Abstract
Recent studies have shown that administering the aromatase inhibitor exemestane after 2–3 years of tamoxifen therapy significantly improves disease-free survival in postmenopausal women with primary breast cancer in comparison with standard 5-year tamoxifen treatment. Although many of the adverse effects associated with exemestane and tamoxifen have been analysed, there are no comparative data concerning body weight and body composition. The aim of this randomised study was to evaluate the longitudinal changes in body composition and lipid profiles in postmenopausal women switched from tamoxifen to exemestane. In total, 60 overweight or obese postmenopausal patients were enrolled. Their anthropometric data, body composition, including fat mass (FM) and fat-free mass (FFM), and lipid profiles, caloric intake and physical activity were assessed 1 week before randomisation, and 6 and 12 months later. In all, 55 patients (27 on tamoxifen and 28 on exemestane) completed the 1-year study period. Fat mass had significantly decreased by month 12 in the exemestane, but not in the tamoxifen group; the between-group difference was statistically significant (P<0.01). The FFM/FM ratio had significantly increased in the exemestane group, but not the tamoxifen group; the between-group difference was statistically significant (P<0.05). Triglycerides and high-density lipoprotein cholesterol significantly decreased (P<0.01; P<0.05), and low-density lipoprotein cholesterol significantly increased (P<0.01) in the exemestane group at the end of the 1-year study period. Our findings suggest that switching patients to adjuvant exemestane treatment after at least 2 years of tamoxifen therapy may be associated with an advantage over continuing adjuvant tamoxifen treatment in terms of body composition.
Keywords: body weight, exemestane, hormonal therapy, lipid, tamoxifen
Tamoxifen is currently used to treat all stages of oestrogen receptor-positive breast cancer and, in the adjuvant setting, has substantial benefits in terms of disease-free and overall survival (Early Breast Cancer Trialists' Collaborative Group, 1992).
The major symptoms attributable to tamoxifen therapy are hot flushes, sweating and vaginal discharges. Although serious adverse medical effects are rare, women are often concerned about some of the risks of tamoxifen therapy, such as blood clots, strokes and endometrial cancer (Meier and Jick, 1998; Ganz, 2001). Moreover, they have often reported a range of events attributed to tamoxifen therapy, including weight gain (Hoskin et al, 1992; Rose et al, 1993). A checklist-based study of patients on endocrine therapy found that weight gain was a common problem in patients on tamoxifen (Malinovszky et al, 2004), but the results of large-scale, randomised clinical trials such as NSABP-B14 and NSABP-P1 suggest that adjuvant tamoxifen therapy is not associated with an increased risk of weight gain, although it must be pointed out that they considered only body weight and not body composition (Fisher et al, 1989, 1998). Nevertheless, adult weight gain is largely reflected in increased body fat, which may be more suitable for assessing adiposity and its metabolic consequences than body weight or the body mass index (BMI), which reflect both lean and fat body mass (Abu-Abid et al, 2002).
The negative effects of excess body weight and fat mass (FM) on the recurrence of breast cancer, and the survival of both pre- and postmenopausal women, has recently been underlined (Daling et al, 2001).
Third-generation aromatase inhibitors, which are considered a new development in the endocrine treatment of oestrogen receptor-positive breast cancer in postmenopausal women, prevent the conversion of adrenal androgens into oestrogens by inhibiting the aromatase of the cytochrome P450-dependent enzyme that is responsible for the majority of oestrogen production in postmenopausal women as well as in men (Smith and Dowsett, 2003).
One of the three newest aromatase inhibitors is exemestane, whose irreversible binding to the aromatase enzyme causes permanent inactivation even after the drug is cleared from the circulation (Goss and Strasser, 2001). Recent findings have shown that, in comparison with standard 5-year tamoxifen therapy, the administration of exemestane after 2–3 years of tamoxifen significantly improves disease-free survival in postmenopausal women with primary breast cancer (Coombes et al, 2004). Although many of the adverse effects associated with tamoxifen and exemestane have been analysed, there are no comparative data concerning their effects on body composition and body weight; furthermore, there are few published data concerning the impact of adjuvant exemestane therapy on lipid profiles (Atalay et al, 2004; Markopoulos et al, 2005). The aim of this study was to evaluate the changes in the body composition and lipid profiles of postmenopausal women switched from tamoxifen to exemestane.
