Abstract
The gene SASH1 (SAM- and SH3-domain containing 1) has originally been identified as a candidate tumour suppressor gene in breast cancer. SASH1 is a member of the SH3-domain containing expressed in lymphocytes (SLY1) gene family that encodes signal adapter proteins composed of several protein–protein interaction domains. The other members of this family are expressed mainly in haematopoietic cells, whereas SASH1 shows ubiquitous expression. We have used quantitative real-time PCR to investigate the expression of SASH1 in tissue samples from 113 patients with colon carcinoma, and compared the expression with 15 normal colon tissue samples. Moreover, nine benign adenomas and 10 liver metastases were analysed. Expression levels of SASH1 were strongly and significantly reduced in colon cancer of UICC stage II, III, and IV, as well as in liver metastases. Moreover, SASH1 was also found to be downregulated on protein levels by immunoblot analysis. However, SASH1 expression was not significantly deregulated in precancerous adenomas and in earlier stage lesions (UICC I). Overall, 48 out of 113 primary colon tumours showed SASH1 expression that was at least 10-fold lower than the levels found in normal colon tissue. Downregulation of SASH1 expression was correlated with the formation of metachronous distant metastasis, and multivariate analysis identified SASH1 downregulation as an independent negative prognostic parameter for patient survival. This study demonstrates for the first time that expression of a member of the SLY1-gene family has prognostic significance in human cancer.
Keywords: colorectal cancer, prognosis, signalling adapter protein, metastasis
Many of the molecular changes associated with tumour formation have been defined for colorectal cancer, owing to the high frequency of the disease and the relatively simple access to virtually all tumour stages by endoscopy (Fearon and Vogelstein, 1990; Kinzler and Vogelstein, 1996). However, many more genes, some still uncharacterised, are likely to be involved in tumour progression and metastasis formation. We have focused on the gene SASH1 (SAM- and SH3-domain containing 1) that has previously been described as candidate tumour suppressor gene in breast cancer (Zeller et al, 2003). SASH1 was mapped to chromosome 6q24.3, loss of heterozygosity (LOH) of this region (occurring in 30% of primary breast cancers) was associated with poor survival and increase in tumour size. Moreover, a strong reduction of SASH1 expression was observed in the majority of breast tumours when compared to normal mammary epithelia (Zeller et al, 2003). Loss of chromosomal region 6q24–25 has also been detected in a study on primary colorectal tumours, and was specifically associated with invasive tumours extending to the serosal fat (Alcock et al, 2003). However, the predictive capacity of SASH1 deregulation in human cancer has not been determined previously. The domain structure and strong sequence similarities places SASH1 in the SH3-domain containing expressed in lymphocytes (SLY1) family of signal adapter proteins, which was first identified in haematopoietic cells (Beer et al, 2001), and comprises in addition the proteins SLY1, and HACS1 (or SLY2, NASH1). SLY1 is involved in regulation of the adaptive immune system (Beer et al, 2001, 2005). HACS1 (or NASH1) is expressed in mast cells, and was reported to be differentially expressed in malignant haematopoietic cells (Claudio et al, 2001; Uchida et al, 2001; Zhu et al, 2004). Whereas the expression of SLY1 and HACS1 seems to be restricted to haematopoietic cells, SASH1 shows ubiquitous expression in human tissue (Zeller et al, 2003). Here, we provide the first evidence that downregulation of SASH1 constitutes an independent prognostic parameter in colon cancer.
PATIENTS, MATERIALS AND METHODS
Patients
Informed, written consent regarding the use of the tissue samples was obtained from each subject before the study. Tissue samples were obtained from 113 patients admitted to our Department of Surgery with the diagnosis colon carcinoma. The group consisted of 69 male and 44 female patients, mean age was 64 years. None of the patients suffered of a known second neoplastic disease; only complete resected tumours (R0) were included in the study. Median survival after surgery was 91 months (range: 44–131 months). During this period, 38 patients died owing to tumour-related causes. Disease recurred in 15 patients, 34 patients developed metachronous distant metastases, and 23 patients showed disease progression. Tumour localisation was: ascending colon (41 cases), transverse colon (12 cases), descending colon (18 cases) and sigmoid colon (42 cases). Tumour grading was: G1 (three cases), G2 (74 cases), G3 (33 cases) and G4 (three cases). Tumour stages according UICC classification were: stage I (12 cases), stage II (45 cases), stage III (23 cases), and stage IV (33 cases). Sixty-five patients had no adjuvant treatment, and 48 patients received systemic chemotherapy. As a control, we examined normal colon tissue (15 patients), benign colonic adenomas (nine patients), and liver metastases from 10 patients. Samples were frozen in liquid nitrogen immediately after surgery and stored at −80°C.
