Abstract
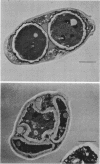
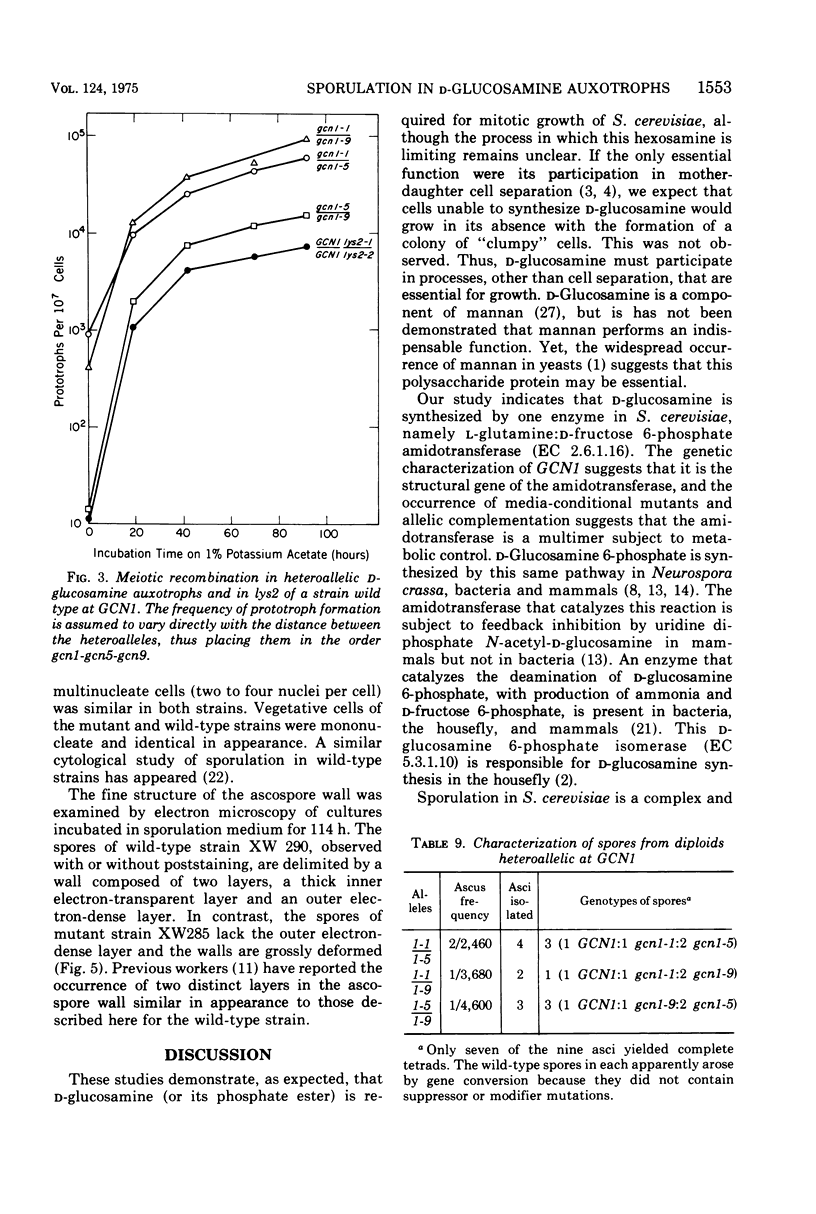
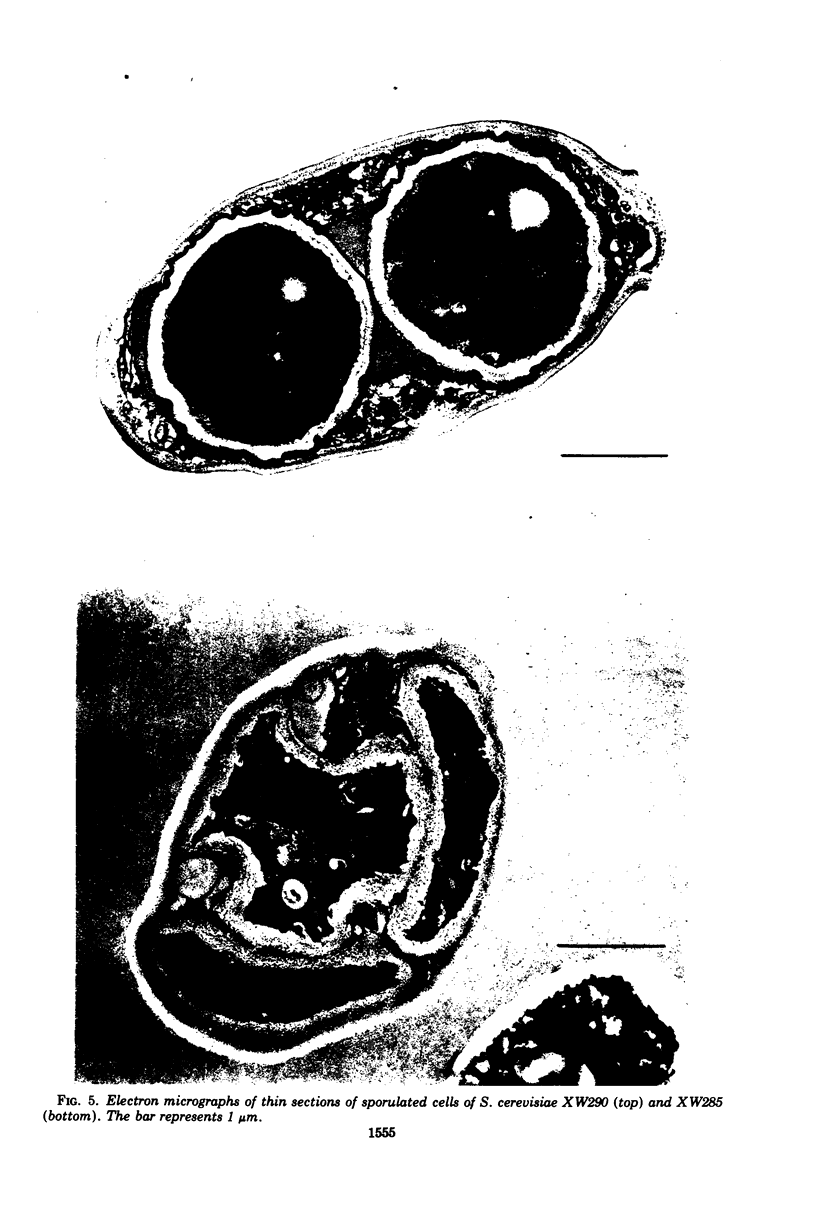
Mutants that require exogenous D-glucosamine for growth were isolated from Saccharomyces cerevisiae X2180-1A after ethyl methane sulfonate mutagenesis. Class A auxotrophs fail to grow on yeast extract-peptone-dextrose and minimal media, whereas class B auxotrophs grow on minimal medium and readily revert to grow on yeast extract-peptone-dextrose medium. Class B auxotrophs are suppressible by a class of suppressors distinct from nonsense suppressors, and their properties suggest that they are defective in a regulatory function. All 23 mutants studied were recessive and allelic, and they define a new gene designated gcn1. An analysis of a class A auxotroph revealed that it lacked L-glutamine:D-fructose 6-phosphate amidotransferase (EC 2.6.1.16) activity and indicates that GCN1 codes the amino acid sequence of this enzyme. The finding that all mutants were allelic indicates that the amidotransferase is the only enzyme responsible for D-glucosamine synthesis in S. cerevisiae. The occurrence of allelic complementation and media-conditional mutants suggests that the amidotransferase is a multimeric enzyme with an activity subject to metabolic control. Diploids homozygous for gcn1 fail to complete sporulation. They proceed through meiosis normally, as judged by the occurrence of meiotic recombination, the production of haploid nuclei, and the formation of multinucleate cells visible after Giemsa staining. However, the formation of glusulase-resistant ascospores is blocked, and deformed spores lacking the electron-dense outer layer characteristic of the normal spore wall are observed by electron microscopy. Cells that acquire the ability to synthesize D-glucosamine, because of gene conversion during meiosis, complete sporulation in a normal fashion. Thus, the GCN1 gene product appears to be synthesized late in sporulation and may prove to be a useful developmental landmark for the termination of ascospore development.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ballou C. E., Raschke W. C. Polymorphism of the somatic antigen of yeast. Science. 1974 Apr 12;184(4133):127–134. doi: 10.1126/science.184.4133.127. [DOI] [PubMed] [Google Scholar]
- Benson R. L., Friedman S. Allosteric control of glucosamine phosphate isomerase from the adult housefly and its role in the synthesis of glucosamine 6-phosphate. J Biol Chem. 1970 May 10;245(9):2219–2228. [PubMed] [Google Scholar]
