Abstract
α-Melanocyte stimulating hormone (α-MSH) analogs, cyclized through site-specific rhenium (Re) and technetium (Tc) metal coordination, were structurally characterized and analyzed for their abilities to bind α-MSH receptors present on melanoma cells and in tumor-bearing mice. Results from receptor-binding assays conducted with B16 F1 murine melanoma cells indicated that receptor-binding affinity was reduced to approximately 1% of its original levels after Re incorporation into the cyclic Cys4,10, d-Phe7–α-MSH4-13 analog. Structural analysis of the Re–peptide complex showed that the disulfide bond of the original peptide was replaced by thiolate–metal–thiolate cyclization. A comparison of the metal-bound and metal-free structures indicated that metal complexation dramatically altered the structure of the receptor-binding core sequence. Redesign of the metal binding site resulted in a second-generation Re–peptide complex (ReCCMSH) that displayed a receptor-binding affinity of 2.9 nM, 25-fold higher than the initial Re–α-MSH analog. Characterization of the second-generation Re–peptide complex indicated that the peptide was still cyclized through Re coordination, but the structure of the receptor-binding sequence was no longer constrained. The corresponding 99mTc- and 188ReCCMSH complexes were synthesized and shown to be stable in phosphate-buffered saline and to challenges from diethylenetriaminepentaacetic acid (DTPA) and free cysteine. In vivo, the 99mTcCCMSH complex exhibited significant tumor uptake and retention and was effective in imaging melanoma in a murine-tumor model system. Cyclization of α-MSH analogs via 99mTc and 188Re yields chemically stable and biologically active molecules with potential melanoma-imaging and therapeutic properties.
Metal ions often play critical roles in protein structure and function. Engineered metal-binding sites in peptides and proteins have been widely used to enhance structural integrity, stabilize biologically active conformations, and confer novel enzymatic activities (1–3). Biochemical and structural analyses of transition-metal coordination by proteins and peptides have traditionally focused on zinc, copper, manganese, and iron because of their roles in important biological processes (4–7). Other transition metals not found in natural proteins have coordination, isotopic, and chemical properties that make them attractive for peptide and protein engineering. Rhenium (Re) and technetium (Tc) are group VIIB transition metals that share similar coordination geometries and form stable complexes with amine and amide nitrogens, carboxylate oxygens, and thiolate and thioether sulfurs, with a strong preference for thiolate sulfurs (8). Radioactive isotopes of Re and Tc have significant medical applications because of the nature of their associated radiation and physical half-life properties.
The synthesis and characterization of radiolabeled antibodies, peptides, and steroid hormones as in vivo tumor-imaging and therapeutic agents is an active area of cancer research today. These molecules specifically target tumor cells by virtue of their high specificities for receptors and antigens present on the surfaces of these cells. In one commonly used approach, metallic radionuclides such as 186Re, 188Re, and 99mTc are appended to the tumor-targeting molecule through bifunctional chelate groups that consist of a metal chelate and an activatable crosslinker (9–11). The resulting radiolabeled proteins, peptides, and small molecules are decorated with one or more chelating groups. The presence of bulky metal-chelating groups and their associated crosslinkers may affect receptor affinity and biodistribution in vivo (12, 13). An alternative approach to the design of radiolabeled tumor-imaging and therapeutic agents involves incorporating the metal directly into the molecule’s structure (14–16). Metal centers with defined coordination geometries can in this way provide a foundation for the construction of stable molecular structures that have high affinities for specific receptors or antigenic sites. Protein, peptide, and small-molecule structures can be designed to use metal coordination to reduce conformational freedom, stabilize active conformations, or mimic native conformations.
α-Melanocyte stimulating hormone (α-MSH) is a tridecapeptide [Ac-Ser-Tyr-Ser-Met-Glu-His-Phe-Arg-Trp-Gly-Lys-Pro-Val-NH2] that regulates skin pigmentation in most vertebrates (17). The core α-MSH sequence His-Phe-Arg-Trp, conserved in a number of species, is sufficient for receptor recognition (17). The presence of α-MSH receptors on both murine and human melanoma cells (18, 19) suggests that α-MSH analogs could be developed into targeted melanoma-imaging or therapeutic agents. Several analogs of α-MSH have been radiolabeled either with halogens or with transition-metal radionuclides coordinated by bifunctional chelates (20–23). Our goal was to design 188Re- or 99mTc-radiolabeled α-MSH analogs in which metal coordination was an integral part of the molecules’ structure. It was hypothesized that cyclic α-MSH analogs, engineered to incorporate radionuclides directly into their structures, would display exceptional stability, biodistribution, and tumor-targeting properties. Rhenium-bound α-MSH analogs were synthesized and characterized to determine whether different metal-incorporation strategies resulted in molecules with different structural stabilities and bioactivities. Incorporation of Re into an initial α-MSH analog resulted in decreased stability and receptor affinity. Structural characterization of the rhenium-bound α-MSH complex Re[(Cys4,10, d-Phe7)–α-MSH4-13] (ReMSH) indicated that metal coordination dramatically altered the core receptor-binding sequence. Correlation of structural changes with functional changes in the ReMSH complex provided the foundation for the design of a second-generation Re[(Cys3,4,10, d-Phe7)–α-MSH3-13] (ReCCMSH) analog with greatly improved chemical stability and bioactivity. Biodistribution and tumor-targeting studies showed rapid tumor uptake and significant tumor retention of the corresponding 99mTc complex 99mTc[(Cys3,4,10, d-Phe7)–α-MSH3-13] (99mTcCCMSH) in melanoma-bearing C57 mice. In addition, initial tumor-imaging experiments showed that the corresponding 99mTcCCMSH analog was able to image melanoma in a murine-tumor model system. These results indicated that 99mTc- and 188ReCCMSH analogs have potential as melanoma-imaging and therapeutic agents.