PATIENTS AND METHODS
Eligibility criteria
The study was approved by our Institutional Review Board, and all of the patients gave their written informed consent.
The eligible patients were postmenopausal oestrogen receptor-positive women with resected breast cancer aged less than 75 years, who had a BMI of 25–35 (weight(kg)/height(m)2) and had received at least 2 years' adjuvant tamoxifen treatment. Postmenopausal status was defined as an age of 55 years or more with amenorrhea for more than 2 years, or amenorrhea for more than 1 year at the time of diagnosis. The exclusion criteria were any evidence of a local relapse or distant metastasis, clinical signs of severe osteoporosis, the use of medications that could affect lipid levels, or the presence of serious medical conditions that could alter food absorption or prognosis. The subjects were randomised using a computer-generated sequence; the person responsible for the randomisation and the people making the study measurements were blinded. The study was not nested in a larger randomised trial.
In total, 60 patients met the eligibility criteria and were randomised to continue tamoxifen 20 mg day−1 (n=30) or to switch to exemestane 25 mg day−1 (n=30). The two groups were well balanced in terms of their main baseline characteristics, including height, weight and BMI (Table 1). All of the enrolled women were overweight or obese.
Table 1. Baseline characteristics of the study population.
| Tamoxifen (n=27) | Exemestane (n=28) | |
|---|---|---|
| Age (years) | 61.15±2.74 | 61.89±4.45 |
| Tumour stage | ||
| T1 | 46% | 38% |
| T2 | 36% | 40% |
| T3 | 18% | 22% |
| Node status | ||
| N+(1–3) | 21% | 18% |
| N+(>4) | 8% | 15% |
| Negative | 71% | 67% |
| Estrogen receptor status | ||
| Positive | 100% | 100% |
| Negative | 0 | 0 |
| Progesteron receptor status | ||
| Positive | 72% | 65% |
| Negative | 28% | 35% |
| Primary treatment | ||
| Mastectomy | 61% | 54% |
| Radiotherapy | 42% | 51% |
| Chemotherapy | 24% | 27% |
| Height, cm (mean±s.d.) | 156.4±2.06 | 155.96±1.92 |
| Weight, kg (mean±s.d.) | 69.75±6.21 | 69.88±7.58 |
| BMI, kg/m2 (mean±s.d.) | 28.97±1.88 | 29.17±2.12 |
BMI=body mass index.
Measurements
The patients' anthropometric data (including height, weight and BMI), body composition, lipid profiles, physical activity, food intake and quality of life (QOL) were assessed 1 week before randomisation, and after 6 and 12 months of treatment. Weight was measured using a balance beam scale after an overnight fast.
Lipid metabolism
Fasting venous blood samples were drawn at all visits in order to assess the levels of total cholesterol (TC), triglycerides (TG) and high- and low-density lipoprotein cholesterol (HDL-C and LDL-C), which except for LDL-C (calculated using Friedwald's formula) were measured by means of colorimetry (Autoanalyzer, Menarini, Italy). The intra- and interassay coefficients of variation in our Institution are, respectively, 1.8 and 3.9% for TC, 1.7 and 3.0% for TG, and 2.0 and 3.0% for HDL-C.
All of the individual samples were stored at −80°C until analysis when they were assayed using one batch of reagent.
Body composition
Body composition was evaluated by means of a single, whole-body, dual-energy X-ray absorptiometry scan made using a QDR 4500 Hologic machine (DXA Hologic Inc., Waltham, MA, USA), and the results were used to derive FM, and fat-free mass (FFM).
The DXA measurements were of high quality. Although the method has good long-term stability and precision, consistent performance was further ensured by means of a daily quality control scan using a known standard (the manufacturer's phantom).
QOL assessment
In order to assess their health-related QOL, all of the patients were asked to complete the European Organisation for Research and Treatment of Cancer (EORTC) core questionnaire (EORTC/QLQ-C30) at trial entry, and after 6 and 12 months (Aaronson et al, 1993). The questionnaire consists of 30 items covering general physical symptoms and function, fatigue/malaise, and social and emotional functioning: higher function scores indicate better functioning, whereas higher symptom scores indicate worse symptoms.
