Abstract
To evaluate the single agent activity, pharmacokinetics and tolerability of the novel tubulin targeted agent vinflunine (VFL) (320 mg m−2 q 21 days) as second-line chemotherapy in patients with metastatic breast carcinoma (MBC). All patients had disease progression after anthracycline/taxane (A/T) therapy. They could have received a nonanthracycline adjuvant treatment and subsequently received a first-line A/T combination for advanced/metastatic disease; or relapsed >6 months after completion of adjuvant A/T therapy and were subsequently treated with the alternative agent; or relapsed within 6 months from an adjuvant A/T combination. Objective response was documented in 18 of 60 patients enrolled (RR: 30% (95% confidence interval (CI): 18.9–43.2%)). Among the responders, seven patients had relapsed during a period of <3 months from taxane-based regimen yielding a RR of 33.3%. The median duration of response was 4.8 months (95% CI: 4.2–7.2), median progression-free survival was 3.7 months (95% CI: 2.8–4.2) and median overall survival was 14.3 months (95% CI: 9.2–19.6). The most frequent adverse event was neutropenia (grade 3 in 28.3% and grade 4 in 36.7% of patients). No febrile neutropenia was observed. Fatigue (grade 3 in 16.7% of patients) and constipation (grade 3 in 11.7% of patients) were also common; these were non-cumulative and manageable permitting achievement of a good relative dose intensity of 93.5%. Vinflunine is an active agent with acceptable tolerance in the management of MBC patients previously treated with (A/T)-based regimens. These encouraging phase II results warrant further investigation of this novel agent in combination with other active agents in this setting or in earlier stages of disease.
Keywords: advanced breast cancer, vinflunine
Anthracyclines, taxanes and vinorelbine are among the most active agents in the management of advanced breast cancer. Despite the high level of activity of these agents, metastatic breast cancer (MBC) is essentially an incurable disease; disease progression during or after chemotherapy is unavoidable (Hamilton and Hortobagyi, 2005). No consensus exists for the use of alternative chemotherapy after anthracycline and taxane failure in MBC; only capecitabine is currently registered as a standard treatment in this setting. Therefore, there is a need for new chemotherapy strategies and new agents in this setting.
Vinflunine (VFL) (Javlor®, Pierre Fabre Médicament, Boulogne-Billancourt, France) is a novel microtubule inhibitor agent obtained by semisynthesis using superacidic chemistry to selectively introduce two fluorine atoms at the 20’ position of catharanthine moiety of Vinca alkaloid (Fahy et al, 1997). The diverse actions of VFL on microtubules are likely to produce different effects on mitotic spindle functions leading to modifications of cell cycle progression and cell killing (Ngan et al, 2000). Vinflunine prevents microtubule assembly during mitosis and induces apoptosis (Kruczynski et al, 1998, 2002). Vinflunine exerts effects on microtubule dynamic instability suppressing the rate and extent of microtubule growing events (Ngan et al, 2001). The affinity profile of VFL shows features which suggest that it will have greater effects on mitotic rather than axonal tubulin and so will not cause significant neurotoxicity (Lobert et al, 1998). Vinflunine showed definite high antitumour activity compared to vinorelbine in xenografts; against the human MX-1 breast xenografts, VFL produced an overall growth inhibition of 61%, whereas vinorelbine did not result in any significant inhibition (Hill et al, 1999; Kruczynski et al, 1999). Moreover, in the first Phase I study, two out of three patients with heavily pretreated MBC achieved partial response (PRs), therefore, VFL was considered to be a good candidate for further investigation in patients with MBC. Vinflunine treatment every 3 weeks was considered optimal, based on clinical and pharmacokinetic data from the three dose schedules evaluated in Phase I trials. Vinflunine was administered at the beginning of the phase I trials at 350 mg m−2 in normal saline as a 10-min infusion; after an early safety analysis this dose was adjusted to 320 mg m−2 which is the recommended dose for all subsequent patients included in clinical trials (Bennouna et al, 2003).
MATERIALS AND METHODS
Objectives
This study was a multicentre, international, nonrandomised Phase II trial, designed to determine the objective response of intravenous VFL administered once every 3 weeks in patients with MBC who had received prior anthracycline/taxane (A/T)-based regimens. Secondary objectives were to assess duration of response, progression-free survival (PFS) and overall survival (OS) and to evaluate the treatment-related toxicity. This trial also included assessment of the pharmacokinetic profile of VFL and planned to document pharmacokinetic/pharmacodynamic relationships in a subset of patients.
The protocol and its amendments were submitted to Independent Ethics Committees and Health Authorities according to local requirements. The study was conducted in accordance with the principles set forth in the Declaration of Helsinki (Somerset West October 1996) and in compliance with Good Clinical and Laboratory Practices. Written informed consent was obtained from each participating patient before enrolment.