MATERIALS AND METHODS
Synthesis and Purification of Peptide-Metal Complexes.
The peptides NAc-Cys-Glu-His-d-Phe-Arg-Trp-Cys-Lys-Pro-Val-NH2 (24) and NAc-Cys-Cys-Glu-His-d-Phe-Arg-Trp-Cys-Lys-Pro-Val-NH2 were synthesized by using standard fluorenylmethoxycarbonyl (Fmoc) chemistry. The bombesin analog Trp-Ala-Val-Gly-His-Leu-Met-NH2 was purchased from Sigma. ReMSH was synthesized by using the exchange complex ReOCl3(Me2S)(OPPh3) (25, 26) by incubation of 1:1 peptide/exchange complex in 62% MeOH, pH = 8–9 at 65–70°C for 1 hr. ReCCMSH was synthesized by using the exchange complex Re-glucoheptonate by incubation of 1:1 peptide/exchange complex in 62% MeOH, pH = 8–9 at 65–70°C for 1 hr. The Re–peptide complexes were purified by C18 reverse-phase HPLC. ReMSH was further purified by normal-phase HPLC with a polysulfoethyl aspartamide column from Poly LC (Columbia, MD). Both complexes underwent Ellman’s analysis to test for the presence of free sulfhydryl groups (27). Mass spectrum analyses were performed by electrospray ionization mass spectroscopy at Mallinckrodt for ReCCMSH and by fast atom bombardment-mass spectroscopy for ReMSH at the University of Kansas (Lawrence, KS) mass spectrometry facility.
99mTcO4− was obtained from a 99Mo/99mTc generator (Mallinckrodt, St. Louis). 188Re was obtained from a 188W/188Re generator produced by the University of Missouri Research Reactor. Radiolabeled peptides were prepared by substitution from either 99mTc- or 188Re-glucoheptonate. For 188Re complexes, 500 μl of generator eluent was added to a tube containing 50 mg of sodium glucoheptonate and 10 mg of SnCl2⋅2H2O dissolved in 200 μl of N2-purged 5% EDTA and heated at 70–80°C for 30 min. Fifty microliters of a 1 mg/ml peptide solution was then added, the pH was adjusted to 7–8 with 0.5 M NaOH, and the mixture was allowed to incubate for 90 min at 70–80°C. Syntheses of 99mTc-labeled peptides differed only in that they were carried out at 25°C by using 1/10 the concentration of SnCl2⋅2H2O. Radiolabeled complexes were analyzed by coinjection on a C18 reverse-phase HPLC column, and in all cases were found to coelute with the previously characterized nonradioactive standards. Tracer-level stability tests were performed by diluting the tracer-level reaction mix 1:2 into a 20 mM solution of cysteine or diethylenetriaminepentaacetic acid (DTPA) at t = 0.
NMR Data Collection and Processing.
NMR data were collected on a 500-MHz Bruker AMX or DMX spectrometer and processed on a Silicon Graphics (Mountain View, CA) XZ4000 workstation with the sybyl program (Tripos Associates, St. Louis). The 1H data were collected in 9:1 H2O/D2O at concentrations ranging from 4 to 10 mg/ml. All spectra were recorded by using the time-proportional phase incrementation (TPPI) method. In most cases, the spectral width was 6,000 Hz. Typically the data set size was 2,048 (t2) × 256 (t1) blocks with between 16 and 64 scans per FID (free induction decay). Two-dimensional spectra for the peptide-metal complexes were collected at 298 K.
Receptor-Binding Assay.
Quantitative receptor-binding assays were carried out following a previously described method (28, 29). Cells were seeded at a density of 5 × 105 cells per well in 24-well tissue culture plates and exposed to various concentrations of peptides in 0.5 ml of binding media (25 mM Hepes/NaOH, pH 7.4/0.2% BSA/0.3 mM 1, 10-phenanthroline/≈100,000 cpm of 125I-[Nle4,d-Phe7]-α-MSH) (30, 31) at 4°C for 8 hr. The cells were rinsed with PBS, lysed with 1 M NaOH, and counted in a 1275 Minigamma gamma counter from LKB.
