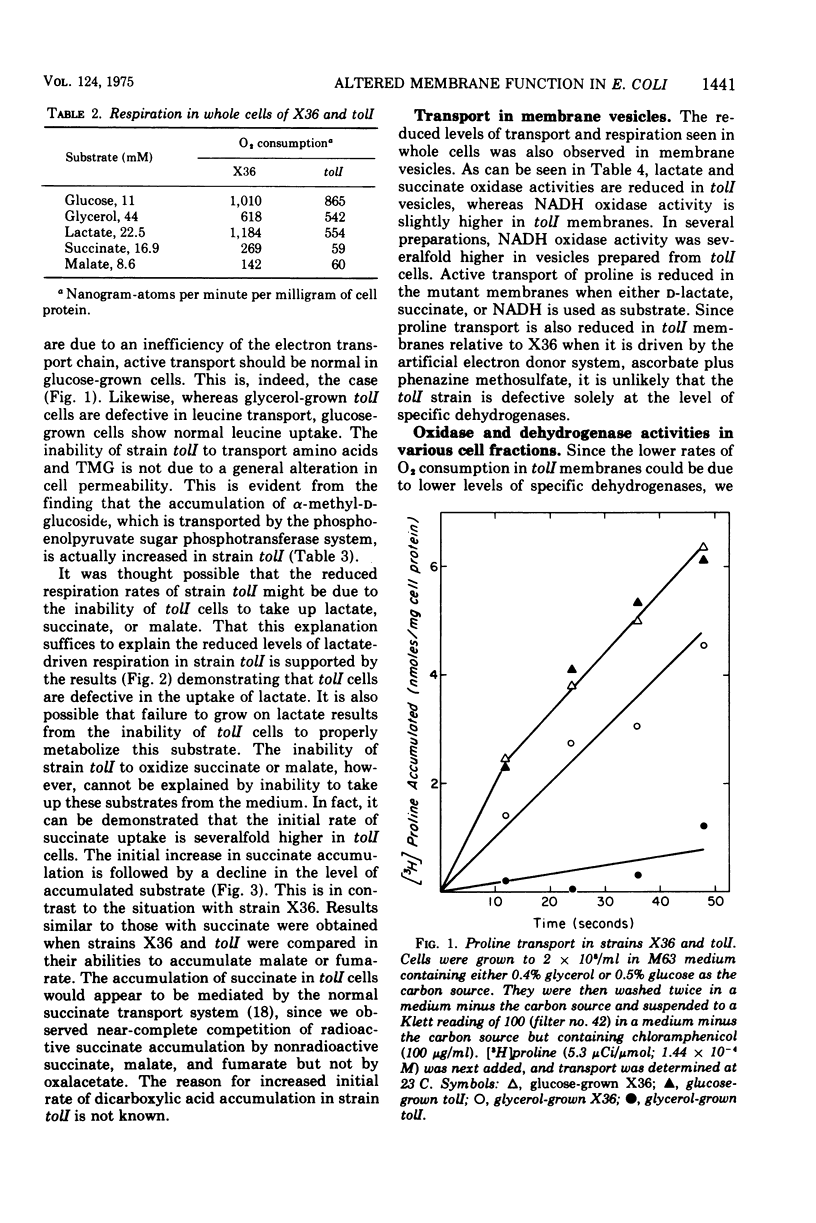
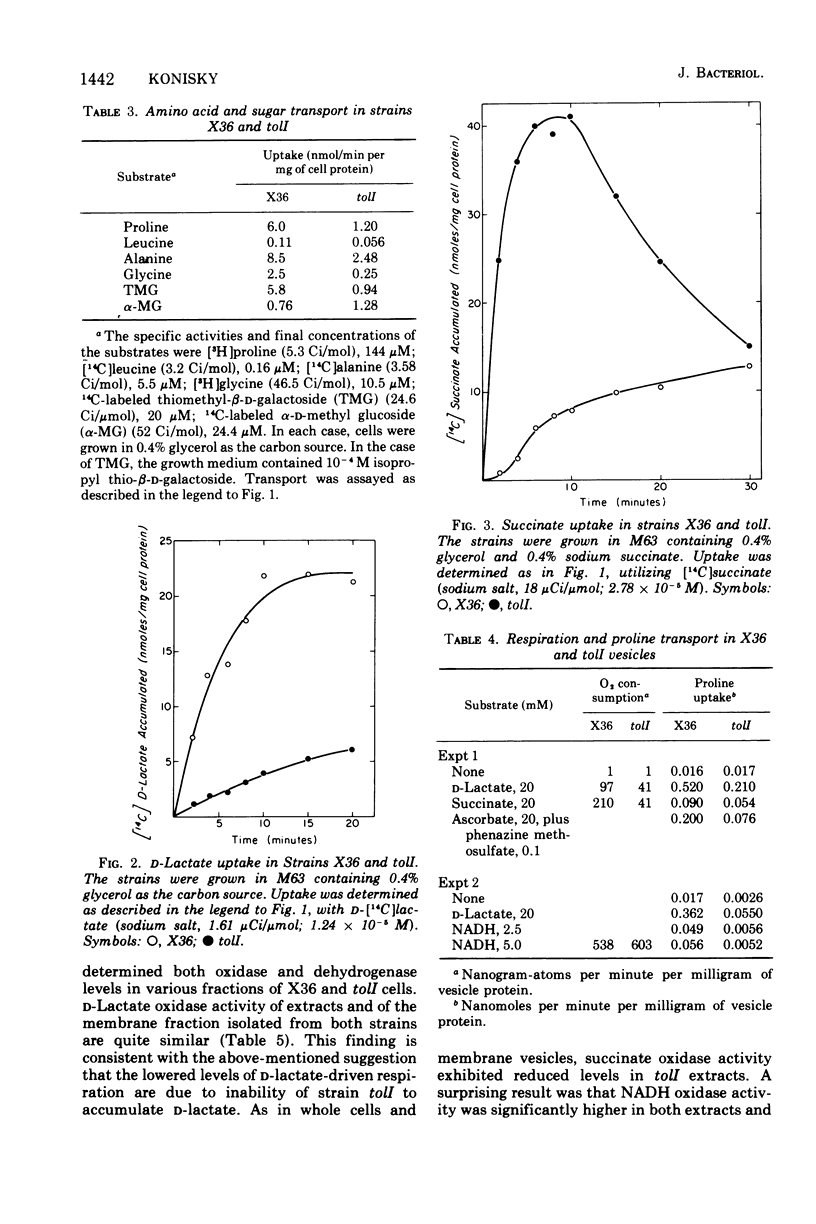
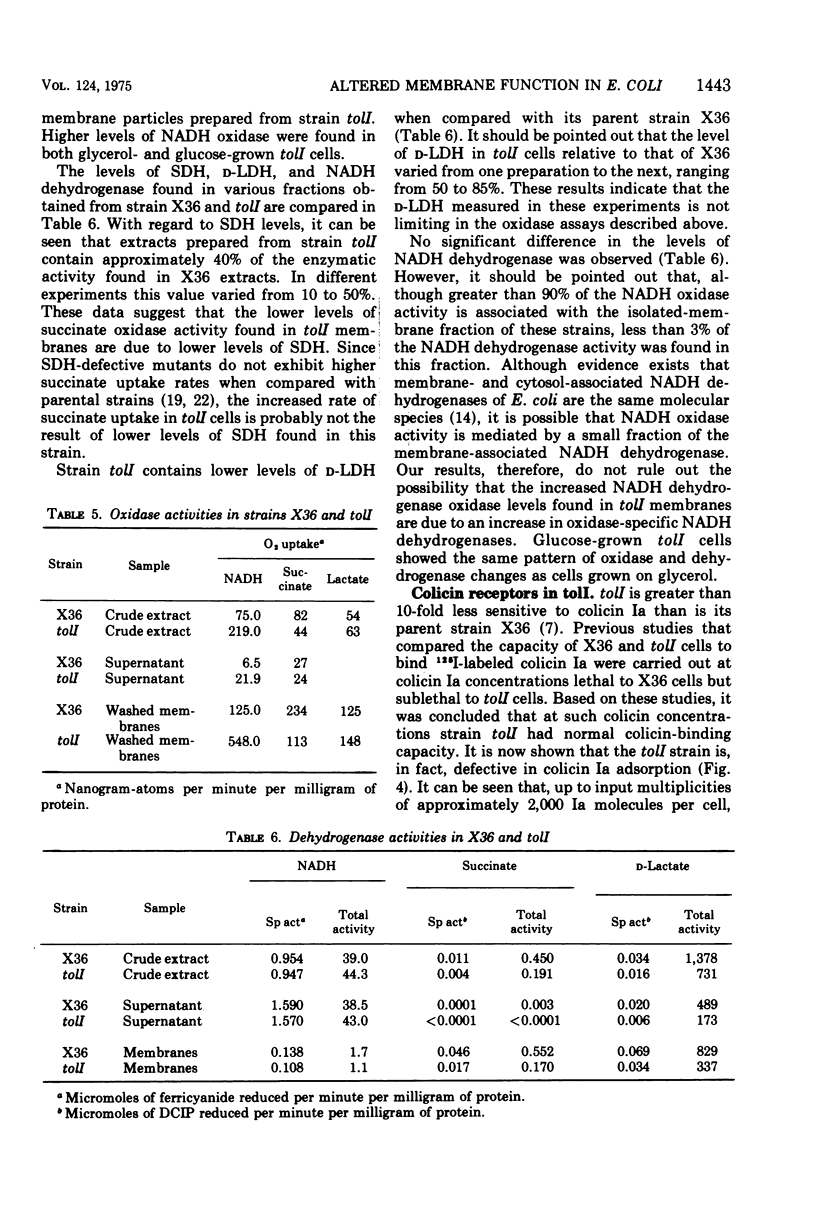
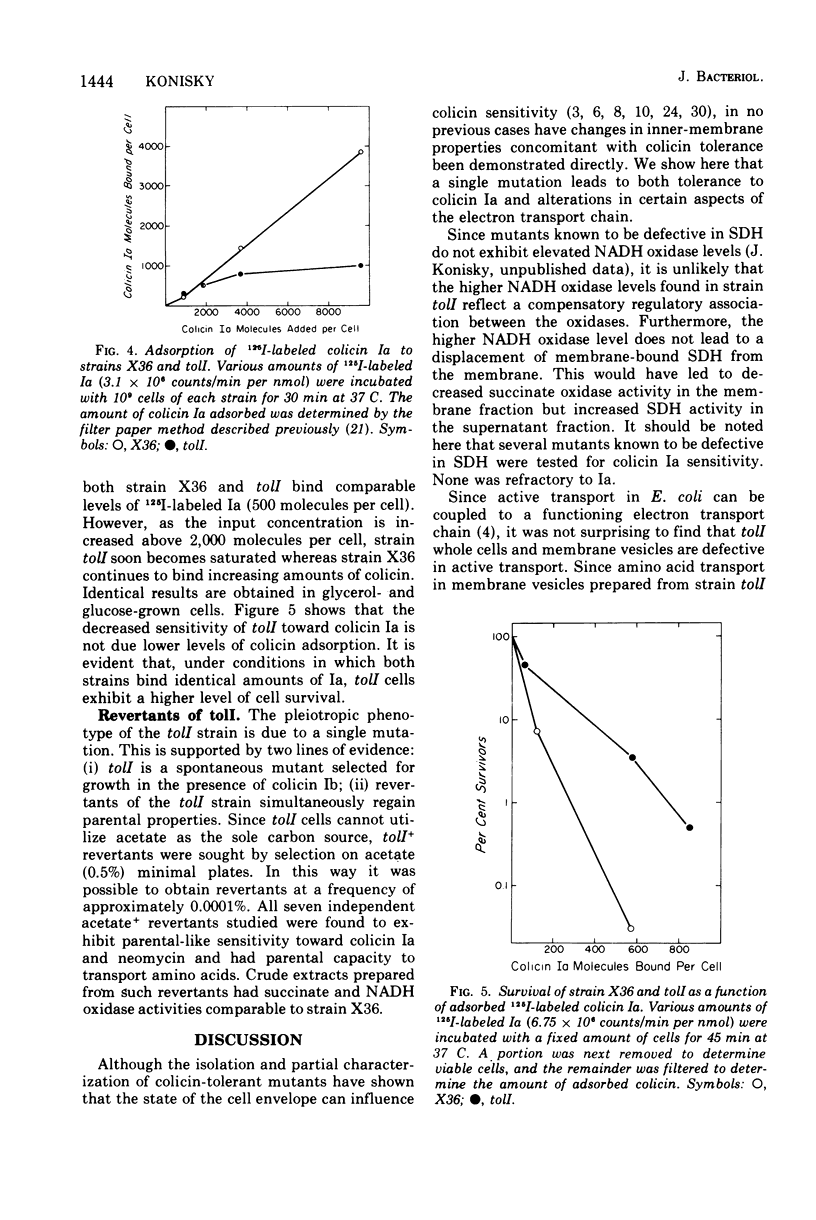
Abstract
An Escherichia coli mutant (tolI) previously shown to be tolerant to colicins Ia and Ib is defective in several functions of the bacterial cytoplasmic membrane. When compared with its parental strain, X36, whole cells of tolI show reduced rates of respiration with succinate, malate, or lactate as the substrate but near-normal rates with glucose or glycerol. Cell membrane preparations prepared from tolI cells exhibit reduced succinate and D-lactate oxidase activity but elevated levels of reduced-form nicotinamide adenine dinucleotide (NADH) oxidase. tolI cells have reduced levels of succinate and D-lactate dehydrogenase but normal levels of NADH dehydrogenase. Glycerol-grown tolI cells and membrane vesicles prepared from such cells are defective in the active transport of several amino acids and thiomethyl-beta-D-galactoside; however, they accumulate higher levels of alpha-methylglucoside when compared with X36 whole cells or vesicles. Although tolI cells adsorb less colicin Ia at high colicin concentrations than do X36 cells, it is shown that the adsorption of an Ia molecule to tolI cells has a lower probability of eliciting cell death than does Ia adsorption to strain X36 cells. It is concluded that a single mutation can lead to an alteration in several aspects of cytoplasmic membrane function and colicin I sensitivity.