Patient selection
Patients were recruited from 15 active centres between March 2002 and September 2003. Patients were required to have histologically proven breast adenocarcinoma with documented progressive disease. Eligibility also included either (1) nonanthracycline adjuvant treatment with at least 6 months disease-free interval and subsequent treatment with a first-line A/T combination for advanced/metastatic disease; or (2) disease relapse more than 6 months after completion of adjuvant anthracycline-containing chemotherapy and subsequent therapy with a taxane-containing combination as first-line treatment for advanced/metastatic disease; or (3) relapsed more than 6 months after completion of adjuvant taxane-containing chemotherapy and subsequently treated by an anthracycline-containing combination as first-line treatment for advanced/metastatic disease or (4) relapsed within 6 months from an adjuvant A/T combination therapy. The presence of at least one bidimensionally measurable lesion, not previously irradiated, and assessed by CT-scan was required; lesions were documented by WHO criteria. Patients were required to be women, aged ⩾18 years with Karnofsky Performance Status (KPS) ⩾80% and an estimated life expectancy of ⩾12 weeks with adequate haematological function (absolute neutrophil count ⩾2.0 × 109 l−1, platelets ⩾100 × 109 l−1), hepatic function (bilirubin ⩽1.5 × upper normal limit (UNL), transaminases ⩽2.5 × UNL, unless due to liver involvement), renal function, and a normal electrocardiogram.
Treatment schedule
Vinflunine was administered at the dose of 320 mg m−2 in normal saline as a 10-min infusion every 21 days. The use of haematopoietic growth factors (G-CSF) was allowed for patients with febrile neutropenia or neutropenic infections according to institutional standard and practice.
Vinflunine was delayed by 1 or 2 weeks in case of >grade 2 haematological or nonhaematological toxicity. If febrile neutropenia and/or grade 4 neutropenia (<1.0 × 109 l−1) lasting 7 days or more was observed between two subsequent administrations of VFL, the dose was to be reduced to 280 mg m−2 from the next cycle on. If, after a dose reduction, this toxicity was seen again, the dose was to be further reduced to 250 mg m−2. If at this dose level the same event recurred, the treatment was to be discontinued. No dose re-escalation was allowed after dose reduction. In case of grade 2 mucositis and/or constipation lasting >5 days, or grade ⩾3 mucositis, and/or constipation of any duration, the VFL dose was to be reduced to 280 mg m−2 from the next cycle on. If, after dose reduction, one of these toxicities was seen again, the dose was to be reduced to 250 mg m−2. If at this dose the event recurred, the treatment was discontinued.
Tumour response was assessed after the initial two cycles of therapy. Patients with complete response (CR) or a PR could continue treatment either until progressive disease (PD), toxicity or patient preference precluded further therapy; those with stable disease (SD) received two additional cycles, were assessed a second time and treatment was continued according to the Investigator's opinion. Patients with PD discontinued the treatment.
Baseline and treatment evaluation
Baseline assessments included a detailed medical history; computed tomography and physical examination (superficial lymph node or skin nodule). All positive imaging procedures at study entry had to be repeated every 6 weeks. An assessment of symptoms was made at study entry and then throughout treatment. Physical examination and vital signs were assessed on day one of each 3-week cycle. A complete blood cell count was taken at baseline and before each cycle of treatment. Additional samples were planned on day 8 and 15 of each cycle: in case the ANC was <1.0 × 109 l−1, counts were repeated every 2 days until recovery to ANC ⩾1.0 × 109 l−1. Transaminases, alkaline phosphatases, total bilirubin, LDH, creatinine, electrolytes including Ca2+, Na+ and K+ and total protein were assessed at every cycle. Electrocardiogram was to be performed and recorded before initial administration and repeated at every cycle.
Response was assessed using the WHO criteria (World Health Organization, 1979; Green and Weiss, 1992). In order to be confirmed, CR and PR were to be maintained at 4 weeks. An independent radiologist reviewed all responses and disease stabilisations. According to WHO criteria, the duration of response was calculated for patients with confirmed response (CR or PR) from the date of registration until the date of documented progression, start of new anticancer therapy, date of death, loss to follow-up or last news. The PFS was defined as the time elapsed from registration until progression, death, loss to follow-up or last news; OS was defined as the time elapsed from registration date to death, loss to follow-up or last news. Safety analyses were performed in patients having received at least one dose of study treatment according to the NCI CTC Version 2.0 grading.