Biodistribution and Imaging Studies.
Six-week-old C57BL/6 mice (Harlan–Sprague–Dawley) were injected in the right flank with 1 × 106 B16-F1 mouse melanoma cells. Tumors, approximately 200–400 mg, appeared 10 days postinjection. Biodistribution studies were performed in normal and tumor-bearing mice injected with 2–3 mCi (1 Ci= 37 GBq) of 99mTcCCMSH via the tail vein. The radiochemical purity of the 99mTcCCMSH preparations used for the biodistribution studies was >98% as determined by HPLC. At various times postinjection mice were sacrificed, and individual tissues were counted. The blocking experiment was performed by administering 1 mg of [Nle4, d-Phe7]-α-MSH in 100 μl of saline solution subcutaneously between the scapulae 30 min prior to the injection of 99mTcCCMSH. For the imaging study, each mouse was given 25 mCi of 99mTcCCMSH by tail-vein injection. The mice were sacrificed 30 min postinjection, and the images were acquired by a Prism XP3000 triple-head γ camera (Picker, Cleveland) equipped with a low-energy high-resolution parallel collimator. The images were collected on a 512 matrix with ×3.999 magnification.
RESULTS AND DISCUSSION
Metal-Binding Site Design.
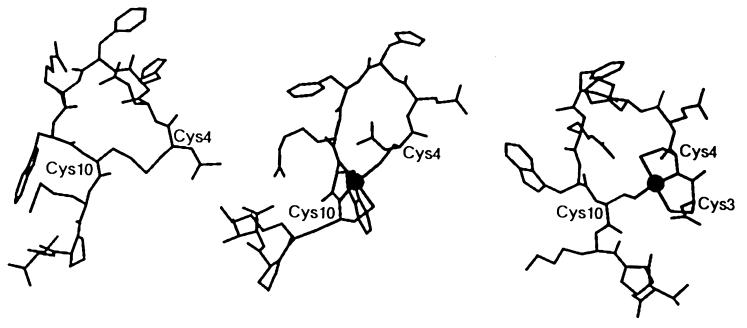
Molecular modeling was used to design and evaluate cyclic α-MSH analogs for their potential to incorporate Re or Tc into their structures. Several different factors were taken into account in the design of Re- and Tc-binding sites, including metal coordination geometry, donor-atom selection, and peptide conformation. Both Tc and Re, when in the +5 oxidation state, prefer a similar square pyramidal coordination geometry. Structural studies of small organic complexes of these metals have shown that donor atoms are located at the corners of a square plane, with a monooxo group located above, at the apex of a square pyramid (32–34). Another determinant of the metal-binding site was the known preference of these metals for specific donor atoms (i.e., S>N≫O) (8). A third important factor was determined not by the metal, but by the structure of the peptide itself. The metal-binding site must be engineered so that metal coordination does not disrupt the biologically active conformation of the molecule. The cyclic α-MSH analog (Cys4,10, d-Phe7)–α-MSH4-13 (APOMSH) was used as the starting point of our studies (24). APOMSH retains a core sequence of residues, His-d-Phe-Arg-Trp that has been found to be sufficient for the bioactivity of α-MSH (17). It was reported that cyclization and incorporation of a d-amino acid in position 7 both increased the potency and prolonged the biological effect of this analog relative to wild-type α-MSH (24).
Initial Rhenium Complexation.
The cyclic Re–α-MSH analog, ReMSH, was synthesized by incorporation of a Re(V)O core into APOMSH. Rhenium was introduced into a prereduced sample of APOMSH via transchelation from ReOCl3(Me2S)(OPPh3). Mass spectroscopy confirmed the expected molecular weight of a 1:1 peptide/metal complex [m/z (M+H)+ observed = 1,546, calculated = 1,544.8]. Initial two-dimensional NMR studies of ReMSH showed that two amide proton resonances were absent compared with the metal-free peptide. The two amide 1H resonances missing in the ReMSH spectrum were assigned to Trp-9 and Cys-10. These results suggested that the thiolate sulfur of Cys-10 and the two preceding amide nitrogens form three corners of a square plane of donor atoms. Chemical-shift alterations of Cys-4 βH’s, as well as Ellman’s analysis, showed that the fourth donor atom in the coordination sphere of Re was the thiolate sulfur of Cys-4. As a result of this binding geometry, ReMSH possesses three metal-containing rings, two five-membered rings and one 18-membered ring (Fig. 1). Thus, the ring size of ReMSH was reduced from a 23-membered ring in APOMSH to an 18-membered ring. The ReMSH metal-coordination geometry included the amide nitrogen of Trp, which is a member of the core sequence of residues responsible for α-MSH binding to its receptor. Some decomposition of the ReMSH complex was observed over time by NMR. Ellman’s analysis of ReMSH NMR samples revealed that approximately 3% of the cysteine residues within the molecule were present as free thiols. These results are compatible with slow rearrangement of the donor atoms in the metal-coordination sphere. Release of one of the Cys sulfurs as a free thiolate would be consistent with the Ellman’s analysis of ReMSH. The same trends in stability were observed at the tracer level. Synthesis of 99mTc/188ReMSH confirmed the relatively low stability of ReMSH. Table 1 shows that in competition with 10 mM free cysteine, the 188ReMSH complex had a t1/2 of approximately 4 hr. The analogous 99mTcMSH complex had an even shorter half-life, with a t1/2 of less than 2 hr, consistent with an increased lability of Tc to substitution reactions.
