Abstract
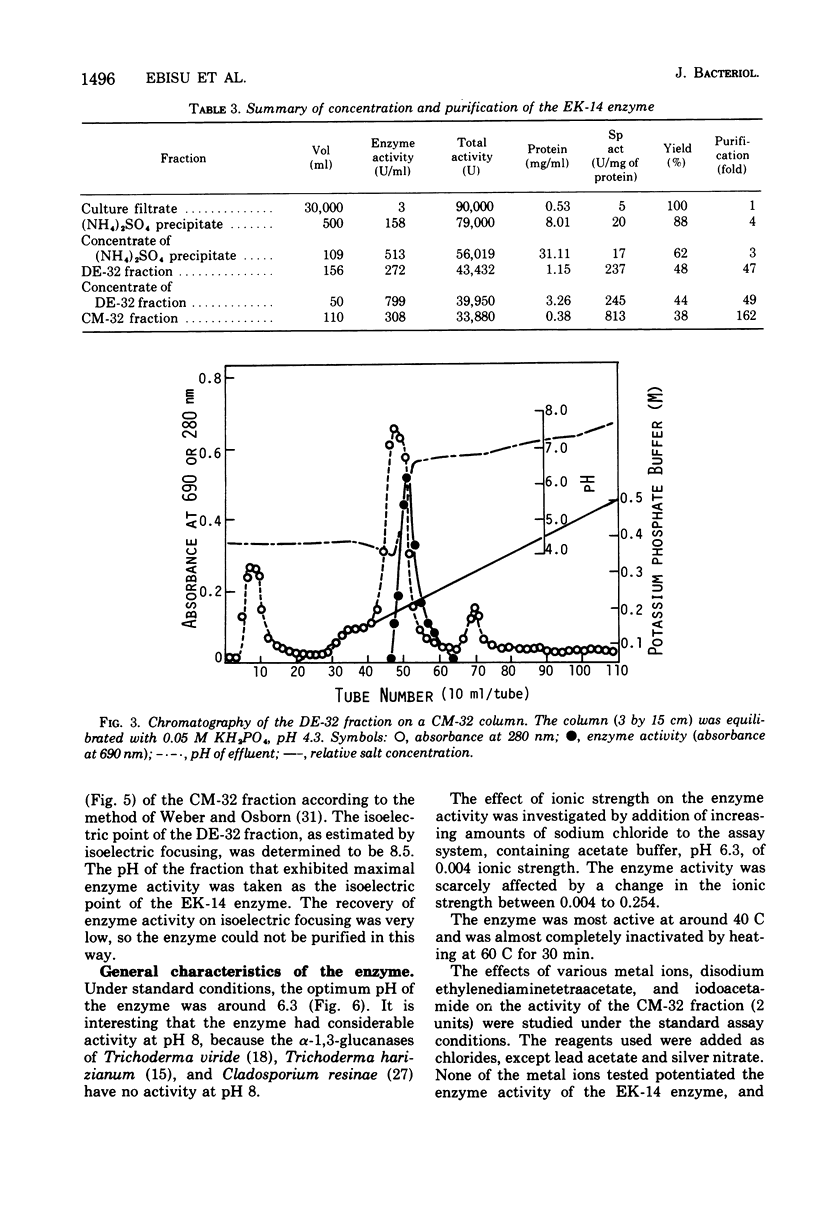
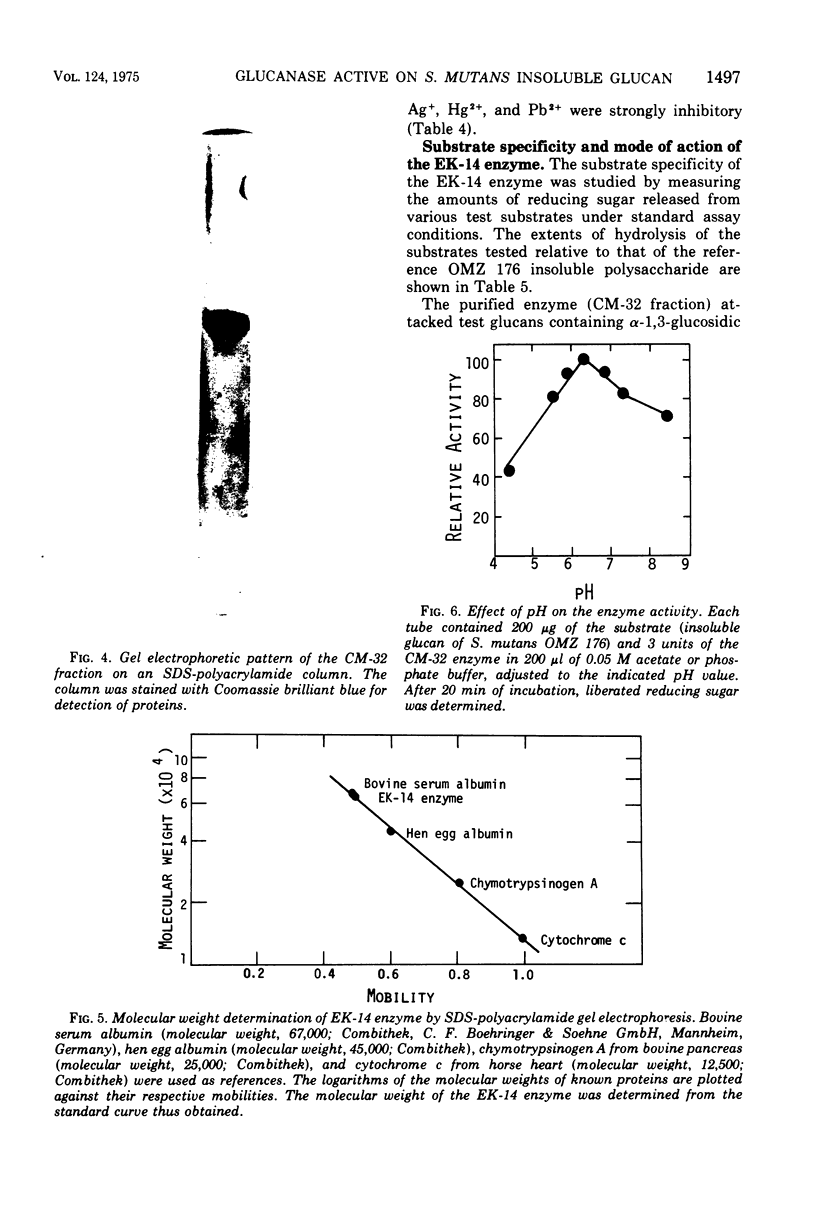
Studies were made on the physical and chemical properties of polysaccharides synthesized by cell-free extracts of Streptococcus mutans, Streptococcus sanguis, and Streptococcus sp. and their susceptibilities to dextranases. Among the polysaccharides examined, insoluble glucans were rather resistant to available dextranase preparations, and the insoluble, sticky glucan produced by S. mutans OMZ 176, which could be important in formation of dental plaques, was the most resistant. By enrichment culture of soil specimens, using OMZ 176 glucans as the sole carbon source, an organism was isolated that produced colonies surrounded by a clear lytic zone on opaque agar plates containing the OMZ 176 glucan. The organism was identified as a strain of Flavobacterium and named the Ek-14 bacterium. EK-14 bacterium was grown in Trypticase soy broth, and an enzyme capable of hydrolyzing the OMZ 176 glucan was concentrated from the culture supernatant and purified by negative adsorption on a diethylaminoethyl-cellulose (DE-32) column and gradient elution chromatography with a carboxymethyl-cellulose (CM-32) column. The enzyme was a basic protein with an isoelectric point of pH 8.5 and molecular weight of 65,000. Its optimum pH was 6.3 and its optimal temperature was 42 C. The purified enzyme released 11% of the total glucose residues of the OMZ 176 glucan as reducing sugars and solubilized about half of the substrate glucan. The products were found to be isomaltose, nigerose, and nigerotriose, with some oligosaccharides. The purified enzyme split the alpha-1,3-glucan endolytically and was inactive toward glucans containing alpha-1,6, alpha-1,4, beta-1,3, beta-1,4, and/or beta-1,6 bonds as the main linkages.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BAKER E. E., WHITESIDE R. E. PREPARATION AND PROPERTIES OF A VI ANTIGEN-DEGRADING ENZYME. J Bacteriol. 1965 May;89:1217–1224. doi: 10.1128/jb.89.5.1217-1224.