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARMSTRONG J. M. THE MOLAR EXTINCTION COEFFICIENT OF 2,6-DICHLOROPHENOL INDOPHENOL. Biochim Biophys Acta. 1964 Apr 4;86:194–197. doi: 10.1016/0304-4165(64)90180-1. [DOI] [PubMed] [Google Scholar]
- Barnes E. M., Jr, Kaback H. R. Mechanisms of active transport in isolated membrane vesicles. I. The site of energy coupling between D-lactic dehydrogenase and beta-galactoside transport in Escherichia coli membrane vesicles. J Biol Chem. 1971 Sep 10;246(17):5518–5522. [PubMed] [Google Scholar]
- Bernstein A., Rolfe B., Onodera K. Pleiotropic properties and genetic organization of the tolA,B locus of Escherichia coli K-12. J Bacteriol. 1972 Oct;112(1):74–83. doi: 10.1128/jb.112.1.74-83.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boos W. Bacterial transport. Annu Rev Biochem. 1974;43(0):123–146. doi: 10.1146/annurev.bi.43.070174.001011. [DOI] [PubMed] [Google Scholar]
- Brewer G. J. Chlorotetracycline as a fluorescent probe for membrane events in the action of colicin K on Escherichia coli. Biochemistry. 1974 Nov 19;13(24):5038–5045. doi: 10.1021/bi00721a027. [DOI] [PubMed] [Google Scholar]
- Burman L. G., Nordström K. Colicin tolerance induced by ampicillin or mutation to ampicillin resistance in a strain of Escherichia coli K-12. J Bacteriol. 1971 Apr;106(1):1–13. doi: 10.1128/jb.106.1.1-13.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardelli J., Konisky J. Isolation and characterization of an Escherichia coli mutant tolerant to colicins Ia and Ib. J Bacteriol. 