Figure 1.
Model structures of APOMSH (Left), ReMSH (Center), and ReCCMSH (Right) derived from NMR analyses. The site of Re coordination is depicted by the filled circle in ReMSH and ReCCMSH molecules.
Table 1.
Stabilities of tracer-level complexes
| Compound | Challenge
|
|||||||
|---|---|---|---|---|---|---|---|---|
| 50 mM Phosphate
|
10 mM Cysteine
|
10 mM DTPA
|
Serum
|
|||||
| 4 hr | 24 hr | 4 hr | 24 hr | 4 hr | 18 hr | 6 hr | 12 hr | |
| 188ReMSH | 90.3% | 66.4% | 48.9% | 34.0% | ND | ND | ND | ND |
| 99mTcMSH | 50.8% | 33.0% | 30.7% | 6.0% | ND | ND | ND | ND |
| 188ReCCMSH | 99.9% | 99.9% | 99.9% | 96.7% | 99.9% | 98.4% | 99.9% | 99.9% |
| 99mTcCCMSH | 99.9% | 99.9% | 95.0% | 91.0% | 96.0% | 94.4% | 99.9% | 99.9% |
Compounds were identified based on reverse-phase HPLC coelution with characterized nonradioactive standards. Serum studies were performed at 37°C. ND, value not determined; DTPA, diethylenetriaminopentacetic acid.
The effect of metal incorporation on the receptor-binding affinity of ReMSH is shown in Table 2. Receptor-binding assays were performed with APOMSH and ReMSH on B16-F1 murine melanoma cells. A decrease was observed in the ability of the ReMSH analog to displace an 125I-labeled α-MSH analog from the cells in a competitive receptor-binding assay (Table 2). The Ki for the ReMSH complex was 6.6 × 10−8, which reflected an apparent receptor binding affinity reduction to 1/100 that of APOMSH (Ki = 6.8 × 10−10). Therefore, it appeared that the presence of the Re(V)O core or its effect on the solution structure of ReMSH significantly reduced the molecule’s receptor affinity.
Table 2.
Ki values for α-MSH analogs
| Compound | Ki, M |
|---|---|
| APOMSH | 6.8 × 10−10 |
| ReMSH | 6.6 × 10−8 |
| CCMSH | 7.6 × 10−9 |
| ReCCMSH | 2.9 × 10−9 |
Metal-Binding Site Redesign.
Two different approaches were followed to create Re⋅MSH conjugates with improved receptor-binding and bioactivity profiles. Structural information relating to ReMSH indicated that the metal-binding site was too close to residues essential for receptor binding. In one attempt to distance the metal-binding site from the His-d-Phe-Arg-Trp core sequence, a spacer Gly residue was inserted between the Trp residue and the C-terminal Cys. It was hypothesized that the addition of the Gly residue would displace the crucial Trp residue from the metal-coordination sphere. The compound was synthesized by using the same conditions used for the synthesis of ReMSH and resulted in a complex that appeared by mass spectroscopy to contain two Re–oxo groups per peptide molecule. Receptor-binding data on this 2:1 Re⋅peptide complex showed no increase in binding affinity over ReMSH (data not shown). That this dimeric complex was the major product of two separate syntheses suggested that there may be an entropic barrier to the cyclization of larger rings via an Re(V)O core.