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacon J. S., Jones D., Farmer V. C., Webley D. M. The occurrence of alpha(1-3)glucan in Cryptococcus, Schizosaccharomyces and Polyporus species, and its hydrolysis by a Streptomyces culture filtrate lysing cell walls of Cryptococcus. Biochim Biophys Acta. 1968 May;158(2):313–315. doi: 10.1016/0304-4165(68)90153-0. [DOI] [PubMed] [Google Scholar]
- Baird J. K., Longyear V. M., Ellwood D. C. Water insoluble and soluble glucans produced by extracellular glycosyltransferases from Streptococcus mutans. Microbios. 1973 Sep-Oct;8(30):143–150. [PubMed] [Google Scholar]
- Bratthall D. Demonstration of five serological groups of streptococcal strains resembling Streptococcus mutans. Odontol Revy. 1970;21(2):143–152. [PubMed] [Google Scholar]
- Ceska M., Granath K., Norrman B., Guggenheim B. Structural and enzymatic studies on glucans synthesized with glucosyltransferases of some strains of oral streptococci. Acta Chem Scand. 1972;26(6):2223–2230. doi: 10.3891/acta.chem.scand.26-2223. [DOI] [PubMed] [Google Scholar]
- Ebisu S., Kato K., Kotani S., Misaki A. Structural differences in fructans elaborated by streptococcus mutans and Strep. salivarius. J Biochem. 1975 Nov;78(5):879–887. doi: 10.1093/oxfordjournals.jbchem.a130993. [DOI] [PubMed] [Google Scholar]
- Ebisu S., Misaki A., Kato K., Kotani S. The structure of water-insoluble glucans of cariogenic Streptococcus mutans, formed in the absence and presence of dextranase. Carbohydr Res. 1974 Dec;38:374–381. doi: 10.1016/s0008-6215(00)82375-7. [DOI] [PubMed] [Google Scholar]
- Freedman M. L., Tanzer J. M. Dissociation of plaque formation from glucan-induced agglutination in mutants of Streptococcus mutans. Infect Immun. 1974 Jul;10(1):189–196. doi: 10.1128/iai.10.1.189-196.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons R. J., Banghart S. B. Synthesis of extracellular dextran by cariogenic bacteria and its presence in human dental plaque. Arch Oral Biol. 1967 Jan;12(1):11–23. doi: 10.1016/0003-9969(67)90137-9. [DOI] [PubMed] [Google Scholar]