RNA Isolation from cell lines and tissue samples
To establish and validate the quantification of SASH1 expression the following human cell lines were used: HEK293 (embryonic kidney epithelial cells), HeLa (cervical carcinoma), SKOV-3 (ovarian adenocarcinoma), CaCo2 (grade II colorectal adenocarcinoma), HT29 (grade I colorectal adenocarcinoma), Jurkat (T-lymphocyte from acute T-cell leukaemia), and Ramos (B-lymphocyte from Burkitt's lymphoma). For RNA isolation from tissue, we used 40 sections of 12 μm thickness (surface approximately, 1–2 cm2). Immunochemistry (haematoxylin (H) and eosin (E) staining) was performed on each first and last section to ensure tumour content above 70%. Accordingly, frozen sections from resected normal colon tissue (as certified by an experienced pathologist) were subjected to the same protocol. RNA was isolated using the RNeasy Kit (Qiagen, Hilden, Germany), quantified, and checked for degradation on a denaturing formaldehyde–agarose gel. cDNA preparation was performed according to standard procedures, using SuperscriptII H-Reverse Transcriptase (Invitrogen, Karlsruhe, Germany) and oligo-dT primers (pd(N)6; Roche Alameda, CA, USA).
Quantitative real-time PCR
Expression of SASH1 and SLY1 transcripts was determined by real-time reverse transcriptase–polymerase chain reaction (RT—PCR) using the ABI PRISM 7300 sequence detection system (Applied Biosystems, Foster City, CA, USA) with the dye SYBRGreen I. Expression of the housekeeping gene HPRT was used as internal reference.
- • HPRT-F:
5′-GCT TTC CTT GGT CAG GCA GTA TAA T-3′
- • HPRT-R:
5′-AAG GGC ATA TCC TAC AAC AAA CTT G-3′
- • SASH1-F:
5′- CGG GAA AGC GTC AAG TCG GA-3′
- • SASH1-R:
5′- ATC TCC TTT CTT GAG CTT GAG-3′
- • SLY1-F:
5′- TCC AGC AGC TTC AAG GAT TT-3′
- • SLY1-R:
5′- CAT CTT GCC CAT CTT CCT GT-3′
Statistical analysis
Analyses were performed using SPSS version 9.0 (SPSS, Munich, Germany). Statistical significance was defined as P⩽0.05. As data sets were too small to assume normal distribution, nonparametric tests were used. The results were analysed with the Kruskal–Wallis test or Mann–Whitney U-test to check for significant differences between individual groups. Expression of SASH1 was assessed in terms of survival by the Cox proportional hazards model using univariate and multivariate analysis. Significance was tested by χ2 analysis.
Preparation of protein lysates
Resected tumours and normal colon tissue samples (as certified by an experienced pathologist) were snap-frozen in liquid nitrogen in lactate buffered Ringer's solution, and stored at −80°C. Tissue samples were thawn quickly and homogenised in pre-cooled Dounce homogenisers in TBST buffer (20 mM Tris/HCl, pH 8.5, 150 mM NaCl, 1% Triton X-100). After homogenisation, samples were extracted overnight at 4°C, and subsequently centrifuged for 1 h, 4°C, at 100 000 g in an ultracentrifuge (Beckman, Krefeld, Germany). Supernatants were collected, frozen in liquid nitrogen, and stored at −80°C. Protein concentration was determined with a Bradford Assay (Biorad, Munich, Germany).
Immunoblotting and antibodies
Equal amounts (40 μg) of protein lysates were boiled in Laemmli buffer for 5 min, separated on 10% polyacrylamide gels, and subjected to immunoblotting according to standard procedures (Towbin et al, 1979). Mouse monoclonal anti-β-tubulin antibody (Oncogene Research Products, Darmstadt, Germany) was used as loading control. A polyclonal rabbit anti-serum was generated by immunisation with two specific peptides corresponding to amino-terminal sequences of murine SASH1, and the serum was further affinity-purified with the peptides. A detailed description of the antiserum will be subject of a future publication. Briefly, the antiserum was used at 1 : 2000 dilution on the immunoblots (at 4°C, overnight), recognising a specific band of an apparent molecular weight of 180 kDa in normal human colon lysates. Secondary antibodies were horseradish peroxidase-conjugated goat anti-mouse IgG and goat anti-rabbit IgG (Jackson Immunoresearch, West Grove, PA, USA), visualised with an enhanced chemiluminescence substrate detection kit (Pierce, Rockford, IL, USA).