During the QOL interview, the women provided information concerning exercise (recorded as the number of hours/week devoted to physical activity) and food intake, which was measured by means of a food frequency questionnaire translated into Italian (Sallis et al, 1985; Block et al, 1986).
Statistical analysis
Assuming an increase of about 15% in serum LDL-C in women switched from tamoxifen to exemestane, a randomised design required 30 patients per group to verify a statistical significant difference in this parameter with an α of 0.05 and a β of 0.80.
Analysis of variance for paired data was used to evaluate the within-group variations in all of the parameters, and analysis of variance for unpaired data to evaluate between-group differences after having calculated the percentage changes at each visit. Nonparametric tests were used as appropriate. A P-value of <0.05 was considered statistically significant for all of the tests, which were made using SPSS 10.1 statistical software.
RESULTS
Of the 60 enrolled patients, 55 (27 on tamoxifen and 28 on exemestane) completed the 1-year study period, three were lost to follow-up, and two had concomitant diseases unrelated to the study drugs (not cardiovascular complications). Table 1 shows the characteristics of the 55 study completers included in the analysis.
Table 2 shows the mean (±s.d.) weight, caloric intake and physical activity in the two groups at baseline, and after 6 and 12 months. Weight remained unchanged in the tamoxifen group (69.75±6.21 kg at baseline and 69.17±7.33 kg at month 12), but decreased in the exemestane group (69.88±7.58 kg at baseline and 67.89±7.45 kg at month 12; P=0.06). The questionnaire data showed no statistically significant differences in baseline caloric intake or physical activity between the tamoxifen and exemestane group, and no statistically significant changes were observed in either group after six and 12 months.
Table 2. Weight, caloric intake and physical activity data: mean values (±s.d.) on tamoxifen and exemestane therapy.
|
Tamoxifen
|
||||||
|---|---|---|---|---|---|---|
| Exemestane | Baseline | 6th month | 12th month | Baseline | 6th month | 12th month |
| Weight (kg) | 69.75±6.21 (CI:67.41–72.09) | 68.33±7.04 (CI:65.67–70.99) | 69.17±7.33 (CI:66.41–71.93) | 69.88±7.58 (CI:67.07–72.09) | 67.95±6.12 (CI:65.68–70.22) | 67.89± 7.45 (CI:65.13–70.65) |
| Caloric intake (kcal day−1) | 1832±326 (CI:1709–1955) | 1852±351 (CI:1720–1984) | 1848±367 (CI:1709–1986) | 1841±365 (CI:1705–1976) | 1837±326 (CI:1716–1957) | 1818±312 (CI:1702–1933) |
| Physical activity (h week−1) | 11.81±7.60 (CI:8.94–14.68) | 12.12±8.25 (CI:9.01–15.23) | 13.22± 8.46 (CI:10.03–16.41) | 10.28±7.26 (CI:7.59–12.97) | 11.80±8.33 (CI:8.71–14.89) | 11.18±7.25 (CI:8.49–13.87) |
CI=confidence interval.
