Abstract
Coenzyme A (CoA) transferase from Peptostreptococcus elsdenii has been purified and crystallized, and some of its properties have been established. The work was facilitated by a newly developed coupled and continuous spectrophotometric assay in which the disappearance of added acrylate could be followed at 245 nm. The rate-limiting conversion of acetyl- and beta-hydroxypropionyl CoA to acrylyl CoA by CoA transferase was followed by the non-rate-limiting conversion to beta-hydroxypropionyl CoA by excess crotonase. Thus, a small priming quantity of acetyl CoA served to generate acrylyl CoA, which, by hydration, generated beta-hydroxypropionyl CoA. This product then served to generate more acrylyl CoA in cyclic fashion. The net result was the CoA transferase-limited conversion of acrylate to beta-hydroxypropionate. The purified transferase has a molecular weight of 125,000 and is composed of two subunits of 63,000 each, as determined by disc gel electrophoresis. Short-chain-length monocarboxylic acids are substrates, whereas dicarboxylic or beta-ketocarboxylic acids are not. The reaction kinetics are typical of a ping-pong bi bi mechanism composed of two half reactions linked by a covalent enzyme intermediate. Incubation of the transferase with acetyl CoA in the absence of a fatty acid acceptor yielded a stable intermediate which, by absorption spectrophotometry, radioactivity measurements, reduction with borohydride, reactivity with hydroxylamine, and catalytic activity, was identified as an enzyme-CoA compound. Kinetic constants for CoA transferase are: final specific activity, 110 U/mg of protein corresponding to 1.38 X 10(4) mumol of acrylate activated per mumol of transferase; Km for acrylate, 1.2 X 10(-3) M; Km for acetyl CoA (beta-hydroxypropionyl CoA), 2.4 X 10(-5) M.
Full text
PDF






Images in this article
Selected References
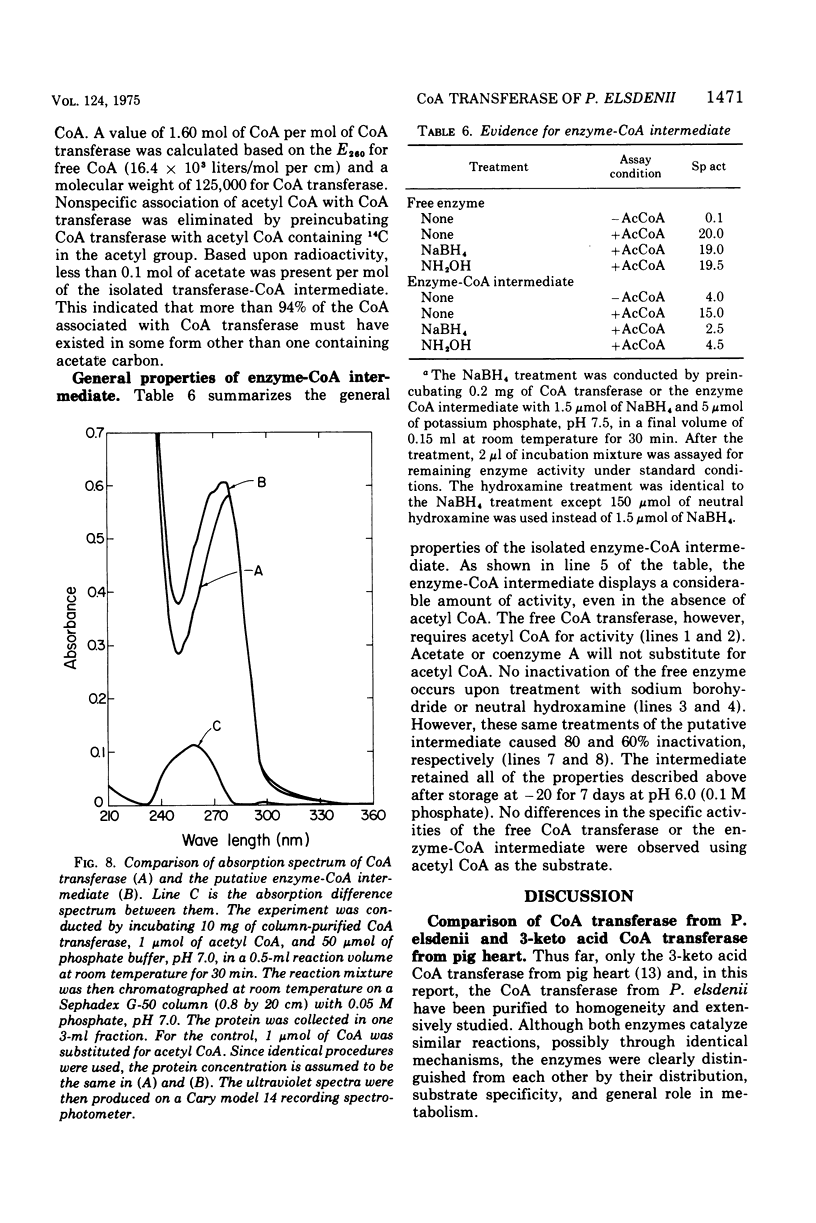
These references are in PubMed. This may not be the complete list of references from this article.