RESULTS
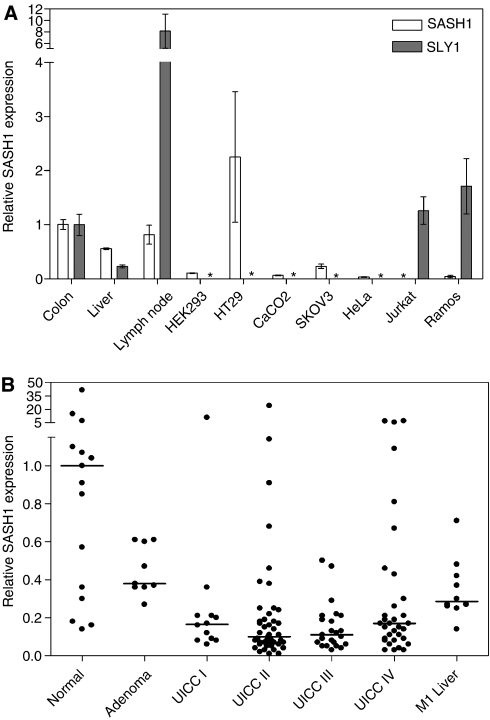
We have first tested normal human tissues and cell lines by qRT–PCR for the expression of SASH1 and its homolog SLY1. SASH1 expression was detected in colon, liver and peripheral lymph nodes, and in reduced amounts as compared to normal colon in various cell lines of epithelial origin (HEK293, SKOV-3, CaCo2) (Figure 1A). Interestingly, expression in HT29 cells (corresponding to well-differentiated colorectal adenocarcinoma) was two-fold higher than in normal colon. SASH1 expression was very low to undetectable in lymphoblastoid cells (Jurkat and Ramos cells) and HeLa cells. The expression patterns of SASH1 and its homologue SLY1 were found to be mutually exclusive, as SLY1 was expressed in the lymphoblastoid cells, but undetectable in epithelial cells. Correspondingly, SLY1 showed highest expression in lymph nodes, and about 10-fold lower expression in colon. It has been reported that protein kinase C (PKC) is a regulator of SASH1 expression (Lindvall et al, 2005). However, we could not observe any difference in expression of SASH1 upon stimulation of PKC with phorbol 12-myristate 13-acetate (PMA)/ionomycin in all cell lines tested (not shown).
Figure 1.
(A) Relative expression of SASH1 and SLY1 determined by qRT–PCR in normal human tissue (n=3 specimen) and cell lines, normalised to the expression in colon. Means±s.d. for three independent experiments. Asterisk: no expression detectable. (B) Relative SASH1 expression in normal colon (n=15), benign adenomas (n=9), and primary colon tumours from stages UICC stage I (n=12), stage II (n=45), stage III (n=23), and stage IV (n=33). Moreover, liver metastases (n=10) and normal liver tissue (n=9) not shown was analysed. SASH1 expression was significantly different from normal tissue in: UICC stage II (P=0.031), UICC stage III (P=0.047), UICC stage IV (P=0.043), and in liver metastases (P=0.030). The bar indicates median expression for each group. Expression was normalised to the median expression of all normal colon samples.
Next, we analysed primary colon carcinomas from 113 patients and compared the expression with 15 normal colon samples (Figure 1). In addition, nine precancerous lesions (adenoma) were analysed. SASH1 was significantly downregulated in tumour stages UICC II, III and IV, but not in adenomas or UICC stage I cancers (Figure 1B). Forty-two per cent of all carcinomas (48 out of 113 samples) showed SASH1 expression that was 10-fold lower in comparison to normal colon tissue. Of note, we detected high levels of expression (>5-fold of the median expression in normal colon) in five tumour samples (4%), and in three normal colon tissues (23%). However, SASH1 expression was not significantly correlated to the anatomical tumour site.
