Abstract
The synthesis by Streptomyces sp. no. 6 of an extracellular chitosanase was induced by glucosamine. The enzyme was purified to homogeneity by Sephadex G-100, carboxymethyl-cellulose, and diethylaminoethyl-cellulose chromatography. The purified enzyme hydrolyzed chitosan (the beta-1,4-linked polymer of glucosamine) but not chitin nor carboxymethyl-cellulose. The only products of the hydrolysis detectable by paper chromatography were di- and triglucosamine. Sephadex G-100 chromatography and sodium dodecyl sulfate-polyacrylamide gel electrophoresis indicated that the molecular weight of the enzyme was between 29,000 and 26,000. Acid hydrolysates of the enzyme contained no cysteic acid or glucosamine or other carbohydrate. At 25 C, maximum activity was obtained between pH 4.5 and 6.5. The enzymatic hydrolysis of chitosan occurred over a wide range of temperatures and was maximal at 60 C. The rate of the reaction was inhibited by concentrations of soluble chitosan higher than 0.5 g/liter. The apparent Km calculated from a Lineweaver-Burke plot was 0.688 g/liter at pH 5.5. The enzyme prevented spore germination and caused a significant decrease in the turbidity of germinated spore suspensions of the Mucor strains tested. Such a decrease was the result of a partial lysis of the cell wall.
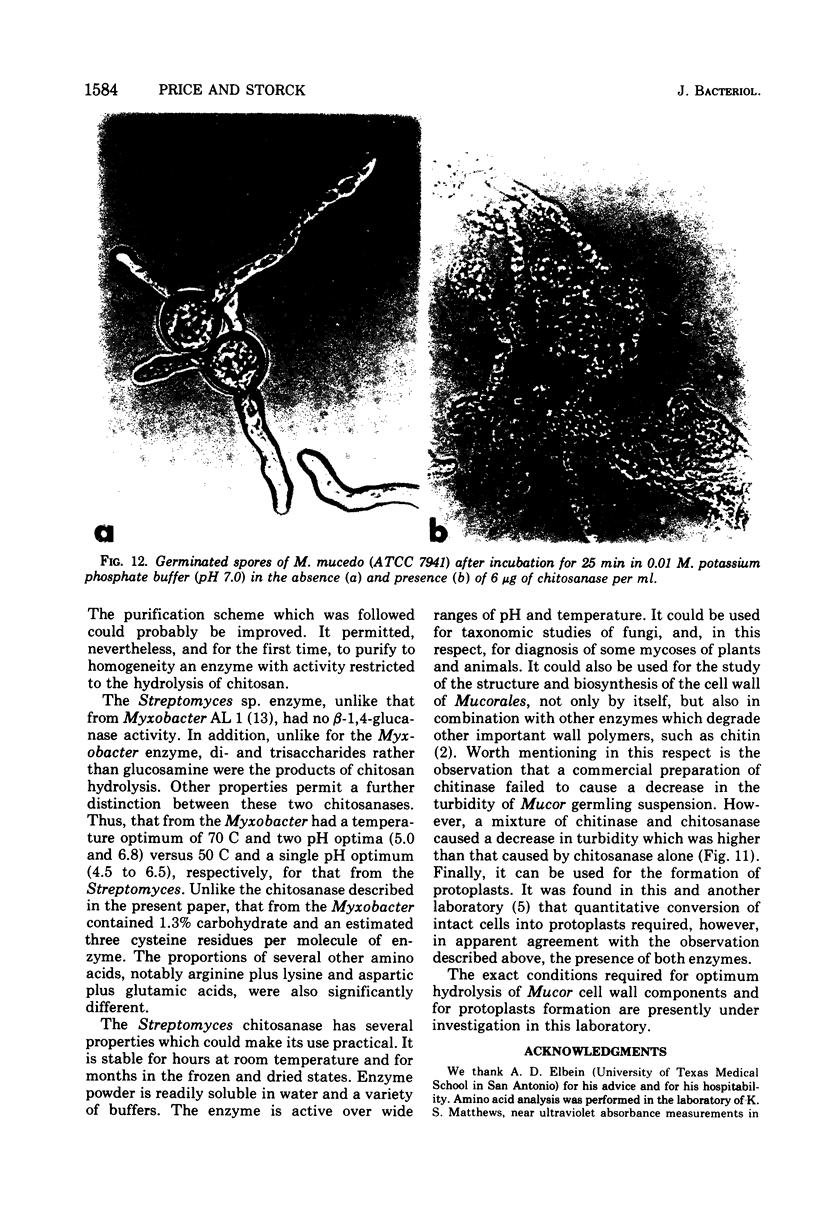
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews P. The gel-filtration behaviour of proteins related to their molecular weights over a wide range. Biochem J. 1965 Sep;96(3):595–606. doi: 10.1042/bj0960595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARTNICKI-GARCIA S., NICKERSON W. J. Isolation, composition, and structure of cell walls of filamentous and yeast-like forms of Mucor rouxii. Biochim Biophys Acta. 1962 Mar 26;58:102–119. doi: 10.1016/0006-3002(62)90822-3. [DOI] [PubMed] [Google Scholar]
- Bartnicki-Garcia S. Cell wall chemistry, morphogenesis, and taxonomy of fungi. Annu Rev Microbiol. 1968;22:87–108. doi: 10.1146/annurev.mi.22.100168.000511. [DOI] [PubMed] [Google Scholar]
- Binding H. The isolation, regeneration and fusion of Phycomyces protoplasts. Mol Gen Genet. 1974;135(3):273–276. doi: 10.1007/BF00268622. [DOI] [PubMed] [Google Scholar]
- DISCHE Z. New color reactions for determination of sugars in polysaccharides. Methods Biochem Anal. 1955;2:313–358. doi: 10.1002/9780470110188.ch11. [DOI] [PubMed] [Google Scholar]