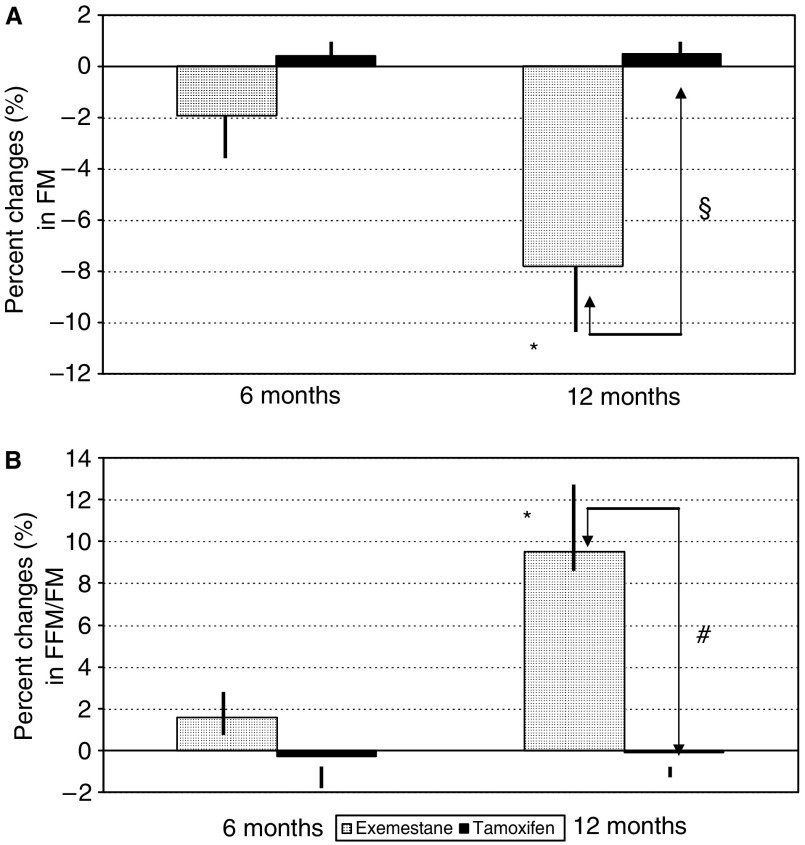
Fat mass had significantly decreased by month 12 in the exemestane group (P<0.01) but not in the tamoxifen group; the between-group difference was statistically significant (P<0.01) (Figure 1A): The FFM/FM ratio significantly increased by month 12 in the exemestane group (P<0.01) but not in the tamoxifen group; the between-group difference was statistically significant (P<0.05) (Figure 1B).
Figure 1.
Percentage changes in FM (A) and the FFM/FM ratio (B) in the exemestane and tamoxifen groups after 6 and 12 months (*P<0.01 vs baseline; #P<0.05, §between groups).
There were no statistically significant changes in the lipid profiles of the patients treated with tamoxifen (Table 3). In the exemestane group, HDL-C decreased by 9.1% at month 6 and by 12.0% at month 12 when the difference became statistically significant (P<0.05), LDL-C significantly increased by 14.8% at month 6 and 16.5% at month 12 (P<0.01), and TG decreased by 12.1% at month 6 and 16.9% at month 12 (P<0.01). The between-group differences were statistically significant only for LDL-C (P<0.05).
Table 3. Lipide profile: mean values (±s.d.) on tamoxifen and exemestane therapy.
|
Tamoxifen
|
||||||
|---|---|---|---|---|---|---|
| Exemestane | Baseline | 6th month | 12th month | Baseline | 6th month | 12th month |
| TC (mg dl−1) | 215.12±10.01 (CI:211.34–218.90) | 216.23±10.44 (CI:212.29–220.17) | 215.32±11.21 (CI:211.09–219.55) | 215.68±9.31 (CI:212.23–219.13) | 224.27±13.33 (CI:219.33–229.21) | 224.17±12.83 (CI:219.42–228.92) |
| HDL-C (mg dl−1) | 58.62±6.17 (CI:56.29–60.95) | 58.22±8.77 (CI:54.91–61.53) | 57.98±8.01 (CI:54.96–61.00) | 58.07±6.40 (CI:55.7–60.44) | 53.41±8.10 (CI:50.41–56.41) | 51.20±8.08* (CI:48.21–54.19) |
| LDL-C (mg dl−1) | 131.15±14.51 (CI:125–68–136.62) | 133.56±19.10 (CI:126.36–140.76) | 132.31±19.24 (CI:125.05–139.57) | 130.91±15.10 (CI:125.32–136.50) | 149.8±17.18* (CI:143.44–156.16) | 152.81±18.12**# (CI:146.10–159.52) |
| TG (mg dl−1) | 124.87±46.1 (CI:107.48–142.26) | 119.30±47.81 (CI:101.27–137.33) | 127.63±40.60 (CI:112.32–142.94) | 124.11±58.27 (CI:102.53–145.69) | 107.21±64.30* (CI:83.39–131.03) | 101.04±43.65** (CI:84.87–117.21) |
CI=confidence interval; HDL-C=high-density lipoprotein cholesterol; LDL-C=low-density lipoprotein cholesterol; TC=total cholesterol; TG=total triglycerides.
