Abstract
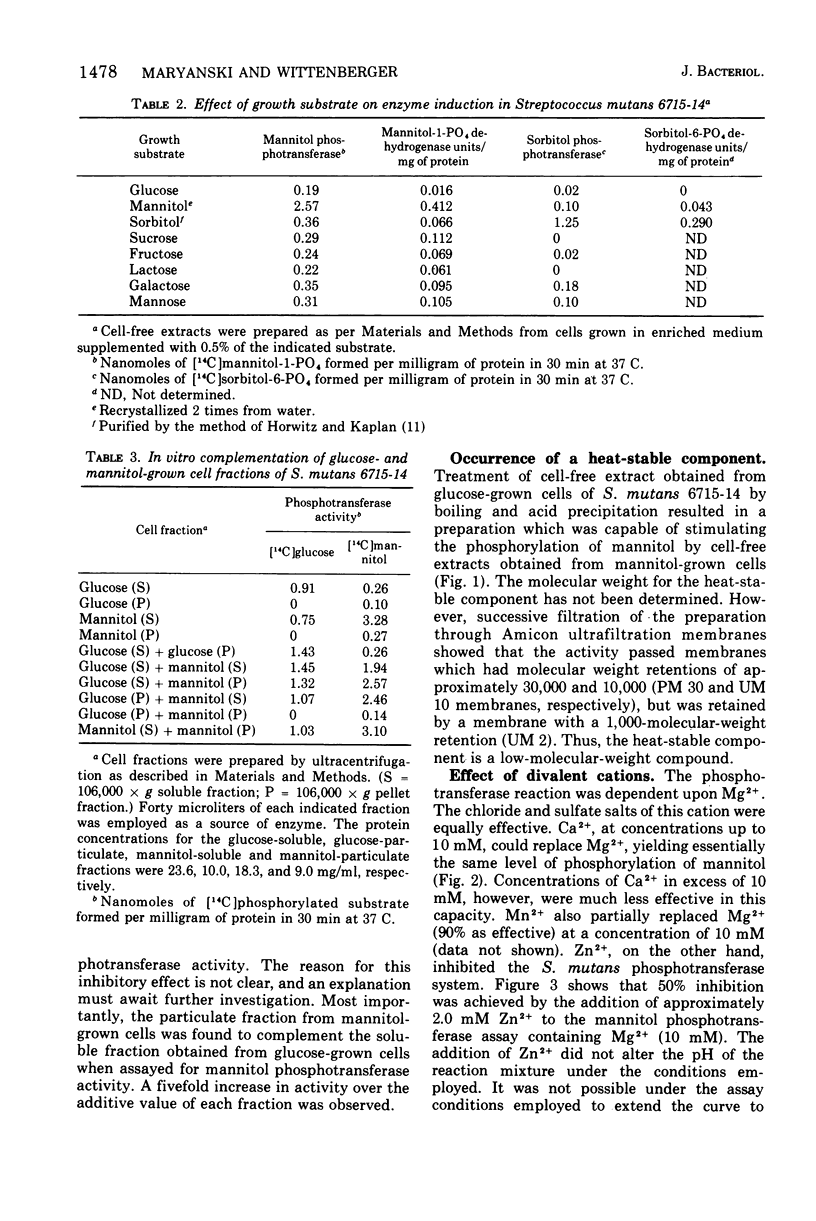
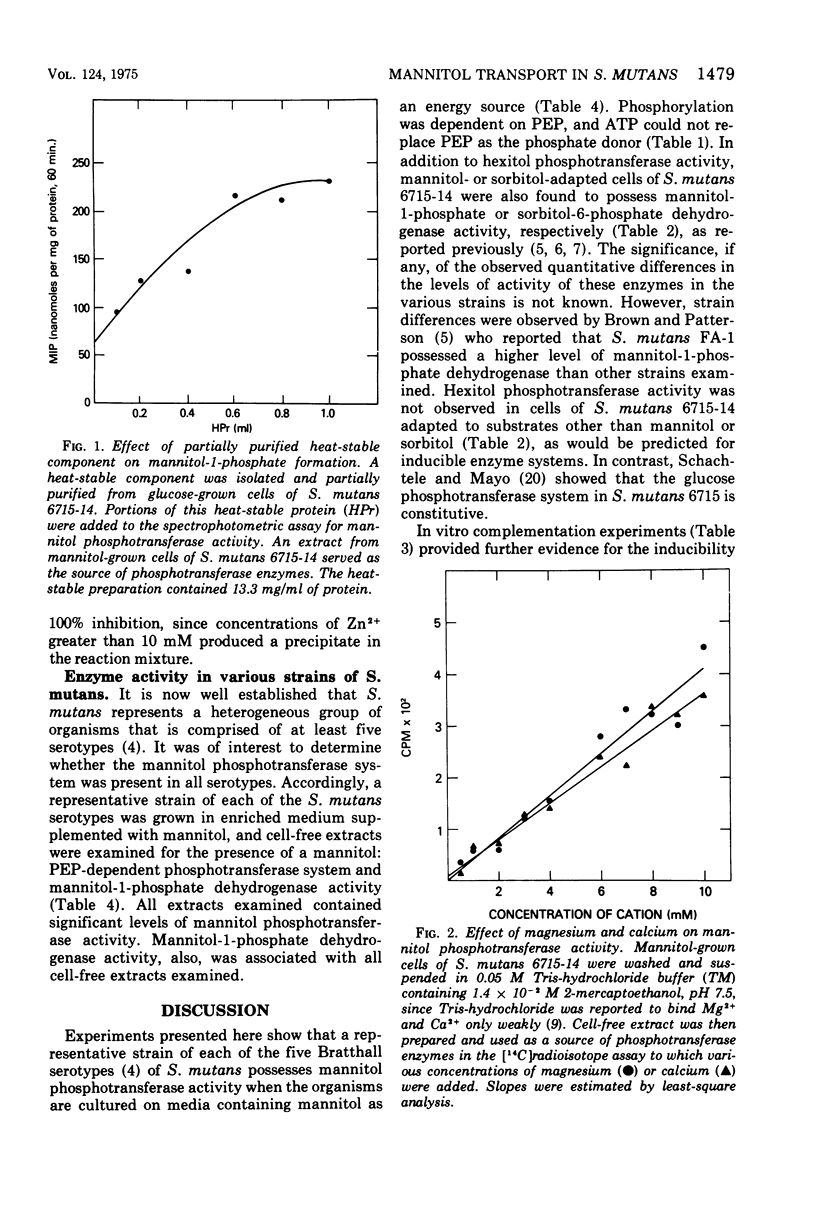
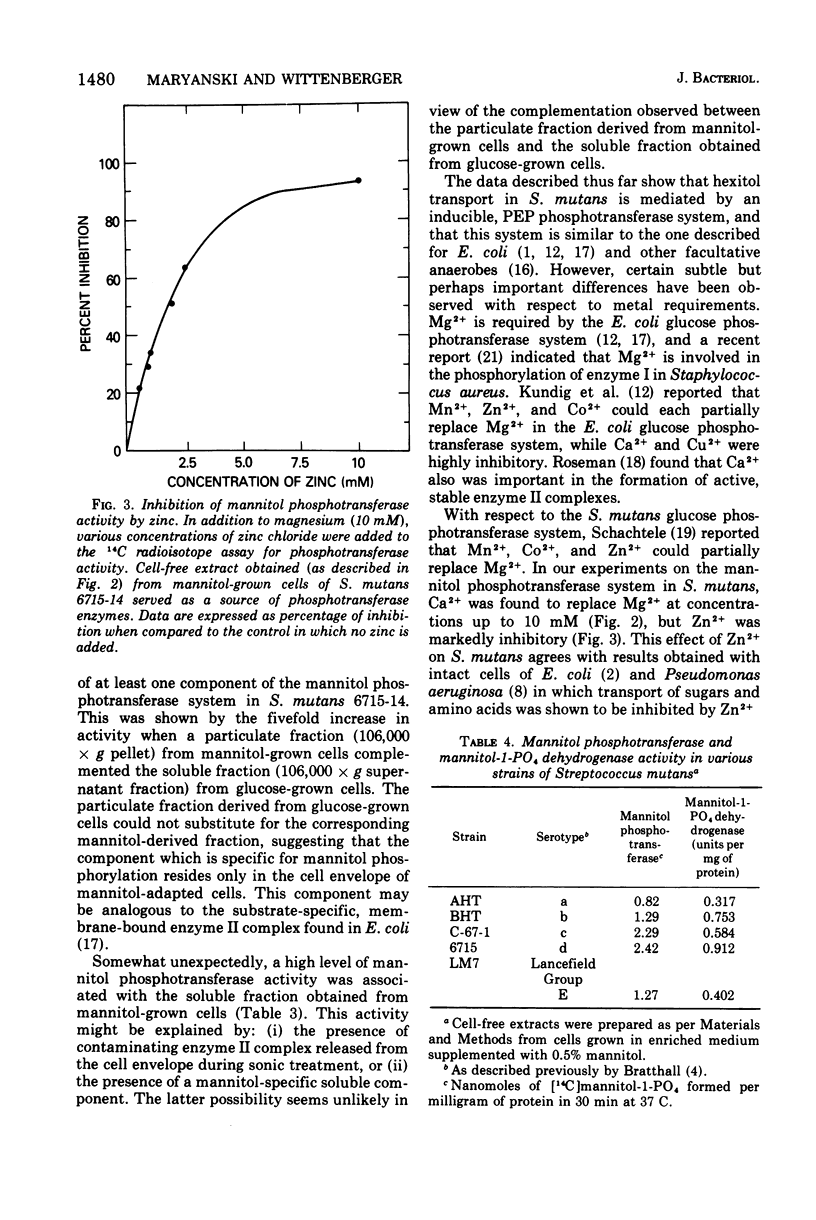
A hexitol-inducible, phosphoenolpyruvate-dependent phosphotransferase system was demonstrated in Streptococcus mutans. Cell-free extracts obtained from mannitol-grown cells from a representative strain of each of the five S. mutans serotypes (AHT, BHT, C-67-1, 6715, and LM7) were capable of converting mannitol to mannitol-1-phosphate by a reaction which required phosphoenolpyruvate and Mg2+. Mannitol and sorbitol phosphotransferase activities were found in cell-free extracts prepared from cells grown on the respective substrate, but neither hexitol phosphotransferase activity was present in extracts obtained from cells grown on other substrates examined. A heat-stable, low-molecular-weight component was partially purified from glucose-grown cells and found to stimulate the mannitol phosphotransferase system. Divalent cations Mn2+ and Ca2+ partially replaced Mg2+, while Zn2+ was found to be highly inhibitory.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson B., Weigel N., Kundig W., Roseman S. Sugar transport. 3. Purification and properties of a phosphocarrier protein (HPr) of the phosphoenolpyruvate-dependent phosphotransferase system of Escherichia coli. J Biol Chem. 1971 Nov 25;246(22):7023–7033. [PubMed] [Google Scholar]