- Cabib E., Bowers B. Chitin and yeast budding. Localization of chitin in yeast bud scars. J Biol Chem. 1971 Jan 10;246(1):152–159. [PubMed] [Google Scholar]
- Cabib E., Farkas V. The control of morphogenesis: an enzymatic mechanism for the initiation of septum formation in yeast. Proc Natl Acad Sci U S A. 1971 Sep;68(9):2052–2056. doi: 10.1073/pnas.68.9.2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esposito M. S., Esposito R. E., Arnaud M., Halvorson H. O. Acetate utilization and macromolecular synthesis during sporulation of yeast. J Bacteriol. 1969 Oct;100(1):180–186. doi: 10.1128/jb.100.1.180-186.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esposito M. S., Esposito R. E., Arnaud M., Halvorson H. O. Conditional mutants of meiosis in yeast. J Bacteriol. 1970 Oct;104(1):202–210. doi: 10.1128/jb.104.1.202-210.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esposito R. E., Frink N., Bernstein P., Esposito M. S. The genetic control of sporulation in Saccharomyces. II. Dominance and complementation of mutants of meiosis and spore formation. Mol Gen Genet. 1972;114(3):241–248. doi: 10.1007/BF01788893. [DOI] [PubMed] [Google Scholar]
- GHOSH S., BLUMENTHAL H. J., DAVIDSON E., ROSEMAN S. Glucosamine metabolism. V. Enzymatic synthesis of glucosamine 6-phosphate. J Biol Chem. 1960 May;235:1265–1273. [PubMed] [Google Scholar]
- Gilmore R. A. Super-suppressors in Saccharomyces cerevisiae. Genetics. 1967 Aug;56(4):641–658. doi: 10.1093/genetics/56.4.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAWTHORNE D. C., MORTIMER R. K. Super-suppressors in yeast. Genetics. 1963 Apr;48:617–620. doi: 10.1093/genetics/48.4.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Illingworth R. F., Rose A. H., Beckett A. Changes in the lipid composition and fine structure of Saccharomyces cerevisiae during ascus formation. J Bacteriol. 1973 Jan;113(1):373–386. doi: 10.1128/jb.113.1.373-386.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane S. M., Roth R. Carbohydrate metabolism during ascospore development in yeast. J Bacteriol. 1974 Apr;118(1):8–14. doi: 10.1128/jb.118.1.8-14.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornfeld R. Studies on L-glutamine D-fructose 6-phosphate amidotransferase. I. Feedback inhibition by uridine diphosphate-N-acetylglucosamine. J Biol Chem. 1967 Jul 10;242(13):3135–3141. [PubMed] [Google Scholar]