P<0.05,
P<0.01 vs baseline values; #P<0.05 between groups.
The comparison of the linearly transformed mean values of the EORTC-QLQ-C30 data was performed: the very similar global health status (P=0.9) and global QOL scores (P=0.9) of the two groups indicated that there were no statistically significant differences in their baseline QOL. In comparison with baseline, none of the P-values in the functional and symptom scales showed any clear between-group trend at month 6 but, at month 12, there was a trend toward improved physical functioning, global health status and global QOL scores in the exemestane group; the between-group difference was not statistically significant.
DISCUSSION
Tamoxifen has long been the gold standard therapy for postmenopausal women with hormone receptor-positive breast cancer but, although the drug is generally well tolerated, its prolonged use may be associated with various side effects, including gynecological and thromboembolic complications (Fisher et al, 1998). Moreover, a few studies have reported some changes in body weight (especially weight gains) in women with resected breast cancer receiving adjuvant treatment including tamoxifen (Camoriano et al, 1990; Chlebowski et al, 2002; Malinovszky et al, 2004).
The present study suggests, for the first time, that switching overweight or obese patients to adjuvant treatment with exemestane after at least 2 years of tamoxifen therapy is associated with a significant decrease in FM (Figure 1), with no statistical significant difference in body weight (Table 2). It must be remembered that the precise pathophysiological mechanisms of body weight changes remain unclear in many patients and that, as body weight is determined by an interplay of caloric intake, activity level and metabolic rate, significant alterations in any of these factors may lead to weight loss or gain (Levine et al, 1991; Goodwin et al, 1998). However, we observed no statistically significant differences in caloric intake or physical activity between our two randomised groups during the study period (Table 2).
One possible explanation of our findings is that the pharmacological properties of exemestane are different from those of tamoxifen. The effects of tamoxifen on the human body vary and can be characterised as a mixture of oestrogenic and anti-oestrogenic properties, whereas exemestane inhibits in vivo aromatisation by about 98% and has no partial agonist activity, thus leading to a profound decrease in serum oestrogen levels (Furr and Jordan, 1984; Geisler et al, 1998). Two-thirds of breast tumours are oestrogen-dependent, and aromatase inhibitors can help block the growth of these tumours by lowering the amount of oestrogen in the body (Yeu and Santen, 1996). As it is known that exemestane and the other aromatase inhibitors reduce circulating oestrogen levels, and that oestrogens have direct effects on adipocytes and the other cellular constituents of adipose tissue, there may be an association between exemestane use, reduced circulating oestrogen levels and body weight changes (Siiteri, 1987; Selby, 1990; Hankinson et al, 1995). However, if such an association exists, our data do not offer any evidence as to whether the expected decrease in oestrogen levels was the cause or effect of body weight changes.
The use of exemestane may also influence appetite, but our QoL questionnaire did not reveal any statistically significant changes in appetite in our study population.
Another intriguing point is the effect of tamoxifen and exemestane on the lipid profiles of postmenopausal women. It has long been known that oestrogens lower serum cholesterol, and that the agonist activity of the selective oestrogen-receptor modulator tamoxifen has protective effects on lipid profiles (Love et al, 1990). Nevertheless, it has recently been suggested that women receiving aromatase inhibitors for breast cancer prevention may be at increased risk of cardiovascular diseases, because the oestrogen-lowering effects of such drugs may have adverse effects on blood lipids (Baum et al, 2003; Goss et al, 2003; Atalay et al, 2004; Esteva and Hortobagyi, 2006; Markopoulos et al, 2005). Our data showed a significant increase in LDL-C in the patients treated with exemestane: this finding may seem to differ from those of other studies indicating that exemestane has a neutral effect on LDL-C, but it must be remembered that our patients had been treated with tamoxifen for at least 2 years, which markedly reduces LDL-C levels (Love et al, 1990; Atalay et al, 2004; Esteva and Hortobagyi, 2006; Markopoulos et al, 2005). The increase in LDL-C may therefore be at least partially explained by the loss of the positive action of tamoxifen. The decrease in HDL-C in the exemestane group is in line with previous studies, and may be explained by a direct action of exemestane and/or by the interruption of tamoxifen therapy (Atalay et al, 2004; Lonning et al, 2005; Markopoulos et al, 2005; Esteva and Hortobagyi, 2006).