- Anraku Y. Transport of sugars and amino acids in bacteria. 3. Studies on the restoration of active transport. J Biol Chem. 1968 Jun 10;243(11):3128–3135. [PubMed] [Google Scholar]
- Berkowitz D. D-Mannitol utilization in Salmonella typhimurium. J Bacteriol. 1971 Jan;105(1):232–240. doi: 10.1128/jb.105.1.232-240.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bratthall D. Demonstration of five serological groups of streptococcal strains resembling Streptococcus mutans. Odontol Revy. 1970;21(2):143–152. [PubMed] [Google Scholar]
- Brown A. T., Patterson C. E. Heterogeneity of Streptococcus mutans strains based on their mannitol-1-phosphate dehydrogenases: criterion for rapid classification. Infect Immun. 1972 Sep;6(3):422–424. doi: 10.1128/iai.6.3.422-424.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A. T., Wittenberger C. L. Mannitol and sorbitol catabolism in Streptococcus mutans. Arch Oral Biol. 1973 Jan;18(1):117–126. doi: 10.1016/0003-9969(73)90026-5. [DOI] [PubMed] [Google Scholar]
- Dallmeier E., Bestmann H. J., Kröncke A. Uber den Abbau von Glukose und Sorbit durch Plaques-Streptokokken. Dtsch Zahnarztl Z. 1970 Sep;25(9):887–898. [PubMed] [Google Scholar]