The second approach used to generate a Re⋅MSH complex with increased receptor-binding affinity involved the synthesis of an analog of APOMSH that contained an additional N-terminal Cys residue, termed Cys-3. It was hypothesized that inclusion of this additional thiolate would drive the metal-coordination sphere away from the His-d-Phe-Arg-Trp core sequence by taking advantage of the increased affinity of Re and Tc for sulfur donor atoms over nitrogen. NMR characterization of this ReCCMSH complex indicated that only a single amide proton resonance had been lost on Re complexation. The single amide 1H resonance lost in ReCCMSH was assigned to Cys-4. Ellman’s analysis of ReCCMSH revealed no free thiols present in the peptide⋅metal complex, suggesting that, as with ReMSH, all thiolates were involved in metal coordination. Mass spectroscopy confirmed the expected molecular weight of a 1:1 peptide/metal complex [m/z (M+H)+ observed = 1648.1, calculated = 1647.9]. In this case, the two N-terminal Cys thiolates, in combination with the intervening amide nitrogen, formed three corners of a square plane. The final donor atom was the remaining Cys-10 thiolate, resulting in cyclization of the molecule. ReCCMSH was also a tricyclic molecule, with rings of 6, 5, and 24 members, respectively (Fig. 1). Increasing the size of the loop containing the core receptor-binding sequence of ReCCMSH by six atoms was designed to relieve the steric stress apparent in the ReMSH molecule. The structural stability of the ReCCMSH molecule was superior to ReMSH. No degradation of the ReCCMSH molecule was observed by NMR over a 3-wk period. Ellman’s analysis of the ReCCMSH NMR sample revealed no detectable free thiols, indicating that three cysteine sulfurs remained coordinated to the ReO core.
The 99mTc- and 188Re-labeled analogs of ReCCMSH were virtually inert to substitution in 10 mM Cys competition assays, whereas the 99mTc- and 188Re-labeled analogs of ReMSH were readily exchanged under the same assay conditions (Table 1). In addition, both 99mTc- and 188ReCCMSH were stable to 10 mM DTPA and incubation in serum at 37°C. The increased stability of ReCCMSH probably resulted from the presence of an additional sulfur donor atom and from decreased backbone strain in the molecule because of the increased ring size. The receptor-binding affinity of ReCCMSH was more than twice that of CCMSH and greatly improved relative to ReMSH (Table 2). Cyclization of CCMSH via metal coordination resulted in a molecule with receptor-binding properties superior to the linear apoCCMSH peptide. The ReCCMSH analog exhibited a dissociation constant of 2.9 nM, approximately 1/25 that of ReMSH. Redesign of the metal-coordination site in ReCCMSH appeared to relieve unfavorable constraints within the core receptor-binding sequence, while enhancing the molecule’s radiochemical stability and receptor-binding affinity.
In Vivo Biodistribution and Imaging.
Biodistribution and tumor-targeting properties of 99mTcCCMSH were examined in normal and melanoma-bearing C57BL/6 mice. The 99mTcCCMSH analog showed rapid clearance from healthy mice, with the kidneys acting as primary route of excretion (data not shown). In melanoma-bearing mice, the 99mTcCCMSH molecule accumulated rapidly in the tumor to a peak level of 10.88 ± 0.54% dose per gram 1 hr postinjection (Table 3). Significant tumor retention of 99mTcCCMSH was observed 4 hr postinjection with an activity level of 9.51 ± 0.51% dose per gram. The tumor/blood ratios (% dose per gram) were 3.12 at 30 min, 6.80 at 1 hr, and 15.34 at 4 hr postinjection. Increasing tumor/blood ratios suggest tumor-specific uptake and retention of 99mTcCCMSH. Subcutaneous injection of 1 mg of [Nle4, d-Phe7]–α-MSH 30 min prior to injection of 99mTcCCMSH reduced tumor uptake from an average of 8.5% injected dose to 0.82% injected dose at the 30-min time point, confirming receptor-mediated uptake in these tissues. Low activity levels present in the stomachs (Table 4) of mice at all time points were consistent with the 99mTcCCMSH complex remaining intact (35). 99mTcCCMSH was cleared rapidly from the tumor-bearing animals with greater than 60 ± 1.89% of the injected dose present in the urine at 30 min (Table 4). There was some kidney retention that represented 6.33 ± 0.47% of the injected dose at 30 min and 5.07 ± 0.50% at 60 min postinjection. It is likely that the presence of two positive charges in the CCMSH analog, Arg-8 and Lys-11, contributed to the nonspecific kidney retention (36). Administration of free lysine before the radiolabeled peptide complex may be effective in reducing kidney retention of the positively charged peptides (37). A 99mTc-labeled bombesin peptide analog was also injected into melanoma-bearing mice as a control for nonspecific tumor uptake. The 99mTc-bombesin analog did not display significant tumor uptake, with levels of 0.71 ± 0.33% dose per gram at 30 min and 0.43 ± 0.22% dose per gram at 60 min postinjection.
Table 3.