The Intergroup Exemestane Study found a trend toward more frequent myocardial infarction in patients treated with exemestane than in those treated with tamoxifen (Coombes et al, 2004). We found that 1-year's use of exemestane led to more changes in cholesterol parameters, which are commonly associated with an increased risk of coronary heart disease (Table 3), thus indicating that the lipid profiles of patients receiving exemestane or other aromatase inhibitors should be monitored. However, it must be borne in mind that the genesis of cardiovascular diseases is multifactorial, and many authors have found that exemestane treatment significantly reduces triglyceride levels, which may help to balance the negative effect of the decrease in HDL-C (Atalay et al, 2004; Markopoulos et al, 2005). Only large-scale randomised trials and a long follow-up can answer the question as to whether postmenopausal women treated with exemestane or other aromatase inhibitors for 3–5 years are at greater cardiovascular risk than those treated with tamoxifen.
In conclusion, the results of our study suggest that adjuvant exemestane treatment may have an advantage over adjuvant tamoxifen treatment in terms of body composition. In addition to recent findings that the use of exemestane after 2–3 years of tamoxifen therapy significantly improves disease-free survival in comparison with standard 5-year tamoxifen treatment, this could be a further factor worth considering when deciding on adjuvant hormonal therapy for postmenopausal women with breast cancer.
References
- Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ, Filiberti A, Flechtner H, Fleishman SB, de Haes JC, Kaasa S, Klee M, Osoba D, Razavi D, Rofe PB, Schraub S, Sneeuw K, Sullivan M, Takeda F (1993) The European Organization for Research and Treatment of Cancer QLQ-C30: A quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst 85: 365–376 [DOI] [PubMed] [Google Scholar]
- Abu-Abid S, Szold A, Klausne J (2002) Obesity and cancer. J Med 33: 73–86 [PubMed] [Google Scholar]
- Atalay G, Dirix L, Biganzoli L, Beex L, Nooij M, Cameron D, Lohrisch C, Cufer T, Lobelle JP, Mattiaci MR, Piccart M, Paridaens R (2004) The effect of exemestane on serum lipid profile in postmenopausal women with metastatic breast cancer: a companion study to EORTC Trial 10951, ‘Randomized phase II study in first line hormonal treatment for metastatic breast cancer with exemestane or tamoxifen in postmenopausal patients'. Ann Oncol 15: 211–217 [DOI] [PubMed] [Google Scholar]
- Baum M, Buzdar A, Cuzick J, Forbes J, Houghton J, Howell A, Sahmoud T, The ATAC (Arimidex Tamoxifen alone or in Combination) Trialists' Group (2003) Anastrozole alone or in combination with tamoxifen versus tamoxifen alone for adjuvant treatment of postmenopausal women with early-stage breast cancer: results of the ATAC (Arimidex, Tamoxifen Alone or in Combination) trial efficacy and safety update analyses. Cancer 98: 1802–1810 [DOI] [PubMed] [Google Scholar]
- Block G, Hartman AM, Dresser CM, Carroll MD, Gannon J, Gardner L (1986) A data-based approach to diet questionnaire design and testing. Am J Epidemiol 124: 453–469 [DOI] [PubMed] [Google Scholar]
- Camoriano JK, Loprinzi CL, Ingle JN, Therneau TM, Krook JE, Veeder MH (1990) Weight change in women treated with adjuvant therapy or observed following mastectomy for node-positive breast cancer. J Clin Oncol 8: 1327–1334 [DOI] [PubMed] [Google Scholar]