- Gibbons R. J., Nygaard M. Synthesis of insoluble dextran and its significance in the formation of gelatinous deposits by plaque-forming streptococci. Arch Oral Biol. 1968 Oct;13(10):1249–1262. doi: 10.1016/0003-9969(68)90081-2. [DOI] [PubMed] [Google Scholar]
- Guggenheim B. Enzymatic hydrolysis and structure of water-insoluble glucan produced by glucosyltransferases from a strain of streptococcus mutans. Helv Odontol Acta. 1970 Nov;14(Suppl):89+–89+. [PubMed] [Google Scholar]
- Guggenheim B. Extracellular polysaccharides and microbial plaque. Int Dent J. 1970 Dec;20(4):657–678. [PubMed] [Google Scholar]
- Guggenheim B., Haller R. Purification and properties of an alpha-(1-3) glucanohydrolase from Trichoderma harzianum. J Dent Res. 1972 Mar-Apr;51(2):394–402. doi: 10.1177/00220345720510022701. [DOI] [PubMed] [Google Scholar]
- Guggenheim B., Schroeder H. E. Biochemical and morphological aspects of extracellular polysaccharides produced by cariogenic streptococci. Helv Odontol Acta. 1967 Oct;11(2):131–152. [PubMed] [Google Scholar]
- HAKOMORI S. A RAPID PERMETHYLATION OF GLYCOLIPID, AND POLYSACCHARIDE CATALYZED BY METHYLSULFINYL CARBANION IN DIMETHYL SULFOXIDE. J Biochem. 1964 Feb;55:205–208. [PubMed] [Google Scholar]
- Hasegawa S., Nordin J. H. Enzymes that hydrolyze fungal cell wall polysaccharides. I. Purification and properties of an endo-alpha-D-(1-3)-glucanase from Trichoderma. J Biol Chem. 1969 Oct 25;244(20):5460–5470. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Mukasa H., Slade H. D. Mechanism of adherence of Streptococcus mutans to smooth surfaces. I. Roles of insoluble dextran-levan synthetase enzymes and cell wall polysaccharide antigen in plaque formation. Infect Immun. 1973 Oct;8(4):555–562. doi: 10.1128/iai.8.4.555-562.