Biodistribution of 99mTcCCMSH in melanoma-bearing mice reported as percent dose per gram
| Tissue | 30 min | 1 hr | 4 hr | 24 hr |
|---|---|---|---|---|
| Blood | 3.44 ± 0.48 | 1.60 ± 0.09 | 0.62 ± 0.21 | 0.06 ± 0.03 |
| Heart | 1.29 ± 0.25 | 0.53 ± 0.17 | 0.25 ± 0.15 | 0.13 ± 0.13 |
| Lung | 3.50 ± 0.20 | 1.39 ± 0.20 | 0.55 ± 0.12 | 0.10 ± 0.06 |
| Liver | 2.12 ± 0.14 | 2.34 ± 2.19 | 0.65 ± 0.08 | 0.06 ± 0.02 |
| Spleen | 1.07 ± 0.36 | 0.46 ± 0.35 | 0.54 ± 0.19 | 0.18 ± 0.28 |
| Kidneys | 28.05 ± 2.32 | 22.60 ± 2.70 | 14.60 ± 1.88 | 1.22 ± 0.15 |
| Muscle | 0.90 ± 0.10 | 0.17 ± 0.22 | 0.08 ± 0.06 | 0.05 ± 0.05 |
| Pancreas | 0.82 ± 0.16 | 0.35 ± 0.08 | 0.14 ± 0.14 | 0.04 ± 0.05 |
| Tumor | 10.74 ± 1.61 | 10.88 ± 0.54 | 9.51 ± 1.97 | 1.38 ± 0.36 |
Reported values ± SD (n = 5).
Table 4.
Biodistribution of 99mTcCCMSH in melanoma-bearing mice reported as percent dose
| Tissue | 30 min | 1 hr | 4 hr | 24 hr |
|---|---|---|---|---|
| Brain | 0.06 ± 0.01 | 0.02 ± 0.01 | 0.01 ± 0.01 | 0.01 ± 0.01 |
| Blood | 3.67 ± 0.33 | 1.76 ± 0.13 | 0.65 ± 0.21 | 0.07 ± 0.04 |
| Heart | 0.10 ± 0.01 | 0.04 ± 0.01 | 0.02 ± 0.01 | 0.01 ± 0.01 |
| Lung | 0.42 ± 0.03 | 0.18 ± 0.02 | 0.06 ± 0.01 | 0.01 ± 0.01 |
| Liver | 1.82 ± 0.24 | 1.12 ± 0.29 | 0.55 ± 0.09 | 0.06 ± 0.01 |
| Spleen | 0.06 ± 0.02 | 0.02 ± 0.01 | 0.02 ± 0.00 | 0.01 ± 0.01 |
| Stomach | 0.57 ± 0.19 | 0.47 ± 0.10 | 0.37 ± 0.20 | 0.01 ± 0.01 |
| Intestine∗ | 3.37 ± 0.33 | 4.53 ± 0.75 | 6.03 ± 1.79 | 0.15 ± 0.01 |
| Kidneys | 6.33 ± 0.47 | 5.07 ± 0.50 | 3.21 ± 0.57 | 0.32 ± 0.03 |
| Urine | 60.52 ± 1.89 | 74.84 ± 2.53 | 81.83 ± 2.45 | 98.49 ± 0.45 |
| Pancreas | 0.15 ± 0.02 | 0.07 ± 0.02 | 0.02 ± 0.02 | 0.01 ± 0.01 |
| Tumor | 3.13 ± 1.54 | 3.49 ± 2.18 | 3.32 ± 1.08 | 0.48 ± 0.27 |
Reported values ± SD (n = 5).
Small and large bowel.
A tumor-imaging study was performed on a melanoma-bearing C57BL/6 mouse. Twenty-five millicuries of 99mTcCCMSH was injected into the tail vein of a mouse bearing a 400-mg melanoma tumor in the right flank. Anterior and lateral scintigraphic images of the mouse were acquired 30 min postinjection. The imaging results were consistent with the biodistribution data. Significant accumulation of the 99mTc-labeled peptide was clearly visible in the melanoma tumor (Fig. 2). Radioactivity was also present in the kidneys and bladder, which serve as the primary route for peptide excretion. These results demonstrated that the redesigned 99mTcCCMSH molecule was capable of selectively targeting melanoma tumors in vivo.
Figure 2.
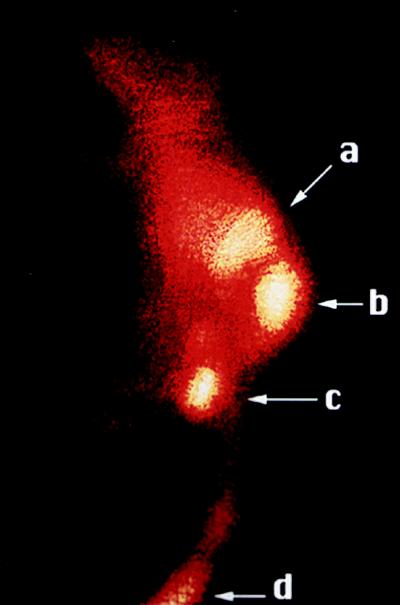
Imaging of a melanoma tumor in a mouse injected with 99mTcCCMSH. The γ-camera image of the melanoma-bearing mouse was acquired 30 min postinjection. The lateral image of the mouse with a 400-mg tumor shows a high degree of radioactivity localized in the tumor (b) with lesser amounts present in the kidneys (a), bladder (c), and tail vein injection site (d). The intensity of the γ-emission is color-coded high-to-low, ranging from white-yellow through orange, with dark red representing lowest values.