1974 Aug;119(2):379–385. doi: 10.1128/jb.119.2.379-385.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chappell J. B. The oxidation of citrate, isocitrate and cis-aconitate by isolated mitochondria. Biochem J. 1964 Feb;90(2):225–237. doi: 10.1042/bj0900225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson-Grennberg K. G., Nordström K. Genetics and physiology of a tolE mutant of Escherichia coli K-12 and phenotypic suppression of its phenotype by galactose. J Bacteriol. 1973 Sep;115(3):1219–1222. doi: 10.1128/jb.115.3.1219-1222.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilchrist M. J., Konisky J. Effects of colicin Ia on transport and respiration in Escherichia coli. J Biol Chem. 1975 Apr 10;250(7):2457–2462. [PubMed] [Google Scholar]
- HATEFI Y., HAAVIK A. G., JURTSHUKP Studies on the electron transport system. XXX. DPNH-cytochrome c reductase I. Biochim Biophys Acta. 1961 Sep 2;52:106–118. doi: 10.1016/0006-3002(61)90908-8. [DOI] [PubMed] [Google Scholar]
- Hempfling W. P. Repression of oxidative phosphorylation in Escherichia coli B by growth in glucose and other carbohydrates. Biochem Biophys Res Commun. 1970 Oct 9;41(1):9–15. doi: 10.1016/0006-291x(70)90461-4. [DOI] [PubMed] [Google Scholar]
- Hendler R. W., Burgess A. H. Fractionation of the electron-transport chain of Escherichia coli. Biochim Biophys Acta. 1974 Aug 23;357(2):215–230. doi: 10.1016/0005-2728(74)90062-0. [DOI] [PubMed] [Google Scholar]