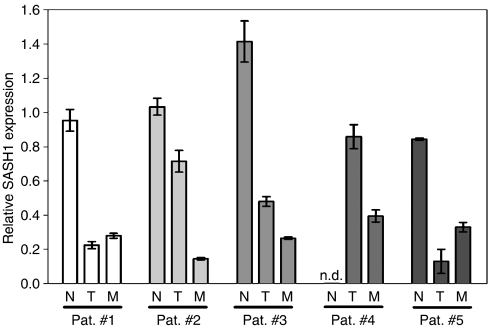
We then compared matched normal colon tissue, primary tumour and colorectal liver metastasis from five individual patients (Figure 2). In all cases, the expression of SASH1 was significantly reduced in the liver metastasis as compared to normal colon tissue. In three cases, SASH1 was already downregulated in the primary tumour, and in two patients, the SASH1 mRNA level of the liver metastasis was significantly lower than that of the matched primary tumour (P<0.01). As the expression levels of SASH1 in normal human liver were not well established so far, we have compared liver metastases to normal liver tissue from nine patients. We could confirm that SASH1 was significantly downregulated in the metastatic tissue also in comparison to the normal surrounding liver tissue.
Figure 2.
Relative expression of SASH1 in matched normal tissue, primary colon tumours and liver metastasis from five patients, normalised to the median expression of all normal colon samples. Mean±s.d. is indicated for three replicate measurements. Note: Normal colon was not available for patient #4.
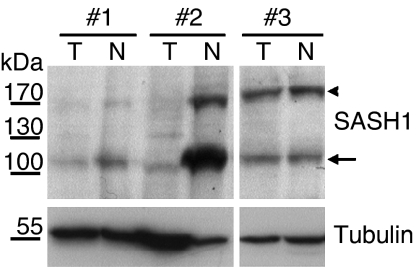
The observed downregulation of SASH1 expression may not necessarily be found on protein levels. Therefore, we have generated a polyclonal rabbit anti-serum that specifically recognises a peptide epitope of SASH1. In a restricted subset of patient samples (n=10), where sufficient material (>50 mg) from both normal and tumour tissue from the same patient was available for protein extraction, we have tested expression by immunoblotting. SASH1 signals were visible at 180 kDa (Figure 3, arrowhead), somewhat exceeding the predicted molecular weight of 140 kDa. Downregulation of SASH1 protein was detectable in eight out of 10 tested tumours. Figure 3 shows representative examples: tumours from patients #1 and #2 show strong downregulation of SASH1, whereas tumour #3 has an equal expression of SASH1 in normal and cancer tissue. Interestingly, a prominent second band with higher electrophoretic mobility (about 110 kDa) was detected in tumour lysates with the anti-SASH1 anti-serum, especially strong in normal tissue from patient #2 (Figure 3, arrow). This lower band may correspond to a degradation product, or to a splice variant of SASH1. After preincubation of the immunoblot with a 10-fold molar excess of the peptides against which the antiserum was generated, both bands were no longer visible, demonstrating the specificity of the immunoreactive bands (not shown).
Figure 3.
SASH1 protein expression in tumour (marked ‘T’) and matched normal tissue (marked ‘N’) from three representative patients. The SASH1 signal has an apparent molecular weight of 180 kDa (arrowhead). SASH1 expression was lower in tumour than in corresponding normal tissue in eight out of 10 tested patients, as seen in patients #1 and #2. In case #3, SASH1 expression was unchanged in the tumour as compared to normal colon. Note the presence of a prominent additional band with higher electrophoretic mobility, which is also reduced in tumours (arrow). Tubulin staining as loading control indicated in the lower panel.
SASH1 transcript expression was not correlated with disease recurrence, but there was a significant correlation of SASH1 expression with metastasis formation: SASH1 downregulation was correlated with the development of metachronous metastasis, defined as metastases occurring more than 6 months after resection of the primary tumour (P=0.014, Mann–Whitney U-test). SASH1 downregulation was also associated with the progression of synchronous metastasis (P=0.046, Kruskal–Wallis H-test). The group of 113 patients was then statistically evaluated by multivariate regression analysis for overall survival using the following factors: age, sex, disease recurrence, pT and nodal status (pN) status, tumour grading, and SASH1 expression (Table 1). SASH1 expression below cutoff (10-fold lower than in normal colon) independently predicted survival in this multivariate analysis using the Cox proportional hazard model, with a relative risk of 2.9 (P=0.005). This analysis also retained known independent predictors for survival, such as disease recurrence, pN, and tumour grading, whereas age and sex were not retained. The pT status was retained in univariate, but not in multivariate analysis as independent parameter.
Table 1. Multivariate Cox regression analysis of prognostic variables.
| Variable | Relative risk | P-value |
|---|---|---|
| pT | — | NS |
| pN | 2.124 | 0.001 |
| Recurrence | 3.735 | <0.001 |
| SASH1 expression below cutoff | 2.908 | 0.005 |
| Grading | 2.413 | 0.002 |
pN=nodal status; pT=primary tumour stage.