Pharmacokinetic sampling
Pharmacokinetic sampling of VFL was optional and was studied during cycles 1 and 2. Three blood samples were collected between T0 and T16 (initial 16 h). The exact time of sampling was randomly assigned to the investigator centres in order to give wide coverage of the time interval. Vinflunine and 4-O-deacetylvinflunine (DVFL) blood concentrations were quantified using a HPLC method with UV detection.
Statistical analysis
A total of sample size of 55 evaluable patients was calculated using a one-sample multiple testing procedure (Fleming, 1982) and was based on a null hypothesis for the true response rate of 15% and an alternative hypothesis of 30% with a type I error alpha of 5% and type II error beta of <20%. Continuous data were summarised using median, minimum and maximum values. Categorical data were presented in contingency tables with frequencies and percentages. Confidence intervals (CI) were calculated at the 95% level. Time dependent parameters were analysed using the Kaplan–Maier method and 95% CI of the median was reported.
Efficacy analyses were performed on the intent to treat (ITT) and evaluable populations. The other efficacy parameters were duration of response, PFS and OS. A subgroup analysis of response was performed according to the time elapsed between the end of treatment with the taxane-containing regimen and progression. Safety analyses were performed in patients having received at least one dose of study treatment for haematological and nonhaematological adverse events.
All statistical analyses were performed with the 8.2 version of SAS® (SAS Institute Inc., Cary, NC USA) for Windows® .
RESULTS
Sixty patients with advanced or metastatic carcinoma of the breast were enrolled. Among these patients, five were not eligible but considered for the study evaluation on an ITT basis (one with no target lesions, one without prior chemotherapy for advanced disease, one patient with more than one prior line of chemotherapy, one received cisplatin in the first-line setting but received A/T in the adjuvant setting; one patient assessed without imaging procedures).
Demographic features of the patients are summarised in Tables 1 and 2. All patients enrolled had received prior A/T-based chemotherapy before study entry in neoadjuvant intent for one patient (1.7%), adjuvant intent for two patients (3.3%) and as first-line treatment for advanced disease in 57 patients (95.0%) (Tables 3 and 4).
Table 1. Demographic data.
| Demographic data | ITT patients |
|---|---|
| Age (years) (N=60) | |
| Median | 55.2 |
| Range | (33.0–75.8) |
| Karnofsky performance status (%) | N=60 (%) |
| 100 | 18 (30.0) |
| 90 | 19 (31.7) |
| 80 | 20 (33.3) |
| 70 | 3 (5.0) |
| No. of organs involved | |
| 1 | 16 (26.7) |
| 2 | 23 (38.3) |
| ⩾3 | 21 (35.0) |
| Organs involved | |
| Liver | 40 (66.7) |
| Lung | 19 (31.7) |
| Lymph nodes | 24 (40.0) |
| Pleural effusion | 13 (21.7) |
| Soft tissue | 8 (13.3) |
| Skin | 8 (13.3) |
| Others | 6 (10.0) |
| Breast | 1 (1.7) |
| Bone | 21 (35.0) |
Abbreviation: ITT=intent to treat.
Table 2. Hormonal receptors at diagnosis.
|
ER
|
||||
|---|---|---|---|---|
| Positive | Negative | Unknown or missing | Total | |
| PR | ||||
| Positive | 22 | 2 | 0 | 24 |
| Negative | 7 | 13 | 0 | 20 |
| Unknown or missing | 9 | 0 | 7 | 16 |
| Total | 38 | 15 | 7 | 60 |
Abbreviation: PR=partial response.
Table 3. Prior chemotherapy.
| (N=60) | % | |
|---|---|---|
| Intent of prior chemotherapy | ||
| Neoadjuvant | 1a | 1.7 |
| Adjuvant | 2a | 3.3 |
| Neoadjuvant+metastatic | 5 | 8.3 |
| Adjuvant+metastatic | 35 | 58.3 |
| Neoadjuvant+adjuvant+metastatic | 2 | 3.3 |
| Metastatic | 15 | 25 |
Two patients were enrolled into the study after adjuvant or neoadjuvant failure; relapsing within 6 months of last administration of neoadjuvant anthracycline and taxane combination.
Table 4. Previous chemotherapy for advanced disease.
| N (%) | % | |
|---|---|---|
| Class of chemotherapy | ||
| Taxane-anthracyclines | 31a,b | 51.6 |
| Taxane single agent | 21 | 35.0 |
| Taxane+other | 3 | 5.0 |
| Anthracycline based | 4 | 6.7 |
| Cisplatin | 1 | 1.7 |
Two patients were enrolled into the study after adjuvant or neoadjuvant failure; relapsing within 6 months of last administration of neoadjuvant anthracycline and taxane combination.
One patient having progressed >6 months after adjuvant treatment (considered a major protocol deviation).
