]
- Goldman S, Glimelius B, Nilsson B, Påhlman L (1989) Incidence of anal epidermoid carcinoma in Sweden 1970–1984. Acta Chir Scand 155: 191–197 [PubMed] [Google Scholar]
- Harris V, Sandridge AL, Black RJ, Brewster DH, Gould A (1998) Cancer Registration Statistics Scotland 1986–1995. Edinburgh: ISD Scotland Publications [Google Scholar]
- Hart GJ, Williamson LM (2005) Increase in HIV sexual risk behaviour in homosexual men in Scotland, 1996–2002: prevention failure? Sex Transm Infect 81: 367–372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson LG, Madeleine MM, Newcomer LM, Schwartz SM, Daling JR (2004) Anal cancer incidence and survival: the surveillance, epidemiology, and end results experience, 1973–2000. Cancer 101: 281–288 [DOI] [PubMed] [Google Scholar]
- Melbye M, Rabkin C, Frisch M, Biggar RJ (1994a) Changing patterns of anal cancer incidence in the United States, 1940–1989. Am J Epidemiol 139: 772–780 [DOI] [PubMed] [Google Scholar]
- Melbye M, Cote TR, Kessler L, Gail M, Biggar RJ (1994b) High incidence of anal cancer among AIDS patients. The AIDS/Cancer Working Group. Lancet 343: 636–639 [DOI] [PubMed] [Google Scholar]
- Morris R, Carstairs V (1991) Which Deprivation? A comparison of selected deprivation indexes. J Public Health Med 13: 318–326 [PubMed] [Google Scholar]
- Office for National Statistics (2001) Cancer Statistics Registrations 1995–1997, England. Series MB1, No. 28, pp 79 London: The Stationery Office [Google Scholar]
- Office for National Statistics (2005) Cancer Statistics Registrations 2003, England. Series MB1, No. 34, pp 80 London: Office for National Statistics [Google Scholar]
- Parkin DM, Whelan SL, Ferlay J, Teppo L, Thomas DB (eds) (2002) Cancer Incidence in Five Continents, volume VIII. IARC Scientific Publication no. 155 Lyon: International Agency for Research on Cancer [Google Scholar]
- Penn I (1986) Cancers of the anogenital region in renal transplant recipients. Analysis of 65 cases. Cancer 58: 611–616 [DOI] [PubMed] [Google Scholar]
- Peters RK, Mack TM, Bernstein L (1984) Parallels in the epidemiology of selected anogenital carcinomas. J Natl Cancer Inst 72: 609–615 [PubMed] [Google Scholar]
- Piketty C, Darragh TM, Heard I, Da Costa M, Bruneval P, Kazatchkine MD, Palefsky JM (2004) High prevalence of anal squamous intraepithelial lesions in HIV-positive men despite the use of highly active antiretroviral therapy. Sex Transm Dis 31: 96–99 [DOI] [PubMed] [Google Scholar]
- Registrar General for Scotland (1977–2003) Annual Reports 1976–2002. Edinburgh: HMSO [Google Scholar]
- Sillman FH, Sentovich S, Shaffer D (1997) Ano-genital neoplasia in renal transplant patients. Ann Transplant 2: 59–66 [PubMed] [Google Scholar]
- Tseng HF, Morgenstern H, Mack TM, Peters RK (2003) Risk factors for anal cancer: results of a population-based case–control study. Cancer Causes Control 14: 837–846 [DOI] [PubMed] [Google Scholar]
- Waterhouse J, Muir C, Correa P, Powell J (1976) Cancer Incidence in Five Continents. volume III, pp 454–459. IARC Scientific Publication no. 15 Lyon: International Agency for Research on Cancer [Google Scholar]
- Young H, Moyes A, Noone A (1997) Gonococcal infections in Scotland 1996. Scottish Centre for Infection and Environmental Health Weekly Report 31(97/49): 264–268 [Google Scholar]