- Eagon R. G., Asbell M. A. Effect of divalent cations on the uptake and oxidation of substrates by Pseudomonas aeruginosa. J Bacteriol. 1969 Feb;97(2):812–819. doi: 10.1128/jb.97.2.812-819.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good N. E., Winget G. D., Winter W., Connolly T. N., Izawa S., Singh R. M. Hydrogen ion buffers for biological research. Biochemistry. 1966 Feb;5(2):467–477. doi: 10.1021/bi00866a011. [DOI] [PubMed] [Google Scholar]
- HORWITZ S. B., KAPLAN N. O. HEXITOL DEHYDROGENASES OF BACILLUS SUBTILIS. J Biol Chem. 1964 Mar;239:830–838. [PubMed] [Google Scholar]
- KUNDIG W., GHOSH S., ROSEMAN S. PHOSPHATE BOUND TO HISTIDINE IN A PROTEIN AS AN INTERMEDIATE IN A NOVEL PHOSPHO-TRANSFERASE SYSTEM. Proc Natl Acad Sci U S A. 1964 Oct;52:1067–1074. doi: 10.1073/pnas.52.4.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin E. C. The genetics of bacterial transport systems. Annu Rev Genet. 1970;4:225–262. doi: 10.1146/annurev.ge.04.120170.001301. [DOI] [PubMed] [Google Scholar]
- Patni N. J., Alexander J. K. Catabolism of fructose and mannitol in Clostridium thermocellum: presence of phosphoenolpyruvate: fructose phosphotransferase, fructose 1-phosphate kinase, phosphoenolpyruvate: mannitol phosphotransferase, and mannitol 1-phosphate dehydrogenase in cell extracts. J Bacteriol. 1971 Jan;105(1):226–231. doi: 10.1128/jb.105.1.226-231.