The median time elapsed between diagnosis of invasive breast cancer and inclusion in the trial was 4.2 years (range: 0.4–17.3). The median progression-free interval after taxane-based regimens was 4.8 months (range: 0–29).
The first stage required five of 30 patients to have an objective response (CR+PR). As nine of the first 30 patients (30%) met this criterion, the study was opened to the second stage and an additional 25 patients were enrolled.
Among the 60 patients included, 18, achieved a tumour objective response of 30% (95% CI: 18.9–43.2%]), and 21 had a stable disease of 35.0% (Table 5).
Table 5. WHO overall response rates.
| Overall response | ITT N=60 (%) |
|---|---|
| PR | 18 (30.0) |
| 95% CI | (18.9–43.2) |
| Stable disease | 21 (35.0) |
| Nonevaluable | 5 (8.3) |
| Disease progression | 16 (26.7) |
Abbreviations: CI=confidence interval; PR=partial response; ITT=intent to treat; WHO=World Health Organization.
Of note, 14 of the responders had visceral involvement (45 patients had visceral involvement at baseline). Five patients could not be evaluated for response but were kept in the denominator for the purpose of the calculation of the response rate: three patients received a single cycle of treatment (one patient due to drug-related toxicity, one case of patient's refusal to continue and one case due to nondrug-related adverse event), one unevaluable due to the fact that CT scan slides were not comparable over the different tumour assessments, and one patient with liver haemangioma as selected target lesion (after panel review).
It is noteworthy that among the 18 responders seven had experienced previous tumour relapse <3 months, achieving a RR of 33.3% (95% CI: 14.6–57.0) in this group.
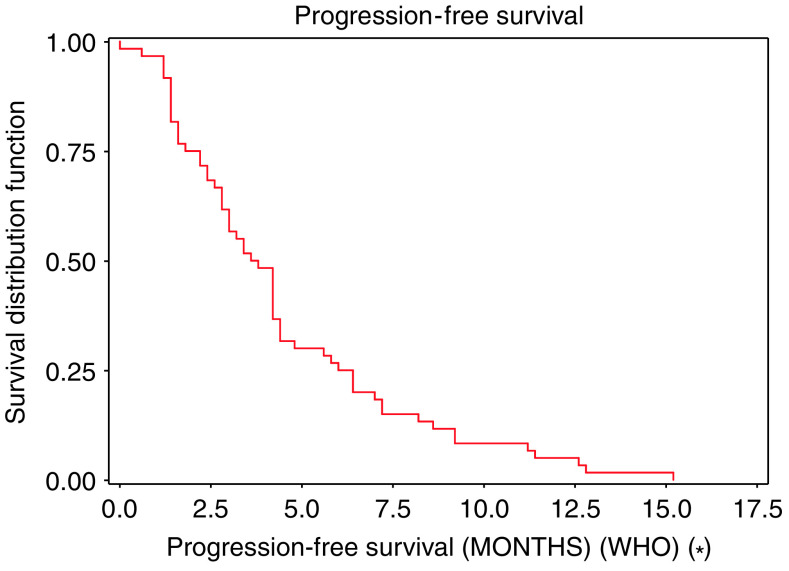
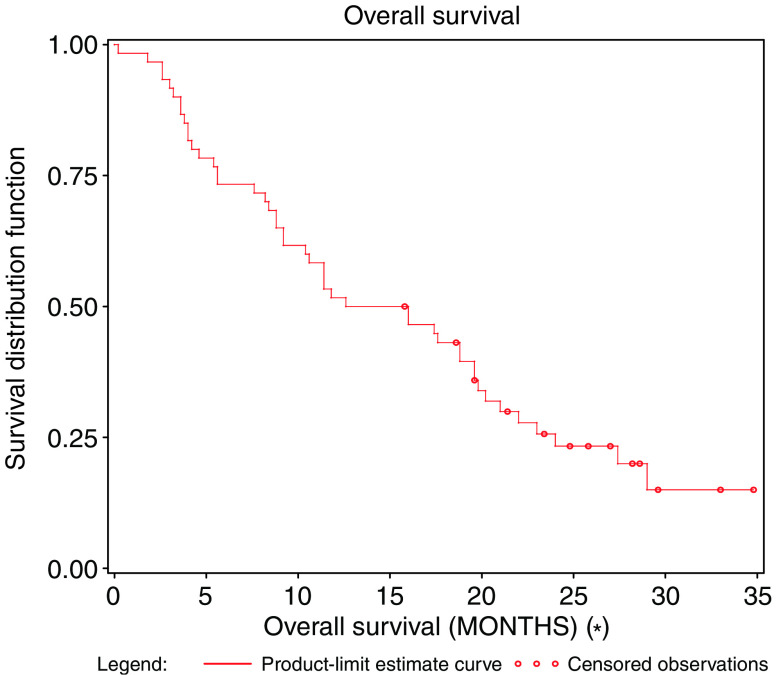
The median duration of response was 4.8 months (95% CI: 4.2–7.2). The median duration of stable disease was estimated at 4.1 months (95% CI: 3.3–5.7). Median PFS was 3.7 months (95% CI: 2.8–4.2) (Figure 1), and the median OS was estimated at 14.3 months (95% CI: 9.2–9.6) (Figure 2).