- ALLEN S. H., KELLERMEYER R. W., STJERNHOLM R. L., WOOD H. G. PURIFICATION AND PROPERTIES OF ENZYMES INVOLVED IN THE PROPIONIC ACID FERMENTATION. J Bacteriol. 1964 Jan;87:171–187. doi: 10.1128/jb.87.1.171-187.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BALDWIN R. L., WOOD W. A., EMERY R. S. LACTATE METABOLISM BY PEPTOSTREPTOCOCCUS ELSDENII: EVIDENCE FOR LACTYL COENZYME A DEHYDRASE. Biochim Biophys Acta. 1965 Feb 15;97:202–213. doi: 10.1016/0304-4165(65)90084-x. [DOI] [PubMed] [Google Scholar]
- Chrambach A., Reisfeld R. A., Wyckoff M., Zaccari J. A procedure for rapid and sensitive staining of protein fractionated by polyacrylamide gel electrophoresis. Anal Biochem. 1967 Jul;20(1):150–154. doi: 10.1016/0003-2697(67)90272-2. [DOI] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- DEN H., ROBINSON W. G., COON M. J. Enzymatic conversion of beta-hydroxypropionate to malonic semialdehyde. J Biol Chem. 1959 Jul;234(7):1666–1671. [PubMed] [Google Scholar]
- Edelhoch H. Spectroscopic determination of tryptophan and tyrosine in proteins. Biochemistry. 1967 Jul;6(7):1948–1954. doi: 10.1021/bi00859a010. [DOI] [PubMed] [Google Scholar]
- Garces E., Cleland W. W. Kinetic studies of yeast nucleoside diphosphate kinase. Biochemistry. 1969 Feb;8(2):633–640. doi: 10.1021/bi00830a026. [DOI] [PubMed] [Google Scholar]
- Hammerstedt R. H., Möhler H., Decker K. A., Wood W. A. Structure of 2-keto-3-deoxy-6-phosphogluconate aldolase. I. Physical evidence for a three-subunit molecule. J Biol Chem. 1971 Apr 10;246(7):2069–2074. [PubMed] [Google Scholar]
- Hedrick J. L., Smith A. J. Size and charge isomer separation and estimation of molecular weights of proteins by disc gel electrophoresis. Arch Biochem Biophys. 1968 Jul;126(1):155–164. doi: 10.1016/0003-9861(68)90569-9. [DOI] [PubMed] [Google Scholar]
- Hersh L. B., Jencks W. P. Isolation of an enzyme-coenzyme A intermediate from succinyl coenzyme A-acetoacetate coenzyme A transferase. J Biol Chem. 1967 Jan 25;242(2):339–340. [PubMed] [Google Scholar]
- Jakoby W. B. A technique for the crystallization of proteins. Anal Biochem. 1968 Nov;26(2):295–298. doi: 10.1016/0003-2697(68)90340-0. [DOI] [PubMed] [Google Scholar]
- LADD J. N., WALKER D. J. The fermentation of lactate and acrylate by the rumen micro-organism LC. Biochem J. 1959 Feb;71(2):364–373. doi: 10.1042/bj0710364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEWIS D., ELSDEN S. R. The fermentation of L-threonine, L-serine, L-cysteine and acrylic acid by a gram-negative coccus. Biochem J. 1955 Aug;60(4):683–692. doi: 10.1042/bj0600683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STADTMAN E. R. The coenzyme A transphorase system in Clostridium kluyveri. J Biol Chem. 1953 Jul;203(1):501–512. [PubMed] [Google Scholar]
- STADTMAN E. R. The coenzyme A transphorase system in Clostridium kluyveri. J Biol Chem. 1953 Jul;203(1):501–512. [PubMed] [Google Scholar]
- STERN J. R., COON M. J., DEL CAMPILLO A., SCHNEIDER M. C. Enzymes of fatty acid metabolism. IV. Preparation and properties of coenzyme A transferase. J Biol Chem. 1956 Jul;221(1):15–31. [PubMed] [Google Scholar]
- STERN J. R., DEL CAMPILLO A. Enzymes of fatty acid metabolism. II. Properties of crystalline crotonase. J Biol Chem. 1956 Feb;218(2):985–1002. [PubMed] [Google Scholar]
- Sokatch J. R., Sanders L. E., Marshall V. P. Oxidation of methylmalonate semialdehyde to propionyl coenzyme A in Pseudomonas aeruginosa grown on valine. J Biol Chem. 1968 May 25;243(10):2500–2506. [PubMed] [Google Scholar]
- Solomon F., Jencks W. P. Identification of an enzyme-gamma-glutamyl coenzyme A intermediate from coenzyme A transferase. J Biol Chem. 1969 Feb 10;244(3):1079–1081. [PubMed] [Google Scholar]
- VAGELOS P. R., EARL J. M. Propionic acid metabolism. III. beta-Hydroxypropionyl coenzyme A and malonyl semialdehyde coenzyme A, intermediates in propionate oxidation by Clostridium kluyveri. J Biol Chem. 1959 Sep;234:2272–2280. [PubMed] [Google Scholar]
- VAGELOS P. R. Propionic acid metabolism. IV. Synthesis of malonyl coenzyme A. J Biol Chem. 1960 Feb;235:346–350. [PubMed] [Google Scholar]
- WILLIAMS D. E., REISFELD R. A. DISC ELECTROPHORESIS IN POLYACRYLAMIDE GELS: EXTENSION TO NEW CONDITIONS OF PH AND BUFFER. Ann N Y Acad Sci. 1964 Dec 28;121:373–381. doi: 10.1111/j.1749-6632.1964.tb14210.x. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]