Abstract
D-Lactate dehydrogenase has been purified to near homogeneity from Peptostreptococcus elsdenii. As isolated, the enzyme contains flavine adenine dinucleotide and a tightly bound metal cofactor. Inactivation by ortho-phenanthroline occurs in two steps and is partially blocked by D-lactate. Reactivation by divalent metal ions occurs, with divalent zinc being the most effective. When ferricyanide is used as the electron acceptor, D-lactate has an apparent K0.5 of 3.3 M0.46; its binding is negatively cooperative with a Hill coefficient of 0.46. Replacement of ferricyanide by the other components of the electron transport system yields hyperbolic kinetics with an apparent Km for D-lactate of 26 mM. The apparent Km for ferricyanide is 2.2 X 10(-4) M. Phosphate and pyrophosphate compounds stimulate the D-lactate:ferricyanide activity. These properties suggest that interaction of this enzyme with other electron transport proteins in the chain may enhance D-lactate binding and, hence, the rate of electron transport.
Full text
PDF
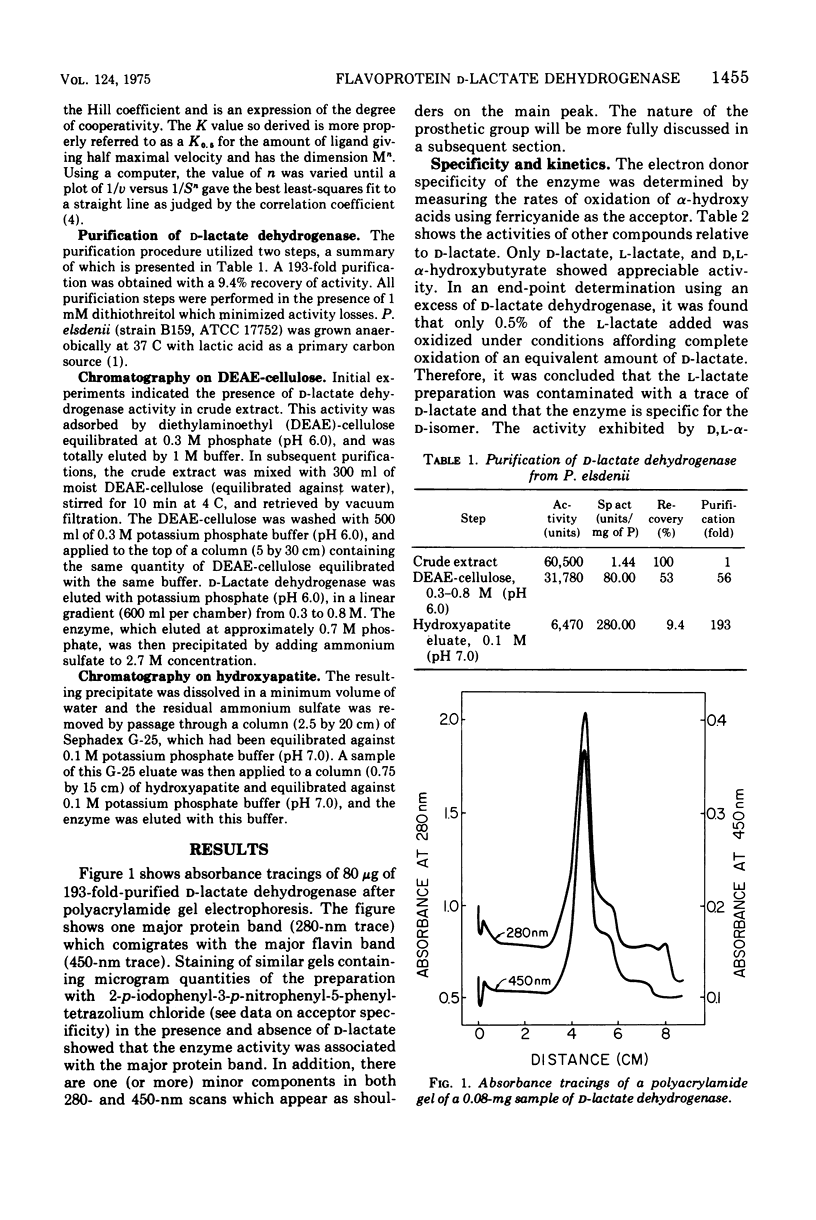
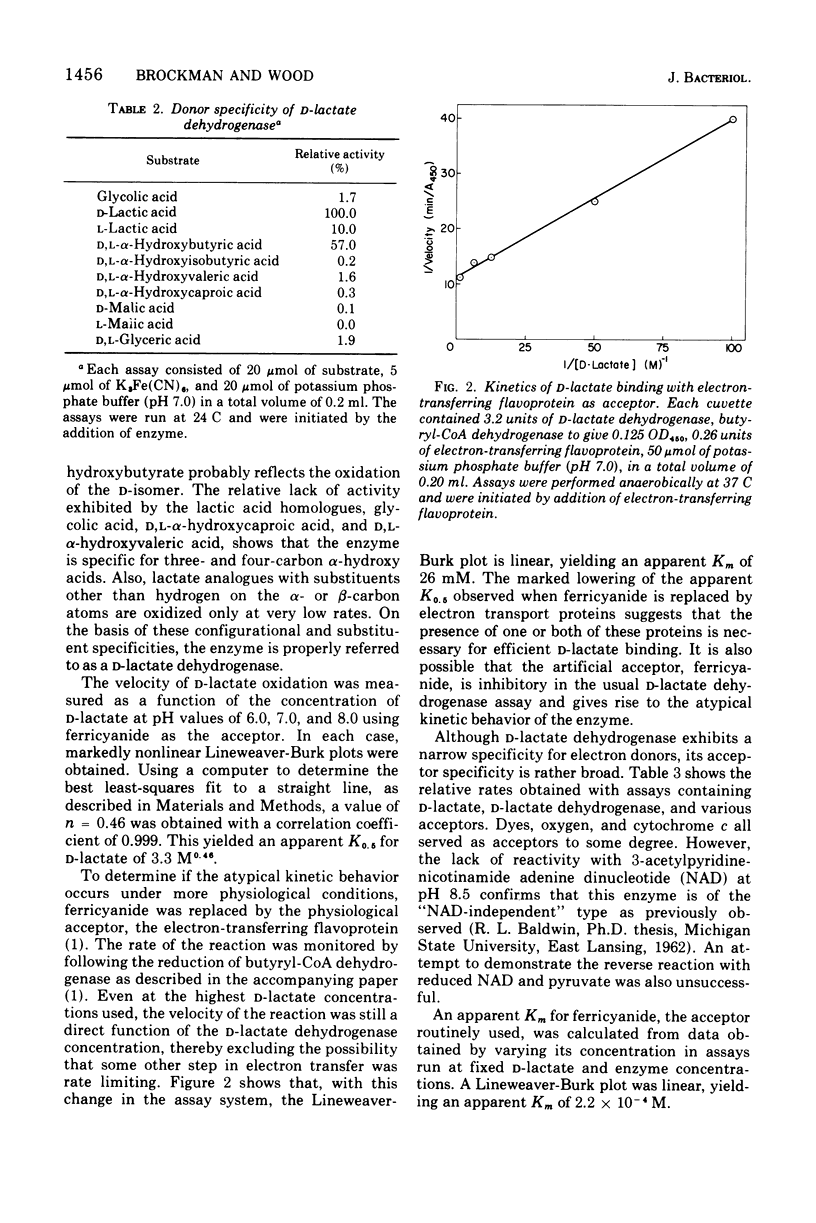

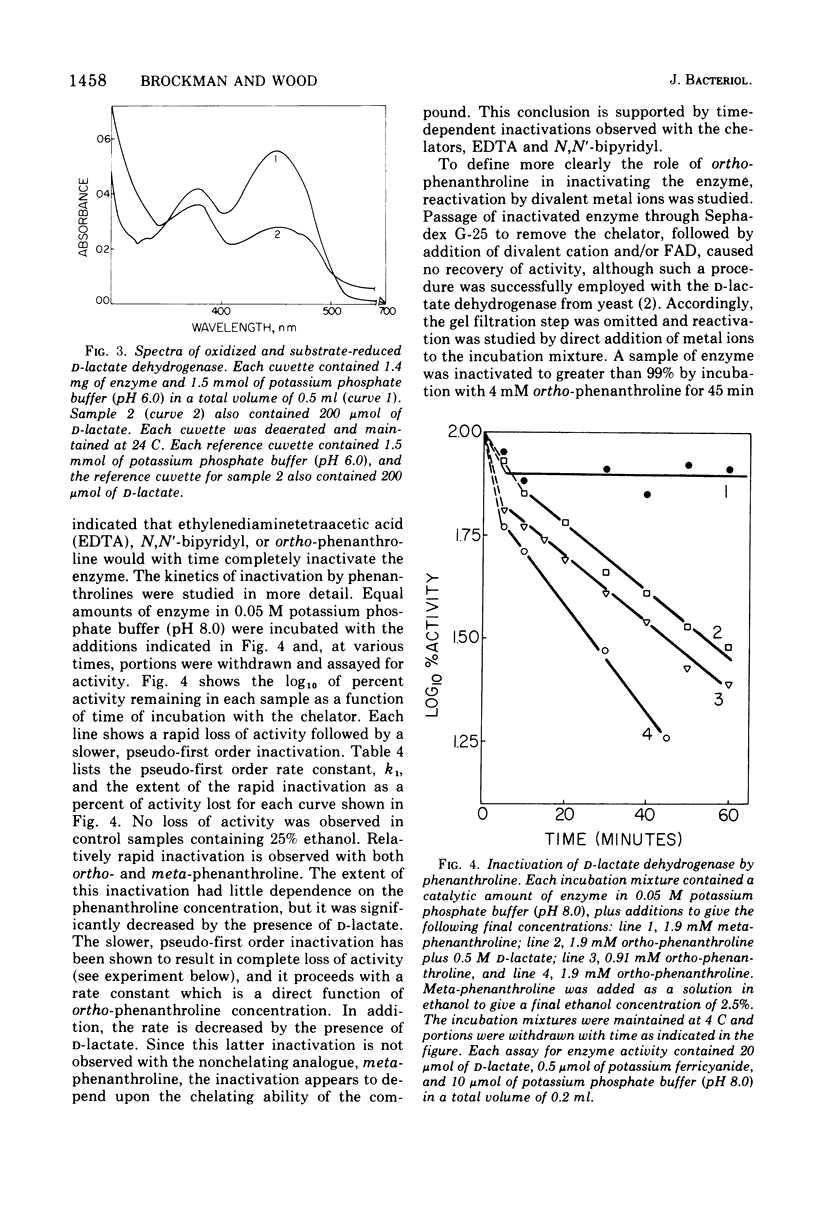
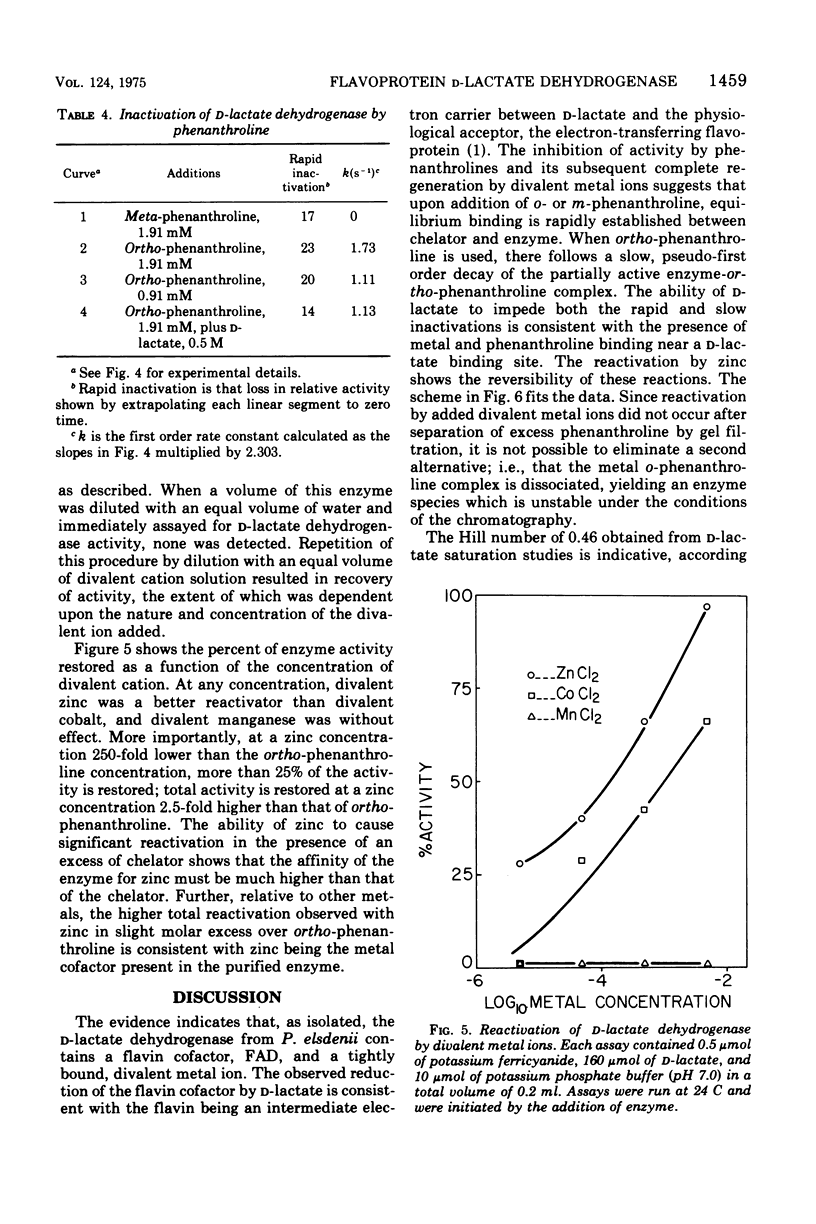


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brockman H. L., Wood W. A. Electron-transferring flavoprotein of Peptostreptococcus elsdenii that functions in the reduction of acrylyl-coenzyme A. J Bacteriol. 1975 Dec;124(3):1447–1453. doi: 10.1128/jb.124.3.1447-1453.