CONCLUSION
We have demonstrated the application of structure-based molecular design in the development of α-MSH receptor-avid peptides that were cyclized through the coordination of the transition metals Re and Tc. Several cycles of peptide design, synthesis, structural analysis, and functional characterization were employed to develop α-MSH analogs that incorporated Re and Tc into their structures while retaining high affinity for their cognate receptors present on melanoma cells. The ability of the 99mTcCCMSH analog to target melanoma in a murine-tumor model system demonstrated the potential use of these compounds for melanoma imaging or therapy.
Acknowledgments
We thank Dr. Wynn Volkert for his helpful discussions and Donna Whitener and Chrys Higganbotham for their technical assistance. We also thank Wei Wycoff and Xinfeng Gao for assistance with the collection of NMR spectra. This work was supported by a grant from the Department of Energy (DOE DE FG02 93ER61661). Instrumentation at the University of Missouri NMR center was supported by a grant from the National Science Foundation (89908304).
ABBREVIATIONS
- α-MSH
α-melanotropin stimulating hormone
- APOMSH
(Cys4,10, d-Phe7)–α-MSH4-13
- ReMSH
Re–(Cys4,10, d-Phe7)–α-MSH4-13
- CCMSH
(Cys3,4,10, d-Phe7)–α-MSH3-13
- ReCCMSH
Re–(Cys3,4,10, d-Phe7)–α-MSH3-13
- DTPA
diethylenetriaminepentaacetic acid
Footnotes
Data deposition: The atomic coordinates and structure factors have been deposited in the Protein Data Bank, Biology Department, Brookhaven National Laboratory, Upton, NY 11973 (PDB ID code 2MSH).
References
- 1. Iverson B L, Iverson S A, Roberts V A, Getzoff E D, Tainer J A, Benkovic S J, Lerner R A. Science. 1990;249:659–662. doi: 10.1126/science.2116666. [DOI] [PubMed] [Google Scholar]
- 2.Regan L. Annu Rev Biophys Biomol Struct. 1993;22:257–281. doi: 10.1146/annurev.bb.22.060193.001353. [DOI] [PubMed] [Google Scholar]
- 3.Kellis J T, Jr, Todd R J, Arnold F H. Bio/Technology. 1991;9:994–995. doi: 10.1038/nbt1091-994. [DOI] [PubMed] [Google Scholar]
- 4.Klemba M, Gardner K H, Marino S, Clarke N D, Regan L. Nat Struct Biol. 1995;2:368–373. doi: 10.1038/nsb0595-368. [DOI] [PubMed] [Google Scholar]
- 5.Kruck T P A, Lau S, Sarkar B. Can J Chem. 1976;54:1300–1308. [Google Scholar]
- 6.Lau S, Laussac J P, Sarkar B. Biochem J. 1989;257:745–750. doi: 10.1042/bj2570745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Franco R, Moura J J G, Moura I. J Biol Chem. 1995;270:26352–26357. doi: 10.1074/jbc.270.44.26352. [DOI] [PubMed] [Google Scholar]
- 8.Vanbilloen H P, Bormans G M, De Roo M J, Verbruggen A M. Nucl Med Biol. 1995;22:325–338. doi: 10.1016/0969-8051(94)00110-6. [DOI] [PubMed] [Google Scholar]
- 9.Bakker W H, Albert R, Bruns C, Breeman W A P, Hofland L J, Marbach P, Pless J, Pralet D, Stoltz B, Koper J W, et al. Life Sci. 1991;49:1583–1591. doi: 10.1016/0024-3205(91)90052-d. [DOI] [PubMed] [Google Scholar]
- 10.Fritzberg A R, Abrams P G, Beaumier P L, Kasina S, Morgan A C, Rao T N, Reno J M, Sanderson J A, Srinivasan A, Wilbur D S, et al. Proc Natl Acad Sci USA. 1988;85:4025–4029. doi: 10.1073/pnas.85.11.4025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu S, Edwards D S, Looby R J, Harris A R, Poirier M J, Barrett J A, Heminway S J, Carroll T R. Bioconjugate Chem. 1996;7:63–71. doi: 10.1021/bc950069+. [DOI] [PubMed] [Google Scholar]
- 12.Krenning E P, Bakker W H, Kooij P P M, Breeman W A P, Oei H Y, de Jong M, Reubi J C, Visser T J, Bruns C, Kwekkeboom D J, et al. J Nucl Med. 1992;33:652–658. [PubMed] [Google Scholar]