- Chlebowski RT, Col N, Winer EP, Collyar DE, Cummings SR, Vogel III VG, Burstein HJ, Eisen A, Lipkus I, Pfister DG, American Society of Clinical Oncology Breast Cancer Technology Assessment Working Group (2002) American Society of Clinical Oncology technology assessment of pharmacologic interventions for breast cancer risk reduction including tamoxifen, raloxifene, and aromatase inhibition. J Clin Oncol 20: 3328–3343 [DOI] [PubMed] [Google Scholar]
- Coombes RC, Hall E, Gibson LJ, Paridaens R, Jassem J, Delozier T, Jones SE, Alvarez I, Bertelli G, Ortmann O, Coates AS, Bajetta E, Dodwell D, Coleman RE, Fallowfield LJ, Mickiewicz E, Andersen J, Lonning PE, Cocconi G, Stewart A, Stuart N, Snowdon CF, Carpentieri M, Massimini G, Bliss JM, Intergroup Exemestane Study (2004) A randomized trial of exemestane after two to three years of tamoxifen in postmenopausal women with primary breast cancer. N Engl J Med 350: 1081–1092 [DOI] [PubMed] [Google Scholar]
- Daling JR, Malone KE, Doody DR, Johnson LG, Gralow JR, Porter PL (2001) Relation of body mass index to tumor markers and survival among young women with invasive ductal breast carcinoma. Cancer 92: 720–729 [DOI] [PubMed] [Google Scholar]
- Early Breast Cancer Trialists' Collaborative Group (1992) Systemic treatment of early breast cancer by hormonal, cytotoxic, or immune therapy. 133 randomised trials involving 31,000 recurrences and 24,000 deaths among 75,000 women. Lancet 339: 1–15 [PubMed] [Google Scholar]
- Esteva FJ, Hortobagyi GN (2006) Comparative assessment of lipid effects of endocrine therapy for breast cancer: implications for cardiovascular disease prevention in postmenopausal women. Breast 15: 301–312 [DOI] [PubMed] [Google Scholar]
- Fisher B, Costantino J, Redmond C, Poisson R, Bowman D, Couture J, Dimitrov NV, Wolmark N, Wickerham DL, Fisher ER, Margolese R, Robidoux A, Shibata H, Terz J, Paterson AHG, Feldman MI, Farrar W, Evans J, Lickley HL, Ketner M (1989) A randomized clinical trial evaluating tamoxifen in the treatment of patients with node-negative breast cancer who have estrogen-receptor-positive tumors. N Engl J Med 320: 479–484 [DOI] [PubMed] [Google Scholar]
- Fisher B, Constantino JP, Wickerham DL, Redmond CK, Kavanah M, Cronin WM, Vogel V, Robidoux A, Dimitrov N, Atkins J, Daly M, Wieand S, Tan-Chiu E, Ford L, Wolmark N (1998) Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project PI study. J Natl Cancer Inst 90: 1371–1388 [DOI] [PubMed] [Google Scholar]
- Furr BJ, Jordan VC (1984) The pharmacology and clinical uses of tamoxifen. Pharmacol Ther 25: 127–205 [DOI] [PubMed] [Google Scholar]
- Ganz PA (2001) Impact of tamoxifen adjuvant therapy on symptoms, functioning, and quality of life. J Natl Cancer Inst 30: 130–134 [DOI] [PubMed] [Google Scholar]
- Geisler J, King N, Anker G, Ornati G, Di Salle E, Lonning PE, Dowsett M (1998) In vivo inhibition of aromatization by exemestane, a novel irreversible aromatase inhibitor, in postmenopausal breast cancer patients. Clin Cancer Res 4: 2089–2093 [PubMed] [Google Scholar]
- Goodwin P, Esplen MJ, Butler K, Winocur J, Pritchard K, Brazel S, Gao J, Miller A (1998) Multidisciplinary weight management in locoregional breast cancer: results of a phase II study. Breast Cancer Res Treat 48: 53–64 [DOI] [PubMed] [Google Scholar]
- Goss PE, Ingle JN, Martino S, Robert NJ, Muss HB, Piccart MJ, Castiglione M, Tu D, Shepherd LE, Pritchard KI, Livingston RB, Davidson NE, Norton L, Perez EA, Abrams JS, Therasse P, Palmer MJ, Pater JL (2003) A randomized trial of letrozole in postmenopausal women after five years of tamoxifen therapy for early-stage breast cancer. N Engl J Med 349: 1793–1802 [DOI] [PubMed] [Google Scholar]