DISCUSSION
A tremendous amount of information has been achieved regarding the molecular and cellular changes associated with colon cancer. However, the overall survival rate for this disease has not substantially improved over the last 20 years (Parker et al, 1997), and patient prognosis still relies mainly on the penetration depth of the tumour and the spreading of the disease to other organs. The identification of new genes functionally involved in tumour development and progression may help to find alternative approaches for prognostic and diagnostic evaluation. Our study focuses on SASH1, a new candidate tumour suppressor gene of the SLY1 family of signal adapter proteins. We have observed broad expression of SASH1 in various normal tissues and epithelial cell lines, with the exception of lymphoblastoid cells. The observed expression pattern of SASH1 was complementary to that of its homologue SLY1.
Interestingly, SASH1 expression was significantly downregulated in invasive and metastasised tumours. The downregulation of SASH1 expression was validated and reproduced independently by immunoblot analysis. SASH1 expression was not reduced in adenomas and locally restricted, low-stage tumours (UICC I). Therefore, it is tempting to speculate that SASH1 does not play a role in the early phases of tumour initiation, but is rather related to processes such as tumour invasion in the surrounding tissue, and dissemination of tumour cells to distant organs. Indeed, SASH1 expression was clearly downregulated in liver metastasis, and the expression levels in the metastases were equal to or lower than the expression in the primary tumour from the same patient. Statistical analysis revealed that downregulation of SASH1 in the primary tumour correlated with the formation of metachronous, postoperative metastasis, and with the progression of synchronous metastases. Moreover, SASH1 downregulation was retained as an independent negative prognostic factor for patient survival by multivariate analysis.
Currently, the molecular mechanisms that regulate SASH1 expression are unknown. Stimulation of PKC in vitro was reported to reduce SASH1 expression in primary B-lymphocytes (Lindvall et al, 2005). Interestingly, PKC may play a role in colorectal cancer formation (Zhang et al, 2004), and the homologue SLY1 is regulated by PKC (Beer et al, 2005). However, we did not detect any regulation of SASH1 expression by PMA/ionomycin stimulation in vitro in various cell lines (HeLa, HEK293, HT29, Ramos). Alternatively, the downregulation of SASH1 in tumours may be explained by genetic mechanisms such as LOH or point mutations. Indeed, frequent LOH at the SASH1 genomic locus 6q24–25 has been reported in primary breast cancer, and also in one study in primary colorectal tumours, with specific association with tumour invasion to the serosal fat (Alcock et al, 2003; Zeller et al, 2003). However, despite extensive sequencing efforts, Zeller and co-workers have not been able to demonstrate invalidating mutations in the second allele that would explain the lack of expression of SASH1 in breast cancer (Zeller et al, 2003). Thus, epigenetic mechanisms such as promoter hypermethylation may underlie the observed downregulation of SASH1 expression.
In the patient collective, a subgroup of tumours displayed normal or elevated levels of SASH1. This might be explained by the different routes that lead to colorectal cancer. Sporadic cancers of the colon are characterised by different forms of genetic instability, which can manifest themselves in either chromosomal instability, or, less frequently, in microsatellite instability (MSI). Interestingly, SASH1 expression was normal in a tumour from a patient diagnosed with hereditary nonpolyposis colorectal cancer (HNPCC) syndrome and MSI-high status (not shown). Even though sporadic MSI-high colon cancer differs from the rare hereditary forms (Jass, 2004), it is tempting to speculate that there may be a connection between normal SASH1 expression in tumours and high MSI levels. However, the tumour samples in this study were not generally characterised for their MSI levels. Therefore, further investigations are necessary to clarify this hypothetical correlation between MSI status and the expression of the tumour suppressor SASH1.
The functional consequences of downregulated SASH1 expression are still unclear. The SASH1 gene encodes a large protein of 1230 amino acids that contains two SAM (sterile α-module) domains, and one SH3-domain (Src homology domain 3). SH3 domains bind to proline-rich motifs in proteins (Pawson, 1995), SAM domains are able to self-associate or to interact with other protein domains (Kim et al, 2001, 2002). Both domains are frequently found in signal adapter proteins or scaffolding factors. Thus, the function of SASH1 may lie in the formation of multiprotein signalling complexes that regulate cell survival, proliferation, or migration. In conclusion, we have demonstrated that downregulation of SASH1 expression in colon cancer is associated with metastasis and bad prognosis.