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CREMONA T., SINGER T. P. THE LACTIC DEHYDROGENASES OF YEAST. V. CHEMICAL PROPERTIES AND FUNCTION OF THE ZINC COMPONENT OF D-LACTIC CYTOCHROME REDUCTASE. J Biol Chem. 1964 May;239:1466–1473. [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- ELSDEN S. R., GILCHRIST F. M., LEWIS D., VOLCANI B. E. Properties of a fatty acid forming organism isolated from the rumen of sheep. J Bacteriol. 1956 Nov;72(5):681–689. doi: 10.1128/jb.72.5.681-689.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GUTIERREZ J., DAVIS R. E., LINDAHL I. L., WARWICK E. J. Bacterial changes in the rumen during the onset of feed-lot bloat of cattle and characteristics of Peptostreptococcus elsdenii n. sp. Appl Microbiol. 1959 Jan;7(1):16–22. doi: 10.1128/am.7.1.16-22.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koshland D. E., Jr, Némethy G., Filmer D. Comparison of experimental binding data and theoretical models in proteins containing subunits. Biochemistry. 1966 Jan;5(1):365–385. doi: 10.1021/bi00865a047. [DOI] [PubMed] [Google Scholar]
- Levitzki A., Koshland D. E., Jr Negative cooperativity in regulatory enzymes. Proc Natl Acad Sci U S A. 1969 Apr;62(4):1121–1128. doi: 10.1073/pnas.62.4.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOLINARI R., LARA F. J. The lactic dehydrogenase of Propionibacterium pentosaceum. Biochem J. 1960 Apr;75:57–65. doi: 10.1042/bj0750057. [DOI] [PMC free article] [PubMed] [Google Scholar]