Figure 1.
Progression-free survival.
Figure 2.
Overall survival.
Karnofsky Performance Status (KPS) was recorded for each patient at baseline and before each treatment cycle during the study (every 3 weeks). During treatment the KPS stayed the same or improved in 43 out of 60 patients; only two patients experienced a decrease of 20% in KPS score.
Pharmacokinetic results
The number of patients evaluable for pharmacokinetics was 17 and 15 in cycles 1 and 2, respectively. All patients were first treated at the dose of 320 mg m−2, seven of them had a dose reduction to 280 mg m−2 in cycle 2; results are shown in Table 6.
Table 6. Main mean (s.d.) blood pharmacokinetic parameters of vinflunine.
| Cycle 2 (n=15 patients) | ||
|---|---|---|
| Cycle 1 (n=17 patients) | 320 mg m−2: 8 patients | |
| Parameters | 320 mg m−2 | 280 mg m−2: 7 patients |
| Cltot (L.h−1) | 41.7 (12.1) | 42.5 (14.4) |
| T1/2 z (h) | 46.6 (7.58) | 46.4 (7.73) |
| Vd (L) | 2749 (269) | 2770 (306) |
The mean total clearance and the range of the individual values were comparable between cycles 1 and 2. The intraindividual variability of Cltot was low between cycles 1 and 2: variability values were all <19% and 11 out 15 values were <10%. No statistically significant difference in Cltot (P=0.871) and T1/2 z (P=0.928), was observed between cycles 1 and 2 using a paired t-test. The four patients who experienced grade 3 and 4 SAEs, did not show any VFL blood overexposure.
Safety evaluation
All 60 patients were evaluable for safety. The total number of cycles administered was 292; (median 5 cycles; (range 1–12)). The median relative dose intensity was 93.5%. Twenty-two out of the 60 patients (36.7, 9.9% of cycles) had dose reductions.
Twenty-four patients (40%) experienced grade 3/4 leucopenia during the study (15.2% of cycles). Grade 3/4 neutropenia occurred in 65% of patients (36.2% of cycles) (Table 7). The median nadir value for leucocytes and neutrophils was 2.3 × 109 l−1 and 0.7 × 109 l−1, respectively. The median cycle at which both leucocyte and neutrophil nadirs occurred was the second cycle of treatment (on days 8 and 11, respectively). The median duration of leucocyte and neutrophil nadir was 7 days. No febrile neutropenia was observed. Grade 3–4 neutropenia complicated with infection was observed in 8.3% of patients (1.7% of cycles). Only 3.1% of patients received growth factor support.
Table 7. Adverse events according to NCI CTC (version 2.0).
|
N=60 patients
|
N=288a cycles |
|||||
|---|---|---|---|---|---|---|
| Adverse events (%) | Overall incidence | Grade 3 | Grade 4 | Overall incidence | Grade 3 | Grade 4 |
| Anaemia | 50 (83.3) | 3 (5.0) | 0 | 180 (62.5) | 3 (1.0) | 0 |
| Leucopenia | 55 (91.7) | 21 (35.0) | 3 (5.0) | 207 (71.9) | 41 (14.2) | 3 (1.0) |
| Neutropenia | 55 (91.7) | 17 (28.3) | 22 (36.7) | 211 (73.3) | 69 (24.0) | 35 (12.2) |
| Thrombocytopenia | 30 (50.0) | 1 (1.7) | 0 | 56 (19.4) | 1 (0.3) | 0 |
| Febrile neutropenia | 0 | 0 | 0 | 0 | 0 | 0 |
| Infection | ||||||
| Infection+G 3/4 neutropenia | 5 (8.3) | 3 (5.0) | 1 (1.7) | 5 (1.7) | 3 (1.0) | 1 (0.3) |
| Infection without neutropenia | 3 (5.0) | 0 | 0 | 4(1.4) | 0 | 0 |
| Gastrointestinal | ||||||
| Nausea | 35 (58.3) | 5 (8.3) | 0 | 79 (27.1) | 5 (1.7) | 0 |
| Vomiting | 28 (46.7) | 4 (6.7) | 0 | 54 (18.5) | 5 (1.7) | 0 |
| Constipation | 38 (63.3) | 7 (11.7) | 0 | 89 (30.5) | 8 (2.7) | 0 |
| Diarrhoea | 8 (13.3) | 1 (1.7) | 0 | 10 (3.4) | 1 (0.3) | 0 |
| Stomatitis | 33 (55.0) | 1 (1.7) | 0 | 54 (18.5) | 1 (0.3) | 0 |
| Dermatological | ||||||
| Alopecia | 21 (35.0) | NA | NA | NA | NA | NA |
| Injection site reaction | 13 (36.1) | 0 | NA | 24 (21.8) | 0 | NA |
| Neurology | ||||||