Acknowledgments
This work was supported by grants from the Deutsche Forschungsgemeinschaft (SFB456, TPA13), and from the KKF/Klinikum rechts der Isar.
References
- Alcock HE, Stephenson TJ, Royds JA, Hammond DW (2003) Analysis of colorectal tumor progression by microdissection and comparative genomic hybridization. Genes Chromosomes Cancer 37: 369–380 [DOI] [PubMed] [Google Scholar]
- Beer S, Scheikl T, Reis B, Huser N, Pfeffer K, Holzmann B (2005) Impaired immune responses and prolonged allograft survival in Sly1 mutant mice. Mol Cell Biol 25: 9646–9660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beer S, Simins AB, Schuster A, Holzmann B (2001) Molecular cloning and characterization of a novel SH3 protein (SLY) preferentially expressed in lymphoid cells. Biochim Biophys Acta 1520: 89–93 [DOI] [PubMed] [Google Scholar]
- Claudio JO, Zhu YX, Benn SJ, Shukla AH, McGlade CJ, Falcioni N, Stewart AK (2001) HACS1 encodes a novel SH3–SAM adaptor protein differentially expressed in normal and malignant hematopoietic cells. Oncogene 20: 5373–5377 [DOI] [PubMed] [Google Scholar]
- Fearon ER, Vogelstein B (1990) A genetic model for colorectal tumorigenesis. Cell 61: 759–767 [DOI] [PubMed] [Google Scholar]
- Jass JR (2004) HNPCC and sporadic MSI-H colorectal cancer: a review of the morphological similarities and differences. Fam Cancer 3: 93–100 [DOI] [PubMed] [Google Scholar]
- Kim CA, Gingery M, Pilpa RM, Bowie JU (2002) The SAM domain of polyhomeotic forms a helical polymer. Nat Struct Biol 9: 453–457 [DOI] [PubMed] [Google Scholar]
- Kim CA, Phillips ML, Kim W, Gingery M, Tran HH, Robinson MA, Faham S, Bowie JU (2001) Polymerization of the SAM domain of TEL in leukemogenesis and transcriptional repression. EMBO J 20: 4173–4182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinzler KW, Vogelstein B (1996) Lessons from hereditary colorectal cancer. Cell 87: 159–170 [DOI] [PubMed] [Google Scholar]
- Lindvall JM, Blomberg KE, Wennborg A, Smith CI (2005) Differential expression and molecular characterisation of Lmo7, Myo1e, Sash1, and Mcoln2 genes in Btk-defective B-cells. Cell Immunol 235: 46–55 [DOI] [PubMed] [Google Scholar]
- Parker SL, Tong T, Bolden S, Wingo PA (1997) Cancer statistics, 1997. CA Cancer J Clin 47: 5–27 [DOI] [PubMed] [Google Scholar]
- Pawson T (1995) Protein modules and signalling networks. Nature 373: 573–580 [DOI] [PubMed] [Google Scholar]
- Towbin H, Staehelin T, Gordon J (1979) Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA 76: 4350–4354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida T, Nakao A, Nakano N, Kuramasu A, Saito H, Okumura K, Ra C, Ogawa H (2001) Identification of Nash1, a novel protein containing a nuclear localization signal, a sterile alpha motif, and an SH3 domain preferentially expressed in mast cells. Biochem Biophys Res Commun 288: 137–141 [DOI] [PubMed] [Google Scholar]
- Zeller C, Hinzmann B, Seitz S, Prokoph H, Burkhard-Goettges E, Fischer J, Jandrig B, Schwarz LE, Rosenthal A, Scherneck S (2003) SASH1: a candidate tumor suppressor gene on chromosome 6q24.3 is downregulated in breast cancer. Oncogene 22: 2972–2983 [DOI] [PubMed] [Google Scholar]
- Zhang J, Anastasiadis PZ, Liu Y, Thompson EA, Fields AP (2004) Protein kinase C (PKC) betaII induces cell invasion through a Ras/Mek-, PKC iota/Rac 1-dependent signaling pathway. J Biol Chem 279: 22118–22123 [DOI] [PubMed] [Google Scholar]
- Zhu YX, Benn S, Li ZH, Wei E, Masih-Khan E, Trieu Y, Bali M, McGlade CJ, Claudio JO, Stewart AK (2004) The SH3-SAM adaptor HACS1 is up-regulated in B cell activation signaling cascades. J Exp Med 200: 737–747 [DOI] [PMC free article] [PubMed] [Google Scholar]