- 13.Wraight E P, Bard D R, Maughan T S, Knight C G, Page-Thomas D P. Br J Radiol. 1992;65:112–118. doi: 10.1259/0007-1285-65-770-112. [DOI] [PubMed] [Google Scholar]
- 14.Varnum J M, Thakur M L, Schally A V, Jansen S A, Mayo K H. J Biol Chem. 1994;209:12583–12588. [PubMed] [Google Scholar]
- 15.Varnum J M, Thakur M, Mayo K H, Jansen S A. J Phys Chem. 1996;100:14630–14636. [Google Scholar]
- 16.Chi D Y, O’Neil J P, Anderson C J, Welch M J, Katzenellenbogen J A. J Med Chem. 1994;37:928–937. doi: 10.1021/jm00033a010. [DOI] [PubMed] [Google Scholar]
- 17.Hruby V J, Sharma S D, Toth K, Jaw J Y, Al-Obeidi F, Sawyer T K, Hadley M E. Ann NY Acad Sci. 1993;680:51–63. doi: 10.1111/j.1749-6632.1993.tb19674.x. [DOI] [PubMed] [Google Scholar]
- 18.Tatro J B, Entwistle M L, Lester B R, Reichlin S. Cancer Res. 1989;50:1237–1242. [PubMed] [Google Scholar]
- 19.Siegrist W, Solca F, Stutz S, Giuffre L, Carrel S, Girard J, Eberle A N. Cancer Res. 1989;49:6352–6358. [PubMed] [Google Scholar]
- 20.Bard D R, Knight C G, Page-Thomas D P. Br J Cancer. 1990;62:919–922. doi: 10.1038/bjc.1990.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bagutti C, Stolz B, Albert R, Bruns C, Pless J, Eberle A N. Int J Cancer. 1994;58:749–755. doi: 10.1002/ijc.2910580521. [DOI] [PubMed] [Google Scholar]
- 22.Garg P K, Alston K L, Welsh P C, Zalutsky M R. Bioconjugate Chem. 1996;7:233–239. doi: 10.1021/bc960001+. [DOI] [PubMed] [Google Scholar]
- 23.Vaidyanathan G, Zalutsky M R. Nucl Med Biol. 1997;24:171–178. doi: 10.1016/s0969-8051(96)00211-9. [DOI] [PubMed] [Google Scholar]
- 24.Cody W L, Mahoney M, Knittel J J, Hruby V J, Castrucci A M de L, Hadley M E. J Med Chem. 1985;28:583–588. doi: 10.1021/jm50001a008. [DOI] [PubMed] [Google Scholar]
- 25.Grove, D. E. & Wilkinson, G. (1966) J. Chem. Soc. 1224–1230.
- 26.Bryan J C, Stenkamp R E, Tulip T H, Mayer J M. Inorg Chem. 1987;26:2283–2288. [Google Scholar]
- 27.Ellman G L. Arch Biochem Biophys. 1959;82:70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- 28.Siegrist W, Oestreicher M, Stutz S, Girard J, Eberle A N. J Recept Res. 1988;8:323–343. doi: 10.3109/10799898809048996. [DOI] [PubMed] [Google Scholar]
- 29.Sahm U G, Olivier G W J, Branch S K, Moss S H, Pouton C W. Peptides. 1994;15:441–446. doi: 10.1016/0196-9781(94)90202-x. [DOI] [PubMed] [Google Scholar]
- 30.Sawyer T K, Sanfilippo P J, Hruby V J, Engel M H, Heward C B, Burnett J B, Hadley M E. Proc Natl Acad Sci USA. 1980;77:5754–5758. doi: 10.1073/pnas.77.10.5754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Giblin M F, Jurisson S S, Quinn T P. Bioconjugate Chem. 1997;8:347–353. doi: 10.1021/bc9700291. [DOI] [PubMed] [Google Scholar]
- 32.Grummon G, Rajagopalan R, Palenik G J, Koziol A E, Nosco D L. Inorg Chem. 1995;34:1764–1772. [Google Scholar]
- 33.Hansen L, Cini R, Taylor A, Marzilli L G. Inorg Chem. 1992;31:2801–2808. [Google Scholar]
- 34.Rao T N, Adhikesavalu D, Camerman A, Fritzberg A R. J Am Chem Soc. 1990;112:5798–5804. [Google Scholar]
- 35.Hnatowich D J. Nucl Med Biol. 1990;17:49–55. doi: 10.1016/0883-2897(90)90007-n. [DOI] [PubMed] [Google Scholar]
- 36.Solling K, Morgenson C E. Proc Eur Dial Transplant Assoc. 1977;14:543–549. [PubMed] [Google Scholar]
- 37.Kobayashi H, Yoo T M, Kim I S, Kim M-K, Le N, Webber K O, Pastan I, Paik C H, Eckelman W C, Carrasquillo J A. Cancer Res. 1996;56:3788–3795. [PubMed] [Google Scholar]