- Jetten A. M., Jetten M. E. Energy requirement for the initiation of colicin action in Escherichia coli. Biochim Biophys Acta. 1975 Apr 14;387(1):12–22. doi: 10.1016/0005-2728(75)90048-1. [DOI] [PubMed] [Google Scholar]
- Kasahara M., Anraku Y. Succinate dehydrogenase of Escherichia coli membrane vesicles. Activation and properties of the enzyme. J Biochem. 1974 Nov;76(5):959–966. [PubMed] [Google Scholar]
- Kay W. W., Kornberg H. L. Genetic control of the uptake of C(4)-dicarboxylic acids by Escherichia coli. FEBS Lett. 1969 Apr;3(2):93–96. doi: 10.1016/0014-5793(69)80105-5. [DOI] [PubMed] [Google Scholar]
- Kay W. W., Kornberg H. L. The uptake of C4-dicarboxylic acids by Escherichia coli. Eur J Biochem. 1971 Jan;18(2):274–281. doi: 10.1111/j.1432-1033.1971.tb01240.x. [DOI] [PubMed] [Google Scholar]
- Kim I. C., Bragg P. D. Some properties of the succinate dehydrogenase of Escherichia coli. Can J Biochem. 1971 Oct;49(10):1098–1104. doi: 10.1139/o71-159. [DOI] [PubMed] [Google Scholar]
- Konisky J., Cowell B. S. Interaction of colicin Ia with bacterial cells. Direct measurement of Ia-receptor interaction. J Biol Chem. 1972 Oct 25;247(20):6524–6529. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lo T. C., Rayman M. K., Sanwal B. D. Transport of succinate in Escherichia coli. I. Biochemical and genetic studies of transport in whole cells. J Biol Chem. 1972 Oct 10;247(19):6323–6331. [PubMed] [Google Scholar]