- Goodwin T. W., Morton R. A. The spectrophotometric determination of tyrosine and tryptophan in proteins. Biochem J. 1946;40(5-6):628–632. doi: 10.1042/bj0400628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haidle C. W., Storck R. Control of dimorphism in Mucor rouxii. J Bacteriol. 1966 Oct;92(4):1236–1244. doi: 10.1128/jb.92.4.1236-1244.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedges A., Wolfe R. S. Extracellular enzyme from Myxobacter AL-1 that exhibits both beta-1,4-glucanase and chitosanase activites. J Bacteriol. 1974 Nov;120(2):844–853. doi: 10.1128/jb.120.2.844-853.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones D., Bacon J. S., Farmer V. C., Webley D. M. Lysis of cell walls of Mucor ramannianus Möller by a Streptomyces sp. Antonie Van Leeuwenhoek. 1968;34(2):173–182. doi: 10.1007/BF02046428. [DOI] [PubMed] [Google Scholar]
- KREGER D. R. Observations on cell walls of yeasts and some other fungi by x-ray diffraction and solubility tests. Biochim Biophys Acta. 1954 Jan;13(1):1–9. doi: 10.1016/0006-3002(54)90264-4. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- LUDOWIEG J., DORFMAN A. A micromethod for the colorimetric determination of N-acetyl groups in acid mucopolysaccharides. Biochim Biophys Acta. 1960 Feb 26;38:212–218. doi: 10.1016/0006-3002(60)91233-6. [DOI] [PubMed] [Google Scholar]
- MARKOVITZ A., KLEIN R. P. Some aspects of the induced biosynthesis of alpha-amylase of Pseudomonas saccharophila. J Bacteriol. 1955 Dec;70(6):641–648. doi: 10.1128/jb.70.6.641-648.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monaghan R. L., Eveleigh D. E., Tewari R. P., Reese E. T. Chitosanase, a novel enzyme. Nat New Biol. 1973 Sep 19;245(142):78–80. doi: 10.1038/newbio245078a0. [DOI] [PubMed] [Google Scholar]
- SEIFTER S., DAYTON S. The estimation of glycogen with the anthrone reagent. Arch Biochem. 1950 Jan;25(1):191–200. [PubMed] [Google Scholar]
- STROMINGER J. L., PARK J. T., THOMPSON R. E. Composition of the cell wall of Staphylococcus aureus: its relation to the mechanism of action of penicillin. J Biol Chem. 1959 Dec;234:3263–3268. [PubMed] [Google Scholar]
- TREVELYAN W. E., PROCTER D. P., HARRISON J. S. Detection of sugars on paper chromatograms. Nature. 1950 Sep 9;166(4219):444–445. doi: 10.1038/166444b0. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Zacharius R. M., Zell T. E., Morrison J. H., Woodlock J. J. Glycoprotein staining following electrophoresis on acrylamide gels. Anal Biochem. 1969 Jul;30(1):148–152. doi: 10.1016/0003-2697(69)90383-2. [DOI] [PubMed] [Google Scholar]