| Ileus | 2 (3.3) | 2 (3.3) | 0 | 2 (0.7) | 2 (0.7) | 0 |
| Neuropathy sensory | 8 (13.3) | 0 | 0 | 22 (7.5) | 0 | 0 |
| Pain | ||||||
| Abdominal pain | 27 (45.0) | 7 (11.7) | 0 | 45 (15.4) | 8 (2.7) | 0 |
| Arthralgia | 14 (23.3) | 1 (1.7) | 0 | 30 (10.3) | 1 (0.3) | 0 |
| Myalgia | 14 (23.3) | 1 (1.7) | 1 (1.7) | 21 (7.2) | 1 (0.3) | 1 (0.3) |
| Jaw pain | 8 (13.3) | 2 (3.3) | 0 | 30 (10.3) | 9 (3.1) | 0 |
| Flu-like symptoms | ||||||
| Fatigue | 49 (81.7) | 10 (16.7) | 0 | 147 (50.3) | 12 (4.1) | 0 |
| Fever without neutropenia | 6 (10.0) | 0 | 0 | 6 (2.1) | 0 | 0 |
Four cycles were not assessed for haematological parameters due to missing data.
Anaemia was rarely severe; only 5.0% of patients developed grade 3 toxicity (1.0% of cycles). The incidence of thrombocytopenia was low, only one out of 60 patients developed grade 3 (0.3% of cycles).
The incidence (Table 5) of severe nausea and vomiting was very low: only 8.3% of patients experienced grade 3 nausea and only 6.7% of patients experienced grade 3 vomiting. The overall incidence of constipation was 63.3%; 16 patients (26.7%) requiring stool softeners or dietary modifications (grade 1), four (6.7%) requiring laxatives (grade 2) and seven patients (11.7%) experienced grade 3 constipation (2.7% of cycles) requiring enema. Ileus was reported in two patients, this event occurred after the first administration and recovered completely allowing continuation of treatment at 280 mg m−2.
Grade 3 or 4 myalgia was observed in only 3.4% of patients (0.6 % of cycles). Grade 3 fatigue was experienced by 16.7% of patients (4.1% of cycles). No grade 3–4 peripheral neuropathy was experienced. Among the 36 patients receiving the drug via peripheral veins, 13 developed mild injection site reactions of grade 1 or 2; no grade 3 reactions were observed. Grade 1–2 alopecia was seen in 35% of patients. No clinically significant alterations in renal or hepatic function were observed. No treatment-related deaths were reported.
DISCUSSION
The data of our study support the hypothesis that VFL is an active and well-tolerated agent for the treatment of patients with MBC. Specifically, these data were derived in the setting of prior therapy with anthracycline and taxane, a situation that is increasingly common in breast cancer management. The value of identifying novel agents for the management of MBC patients previously treated with anthracyclines and taxanes is underscored by the fact that in the eligible patient population only capecitabine is currently registered as a standard treatment in this setting.
The first trial to assess capecitabine was conducted in 162 patients with paclitaxel-refractory MBC; the ORR in this population was 20%, median time to progression (TTP) 3 months, and OS 12.6 months (Blum et al, 1999). An ORR of 15% was obtained in another clinical study (136 patients), with a median TTP and OS of 3.5 months and 10.1 months was observed respectively; the median interval between the last taxane-containing therapy and start of capecitabine treatment in this study was 4.4 months and 17% of patients discontinued the study drug due to drug-related adverse events, mainly hand-foot syndrome (Reichardt et al, 2003). Most recently, in a study of 126 patients with MBC treated with capecitabine after two or three prior chemotherapies including an anthracycline and a taxane, the ORR was 28.0% and the TTP was 4.9 months (95% CI: 4.0–6.4 months) with a median OS of 15.2 months (95% CI: 13.5–19.6 months) (Fumoleau et al, 2004). One single-agent study with gemcitabine at 1200 mg m−2 days 1, 8 and 15 q 4 weeks, failed to demonstrate objective response in 23 patients (Smorenburg et al, 2001). Clearly other active agents are needed.