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukasa H., Slade H. D. Mechanism of the Adherence of Streptococcus mutans to Smooth Surfaces III. Purification and Properties of the Enzyme Complex Responsible for Adherence. Infect Immun. 1974 Nov;10(5):1135–1145. doi: 10.1128/iai.10.5.1135-1145.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murayama Y., Wada H., Hayashi H., Uchida T., Yokomizo E. Effects of dextranase from Spicaria violaceae (IFO 6120) on the polysaccharides produced by oral streptococci and on human dental plaque. J Dent Res. 1973 Jul-Aug;52(4):658–667. doi: 10.1177/00220345730520040401. [DOI] [PubMed] [Google Scholar]
- Newbrun E. Extracellular polysaccharides synthesized by glucosyltransferases of oral streptococci. Composition and susceptibility to hydrolysis. Caries Res. 1972;6(2):132–147. doi: 10.1159/000259785. [DOI] [PubMed] [Google Scholar]
- Newbrun E. Sucrose, the arch criminal of dental caries. Odontol Revy. 1967;18(4):373–386. [PubMed] [Google Scholar]
- PARK J. T., JOHNSON M. J. A submicrodetermination of glucose. J Biol Chem. 1949 Nov;181(1):149–151. [PubMed] [Google Scholar]
- Sakakibara K., Torii M., Misaki A., Miyaji H. Acetolysis of dextran NRRL b1397. Preparation of trisaccharides containing (1 leads to 2)- and (1 leads to 6)-alpha-D-glucose linkages. Carbohydr Res. 1972 Dec;25(2):443–451. doi: 10.1016/s0008-6215(00)81656-0. [DOI] [PubMed] [Google Scholar]
- TREVELYAN W. E., PROCTER D. P., HARRISON J. S. Detection of sugars on paper chromatograms. Nature. 1950 Sep 9;166(4219):444–445. doi: 10.1038/166444b0. [DOI] [PubMed] [Google Scholar]
- Tanzer J. M., Freedman M. L., Fitzgerald R. J., Larson R. H. Diminished virulence of glucan synthesis-defective mutants of Streptococcus mutans. Infect Immun. 1974 Jul;10(1):197–203. doi: 10.1128/iai.10.1.197-203.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Zonneveld B. J. A new type of enzyme, and exo-splitting -1,3 glucanase from non-induced cultures of Aspergillus nidulans. Biochim Biophys Acta. 1972 Feb 28;258(2):541–547. doi: 10.1016/0005-2744(72)90245-8. [DOI] [PubMed] [Google Scholar]