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romano A. H., Eberhard S. J., Dingle S. L., McDowell T. D. Distribution of the phosphoenolpyruvate: glucose phosphotransferase system in bacteria. J Bacteriol. 1970 Nov;104(2):808–813. doi: 10.1128/jb.104.2.808-813.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schachtele C. F. Glucose transport in Streptococcus mutans: preparation of cytoplasmic membranes and characteristics of phosphotransferase activity. J Dent Res. 1975 Mar-Apr;54(2):330–338. [PubMed] [Google Scholar]
- Schachtele C. F., Mayo J. A. Phosphoenolpyruvate-dependent glucose transport in oral streptococci. J Dent Res. 1973 Nov-Dec;52(6):1209–1215. doi: 10.1177/00220345730520060801. [DOI] [PubMed] [Google Scholar]
- Stein R., Schrecker O., Lauppe H. F., Hengstenberg H. The staphylococcal PEP dependent phosphotransferase system: demonstration of a phosphorylated intermediate of the enzyme I component. FEBS Lett. 1974 May 15;42(1):98–100. doi: 10.1016/0014-5793(74)80288-7. [DOI] [PubMed] [Google Scholar]
- Tanaka S., Lerner S. A., Lin E. C. Replacement of a phosphoenolpyruvate-dependent phosphotransferase by a nicotinamide adenine dinucleotide-linked dehydrogenase for the utilization of mannitol. J Bacteriol. 1967 Feb;93(2):642–648. doi: 10.1128/jb.93.2.642-648.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]