A phase II trial of vinorelbine in 40 patients with paclitaxel-refractory MBC demonstrated a median time to progression of 3 months and a median survival of 8 months with an objective response rate of 25% (Livingston et al, 1997). Moderate activity has been observed with irinotecan and pemetrexed yielding ORRs of 14–26% and 10–26%, respectively, in patients with MBC after anthracycline and/ or taxane exposure (Calvert, 2003; Heinemann, 2003; Perez et al, 2004). In a phase II trial with ixabepilone, 37 women with metastatic and locally advanced breast cancer who had received paclitaxel and/or docetaxel as prior neoadjuvant, adjuvant or metastatic therapy, the ORR was 22% and median duration of response 3.8 months; the median PFS was established at 2.8 months (Low et al, 2005).
The present study confirms that VFL is an active and tolerable treatment for the management of MBC previously treated with anthracycline and taxane-based regimens. The overall response rate of 30% and the response rate of 33% in patients who had developed tumour relapse less than 3 months after a taxane containing regimen, place VFL among the most active drugs in this setting. This is supported by the median OS estimated at 14.3 months. Although VFL was associated with some severe toxicity in the form of myelosuppression and constipation these adverse events were predictable, reversible, noncumulative and manageable.
In conclusion, the present study demonstrates that VFL has clinically relevant activity with acceptable toxicity in the treatment of patients with advanced breast cancer who have failed prior A/T containing regimens. These results warrant further investigation in phase III trials of MBC either as monotherapy or in combination with other agents in first-line treatment.
Acknowledgments
We acknowledge Pr Edith Perez, Mayo Clinic Jacksonville, Florida (USA); Dr S Johnson, Musgrove Park Hospital, Taunton (UK); Dr S Hill, Pierre Fabre Ltd, UK; Mr JC Pouget, Biostatistics, Pierre Fabre Médicament (France) for their help in the preparation of this manuscript. The study was supported by Institut de Recherche Pierre Fabre, Boulogne-Billancourt, France and previously presented in part as: Fumoleau P, Campone M, Vorobiof D, Casado M, Ruff P, Cortes-Funes H, Khalfallah S, Caroff I, Colin C (2004). Phase II study of i.v. vinflunine as second line in patients with metastatic breast cancer after anthracycline-taxane failure. Proc Am Soc Clin Oncol 23: abstract 542. Campone M, Vorobiof D, Cotes-Funes H, Verrill M, Khoo KS, Slabber CF, Caroff I, Pouget JC, Fumoleau P (2003). Preliminary results of a phase II study of intravenous vinflunine as second-line therapy in patients with metastatic breast cancer after anthracycline-taxane-based regimen failure. 26th Annual San Antonio Breast Cancer symposium.
References
- Bennouna J, Fumoleau P, Armand JP, Raymond E, Campone M, Delgado FM, Puozzo C, Marty M (2003) Phase I and pharmacokinetic study of the new vinca alkaloid vinflunine administered as a 10-min infusion every 3 weeks in patients with advanced solid tumours. Ann Oncol 14: 630–637 [DOI] [PubMed] [Google Scholar]
- Blum JL, Jones SE, Buzdar AU, Lorusso PM, Kuter I, Vogel C, Osterwalder B, Burger HU, Brown CS, Griffin T (1999) Multicenter phase II study of capecitabine in paclitaxel-refractory metastatic breast cancer. J Clin Oncol 17: 485–493 [DOI] [PubMed] [Google Scholar]
- Calvert H (2003) Pemetrexed (Alimta) a promising new agent for the treatment of breast cancer. Semin Oncol 30: 2–5 [DOI] [PubMed] [Google Scholar]
- Fahy J, Duflos A, Ribet JP, Jacquesy JC, Berrier C, Jounnetaud MP, Zunino F (1997) Vinca alkaloids in superacidic media: a method for creating a new family of antitumor derivatives. J Am Chem Soc 119: 8576–8577 [Google Scholar]
- Fleming TR (1982) One-sample multiple testing procedure for phase II clinical trials. Biometrics 38: 143–151 [PubMed] [Google Scholar]