- LELOIR L. F., CARDINI C. E. The biosynthesis of glucosamine. Biochim Biophys Acta. 1953 Sep-Oct;12(1-2):15–22. doi: 10.1016/0006-3002(53)90119-x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Manney T. R. Evidence for chain termination by super-suppressible mutants in yeast. Genetics. 1968 Dec;60(4):719–733. doi: 10.1093/genetics/60.4.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moens P. B., Esposito R. E., Esposito M. S. Aberrant nuclear behavior at meiosis and anucleate spore formation by sporulation-deficient (SPO) mutants of Saccharomyces cerevisiae. Exp Cell Res. 1974 Jan;83(1):166–174. doi: 10.1016/0014-4827(74)90700-9. [DOI] [PubMed] [Google Scholar]
- Moens P. B. Fine structure of ascospore development in the yeast Saccharomyces cerevisiae. Can J Microbiol. 1971 Apr;17(4):507–510. doi: 10.1139/m71-084. [DOI] [PubMed] [Google Scholar]
- Moens P. B., Rapport E. Spindles, spindle plaques, and meiosis in the yeast Saccharomyces cerevisiae (Hansen). J Cell Biol. 1971 Aug;50(2):344–361. doi: 10.1083/jcb.50.2.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortimer R. K., Hawthorne D. C. Genetic mapping in Saccharomyces. Genetics. 1966 Jan;53(1):165–173. doi: 10.1093/genetics/53.1.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth R. Carbohydrate accumulation during the sporulation of yeast. J Bacteriol. 1970 Jan;101(1):53–57. doi: 10.1128/jb.101.1.53-57.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth R. Chromosome replication during meiosis: identification of gene functions required for premeiotic DNA synthesis. Proc Natl Acad Sci U S A. 1973 Nov;70(11):3087–3091. doi: 10.1073/pnas.70.11.3087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth R., Halvorson H. O. Sporulation of yeast harvested during logarithmic growth. J Bacteriol. 1969 May;98(2):831–832. doi: 10.1128/jb.98.2.831-832.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth R., Lusnak K. DNA synthesis during yeast sporulation: genetic control of an early developmental event. Science. 1970 Apr 24;168(3930):493–494. doi: 10.1126/science.168.3930.493. [DOI] [PubMed] [Google Scholar]
- SHERMAN F., ROMAN H. Evidence for two types of allelic recombination in yeast. Genetics. 1963 Feb;48:255–261. doi: 10.1093/genetics/48.2.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sentandreu R., Northcote D. H. The structure of a glycopeptide isolated from the yeast cell wall. Biochem J. 1968 Sep;109(3):419–432. doi: 10.1042/bj1090419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simchen G. Are mitotic functions required in meiosis? Genetics. 1974 Apr;76(4):745–753. doi: 10.1093/genetics/76.4.745. [DOI] [PMC free article] [PubMed] [Google Scholar]