- Goss PE, Strasser K (2001) Aromatase inhibitors in the treatment and prevention of breast cancer. J Clin Oncol 19: 881–894 [DOI] [PubMed] [Google Scholar]
- Hankinson SE, Willett WC, Manson JE, Hunter DJ, Colditz GA, Stampfer MJ, Longeope C, Speizer FE (1995) Alcohol, height, and adiposity in relation to estrogen and prolactin levels in postmenopausal women. J Natl Cancer Inst 87: 1297–1302 [DOI] [PubMed] [Google Scholar]
- Hoskin PJ, Ashley S, Yarnold JR (1992) Weight gain after primary surgery for breast cancer-effect of tamoxifen. Breast Cancer Res Treat 22: 129–132 [DOI] [PubMed] [Google Scholar]
- Levine EG, Raczynski JM, Carpenter JT (1991) Weight gain with breast cancer adjuvant treatment. Cancer 67: 1954–1959 [DOI] [PubMed] [Google Scholar]
- Lonning PE, Geisler J, Krag LE, Erikstein B, Bremnes Y, Hagen AI, Schlichting E, Lien EA, Ofjord ES, Paolini J, Polli A, Massimini G (2005) Effects of exemestane administered for 2 years versus placebo on bone mineral density, bone biomarkers, and plasma lipids in patients with surgically resected early breast cancer. J Clin Oncol 23: 5126–5137 [DOI] [PubMed] [Google Scholar]
- Love RR, Newcomb PA, Wiebe DA, Surawicz TS, Jordan VC, Carbone PP, DeMets DL (1990) Effects of tamoxifen therapy on lipid and lipoprotein levels in postmenopusal patients with node-negative breast cancer. J Natl Cancer Inst 82: 1327–1332 [DOI] [PubMed] [Google Scholar]
- Malinovszky KM, Cameron D, Douglas S, Love C, Leonard T, Dixon JM, Hopwood P, Leonard RC (2004) Breast cancer patients' experiences on endocrine therapy: monitoring with a checklist for patients on endocrine therapy (C-PET). The Breast 13: 363–368 [DOI] [PubMed] [Google Scholar]
- Markopoulos C, Polychronis A, Zobolas V, Xepapadakis G, Papadiamantis J, Koukouras D, Lappas H, Gogas H (2005) The effect of exemestane on the lipidemic profile of postmenopausal early breast cancer patients: preliminary results of the TEAM Greek sub-study. Breast Cancer Res Treat 93: 61–66 [DOI] [PubMed] [Google Scholar]
- Meier CR, Jick H (1998) Tamoxifen and risk of idiopathic venous thromboembolism. Br J Clin Pharmacol 45: 608–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose DP, Connolly JM, Chlebowski RT, Buzzard IM, Wynder EL (1993) The effects of a low-fat dietary intervention and tamoxifen adjuvant therapy on the serum estrogen and sex hormone-binding globulin concentrations of postmenopausal breast cancer patients. Breast Cancer Res Treat 27: 253–262 [DOI] [PubMed] [Google Scholar]
- Sallis JF, Haskell WL, Wood PD, Fortmann SP, Rogers T, Blair SN, Paffenbarger Jr RS (1985) Physical activity assessment methodology in the Five-City project. Am J Epidemiol 121: 91–106 [DOI] [PubMed] [Google Scholar]
- Selby C (1990) Sex hormone binding globulin: origin, function, and clinical significance. Ann Clin Biochem 27: 532–541 [DOI] [PubMed] [Google Scholar]
- Siiteri PK (1987) Adipose tissue as a source of hormones. Am J Clin Nutr 45(Suppl 1): 277–282 [DOI] [PubMed] [Google Scholar]
- Smith IE, Dowsett M (2003) Aromatase inhibitors in breast cancer. N Engl J Med 348: 2431–2442 [DOI] [PubMed] [Google Scholar]
- Yeu W, Santen RJ (1996) Aromatase inhibitors: rationale for use following antiestrogen therapy. Semin Oncol 23(Suppl 9): 21–27 [PubMed] [Google Scholar]