- Fumoleau P, Largillier R, Clippe C, Dieras V, Orfeuvre H, Lesimple T, Culine S, Audhuy B, Serin D, Cure H, Vuillemin E, Morere JF, Montestruc F, Mouri Z, Namer M (2004) Multicentre, phase II study evaluating capecitabine monotherapy in patients with anthracycline- and taxane-pretreated metastatic breast cancer. Eur J Cancer 40: 536–542 [DOI] [PubMed] [Google Scholar]
- Green S, Weiss GR. (1992) Southwest Oncology Group standard response criteria, endpoint definitions and toxicity criteria. Invest New Drugs 10: 239–253 [DOI] [PubMed] [Google Scholar]
- Hamilton A, Hortobagyi G (2005) Chemotherapy: what progress in the last 5 years. J Clin Oncol 23: 1760–1775 [DOI] [PubMed] [Google Scholar]
- Heinemann V (2003) Role of gemcitabine in the treatment of advanced and metastatic breast cancer. Oncology 64: 191–206 [DOI] [PubMed] [Google Scholar]
- Hill BT, Fiebig HH, Waud WR, Poupon MF, Colpaert F, Kruczynski A (1999) Superior in vivo experimental antitumour activity of vinflunine, relative to vinorelbine, in a panel of human tumour xenografts. Eur J Cancer 35: 512–520 [DOI] [PubMed] [Google Scholar]
- Kruczynski A, Astruc J, Ricome C, Colpaert F, Hill BT (1999) Definite antitumour activity of vinflunine, a novel fluorinated vinca alkaloid, against human tumour xenografts. Contrib Oncol Basel Karger 54: 369–378 [Google Scholar]
- Kruczynski A, Barret JM, Etiévant C, Colpaert F, Fahy J, Hill BT (1998) Antimitotic and tubulin-interacting properties of vinflunine, a novel vinca alkaloid. Biochem Pharmacol 55: 635–648 [DOI] [PubMed] [Google Scholar]
- Kruczynski A, Etiévant C, Perrin D, Chansard N, Duflos A, Hill BT (2002) Characterization of apoptosis induced by vinflunine, the most recent vinca alkaloid in clinical development. BJC 86: 143–150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livingston RB, Ellis GK, Gralow JR, Williams MA, White R, McGuirt C, Adamkiewicz BB, Long CA (1997) Dose-intensive vinorelbine with concurrent granulocyte colony-stimulating factor support in paclitaxel-refractory metastatic breast cancer. J Clin Oncol 15: 1395–1400 [DOI] [PubMed] [Google Scholar]
- Lobert S, Ingram JW, Hill BT, Correia JJ (1998) A comparison of thermodynamic parameters for vinorelbine- and vinflunine-induced tubulin self-association by sedimentation velocity. Mol Pharmacol 53: 908–915 [PubMed] [Google Scholar]
- Low JA, Wedam SB, Lee JJ, Berman AW, Brufsky A, Yang SX, Poruchynsky MS, Steinberg SM, Mannan N, Fojo T, Swain SM (2005) Phase II clinical trial of ixabepilone (BMS-247550), an epothilone B analog, in metastatic and locally advanced breast cancer. J Clin Oncol 23: 2726–2734 [DOI] [PubMed] [Google Scholar]
- Ngan VK, Bellman K, Hill BT, Wilson L, Jordan MA (2001) Mechanism of mitotic block and inhibition of cell proliferation by semisynthetic vinca alkaloids vinorelbine and its new derivative vinflunine. Mol Pharmacol 60: 225–232 [DOI] [PubMed] [Google Scholar]
- Ngan VK, Bellman K, Panda D, Hill BT, Jordan MA, Wilson L (2000) Novel actions of the antitumor drugs vinflunine and vinorelbine on microtubules. Cancer Res 60: 5045–5051 [PubMed] [Google Scholar]
- Perez EA, Hillman DW, Mailliard JA, Ingle JN, Ryan JM, Fitch TR, Rowland KM, Kardinal CG, Krook JE, Kugler JW, Dakhil SR (2004) Randomized phase II study of two irinotecan schedules for patients with metastatic breast cancer refractory to an anthracycline, a taxane, or both. J Clin Oncol 22: 2849–2855 [DOI] [PubMed] [Google Scholar]
- Reichardt P, Von Minckwitz G, Thusss-Patience PC, Jonat W, Kolbl H, Janicke F Kieback DG, Kuhn W, Schindler AE, Mahrmann S, Kaufmann M, Luck HJ (2003) Multicenter phase II study of oral capecitabine (Xeloda®) in patients with metastatic breast cancer relapsing after treatment with a taxane-containing therapy. Ann Oncol 14: 1227–1233 [DOI] [PubMed] [Google Scholar]
- Smorenburg CH, Bontenbal M, Seynaeve C, Van Zuylen C, de Heus G, Verweij J, de Wit R (2001) Phase II study of weekly gemcitabine in patients with metastatic breast cancer relapsing or failing both an anthracycline and a taxane. Breast Cancer Res Treat 66: 83–87 [DOI] [PubMed] [Google Scholar]
- World Health Organization (1979) WHO handbook for reporting results for cancer treatment. Geneva: World Health Organization [Google Scholar]