- Nagel de Zwaig R., Luria S. E. Genetics and physiology of colicin-tolerant mutants of Escherichia coli. J Bacteriol. 1967 Oct;94(4):1112–1123. doi: 10.1128/jb.94.4.1112-1123.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto K. Requirement of heat and metabolic energy for the expression of inhibitory action of colicin K. Biochim Biophys Acta. 1975 May 6;389(2):370–379. doi: 10.1016/0005-2736(75)90329-6. [DOI] [PubMed] [Google Scholar]
- Plate C. A. Effects of temperature and of fatty acid substitutions on colicin K action. Antimicrob Agents Chemother. 1973 Jul;4(1):16–24. doi: 10.1128/aac.4.1.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds B. L., Reeves P. R. Kinetics of adsorption of colicin CA42-E2 and reversal of its bactericidal activity. J Bacteriol. 1969 Oct;100(1):301–309. doi: 10.1128/jb.100.1.301-309.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer M. E., Guest J. R. Proteins of the inner membrane of Escherichia coli: changes in composition associated with anaerobic growth and fumarate reductase amber mutation. J Bacteriol. 1974 Mar;117(3):954–959. doi: 10.1128/jb.117.3.954-959.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Săsărman A., Surdeanu M., Szégli G., Horodniceanu T., Greceanu V., Dumitrescu A. Hemin-deficient mutants of Escherichia coli K-12. J Bacteriol. 1968 Aug;96(2):570–572. doi: 10.1128/jb.96.2.570-572.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitney E. N. The tolC locus in Escherichia coli K12. Genetics. 1971 Jan;67(1):39–53. doi: 10.1093/genetics/67.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]


