Abstract
The impact of the fibroblast growth factor receptor 4 (FGFR4) Gly388Arg polymorphism on bladder cancer is unknown. We found no clear correlations between the FGFR4 genotype and risk of bladder cancer or pathological parameters. Neither the polymorphism nor TP53 mutation status was an independent predictor of prognosis, but they might act jointly on the disease-specific survival of patients.
Keywords: bladder cancer, single nucleotide polymorphism, fibroblast growth factor receptor 4, prognosis, TP53 mutation
Bladder cancer is the fourth most common cancer in men and the ninth most common cancer in women in the United States. In 2006, there will be 61 420 new cases diagnosed and 13 060 will die from the disease (American Cancer Society, 2006). Owing to the highly heterogeneous clinical behaviors of bladder carcinoma, there is a need to stratify patients by molecular markers to optimise management and improve survival for patients with bladder cancer.
The fibroblast growth factor receptor (FGFR) family, which comprises of four structurally related tyrosine kinase receptors, transduces various crucial biological activities required for the growth and survival of cancer cells (Powers et al, 2000). Mutations in the FGFR3 gene have been identified in 40–50% of bladder tumours, and were associated with a favourable prognosis with a lower recurrence rate and disease-specific mortality (Cappellen et al, 1999; Karoui et al, 2001; Sibley et al, 2001; van Rhijn et al, 2001). These mutations in the FGFR3 gene are largely bladder cancer specific, and it is suggested that the FGFR family may play an important role in bladder carcinogenesis.
Recently, a common polymorphism in the transmembrane domain of the FGFR4 gene, Gly388Arg, was identified by Bange's group (Bange et al, 2002). A positive correlation between the presence of the FGFR4 Arg388 allele and prognostic parameters as well as survival time was reported in variant cancer studies, including breast, colon, lung, prostate, head and neck cancers and high-grade soft-tissue sarcoma (Bange et al, 2002; Morimoto et al, 2003; Streit et al, 2004; Wang et al, 2004; Spinola et al, 2005a). However, most studies were carried out with a relatively small sample size or suggested a possible effect only on a specific tumour type (Morimoto et al, 2003) or population (Wang et al, 2004). Furthermore, in subsequent larger studies of melanoma, breast, and colon cancer, no obvious association between the Gly388Arg genotype and cancer prognosis was found (Becker et al, 2003; Jezequel et al, 2004; Spinola et al, 2005b; Streit et al, 2006).
Mutations in the FGFR3 and TP53 genes are the two most frequent events observed in primary bladder tumours and have occurred in 59% and 25% of tumours, respectively (van Rhijn et al, 2004). TP53 mutations are often found in advanced tumours and, thus, are associated with a poor prognosis. On the contrary, FGFR3 mutations are associated with low-grade tumours and a favourable prognosis. This mutually exclusive expression suggests that FGFR3 and TP53 gene mutations may represent two alternative genetic pathways in the pathogenesis of bladder cancer.
To our knowledge, no study has been conducted on the prognostic significance of the FGFR4 genotype in bladder cancer. Given the potential involvement of the FGFR family in bladder carcinogenesis, it is of interest to investigate the potential impact of the FGFR4 Gly388Arg polymorphism and the combination of the FGFR4 genotype and TP53 mutation status on the progression of bladder cancer.
MATERIALS AND METHODS
This study involved 140 newly diagnosed bladder cancer patients who underwent radical cystectomy and 151 healthy controls at the Memorial Sloan-Kettering Cancer Center from October 1993 to June 1997. All cancer patients were pathologically confirmed and staged according to the TNM staging system. After obtaining IRB-approved signed consents, all subjects were interviewed and had blood samples collected. Complete follow-up data were available for 140 cases with a median follow-up time of 33 months (range, 5–120 months). Genotyping of the FGFR4 Gly388Arg polymorphism was determined with the RFLP analysis as described by Bange et al (2002). The status of TP53 mutation was previously detected in the same samples by three essays, including manual sequencing, a commercial genechip-based assay (p53 Genechip) and immunohistochemistry (Lu et al, 2002). The survival time was calculated from the date of cystectomy to the date of disease-specific deaths, the date of recurrence or metastasis, or the date of the last follow-up. Survival curves were plotted according to the Kaplan–Meier estimate and compared using the long-rank test. The proportional hazards model was employed to estimate hazard ratios when adjusting for potential confounding factors. All statistic analyses were performed using SAS 8.1 software.
RESULTS
The present study comprised of 140 bladder cancer patients and 151 healthy controls. Fifteen patients were excluded from the analysis because of poor quality DNA or indistinct genotyping results. One hundred and twenty-five patients who had similar distributions of demographic or pathological factors as the whole study group were included in the analyses. Among the 125 bladder cancer patients, 42.4% were heterozygous and 10.4% were homozygous carriers of the Arg388 allele. Compared with noncarriers, the OR of developing bladder cancer among Arg388 allele carriers was 1.05 (95% CI, 0.5–2.1) (data not shown). No obvious correlation was observed between the FGFR4 Gly388Arg polymorphism and clinicopathological parameters (Table 1).
Table 1. Associations between the FGFR4 genotype and demographic characteristics, smoking status, pathological parameters, and TP53 mutation status in 125 patients with bladder cancer.
| Gly/Gly, n (%) | Gly/Arg, n (%) | Arg/Arg, n (%) | P-value | |
|---|---|---|---|---|
| All patients | 59 (47.2) | 53 (42.4) | 13 (10.4) | |
| Age at diagnosis (years±s.d.) | 67.0±9.6 | 64.7±10.9 | 69.7±9.5 | 0.220 |
| Age(years) | ||||
| >66 | 35 (59.3) | 29 (54.7) | 8 (61.5) | 0.868 |
| ≤66 | 24 (40.7) | 24 (45.3) | 5 (38.5) | |
| Gender | ||||
| Male | 45 (76.3) | 42 (79.3) | 10 (76.9) | 0.952 |
| Female | 14 (23.7) | 11 (20.7) | 3 (23.1) | |
| Smoking status | ||||
| Current | 7 (13.2) | 7 (14.6) | 1 (7.7) | 0.885 |
| Former | 36 (67.9) | 32 (66.7) | 8 (61.5) | |
| Never | 10 (18.9) | 9 (18.7) | 4 (30.8) | |
| Stage | ||||
| I | 4 (6.8) | 3 (5.7) | 1 (7.7) | 0.993 |
| II | 5 (8.5) | 5 (9.4) | 1 (7.7) | |
| III | 45 (76.2) | 41 (77.4) | 11 (84.6) | |
| IV | 5 (8.5) | 4 (7.5) | 0 (0) | |
| Grade | ||||
| G1 | 3 (5.2) | 3 (5.8) | 0 (0) | 0.924 |
| G2 | 9 (15.5) | 11 (21.1) | 2 (15.4) | |
| G3-4 | 46 (79.3) | 38 (73.1) | 11 (54.6) | |
| Lymph node involvement | ||||
| Present | 22 (37.3) | 22 (41.5) | 7 (53.8) | 0.545 |
| Absent | 37 (62.7) | 31 (58.5) | 6 (46.2) | |
| Vascular invasion | ||||
| Yes | 31 (52.5) | 27 (50.9) | 8 (61.5) | 0.842 |
| No | 28 (47.5) | 26 (49.1) | 5 (38.5) | |
| TP53 mutation | ||||
| Yes | 26 (44.1) | 22 (41.5) | 10 (76.9) | 0.076 |
| No | 33 (55.9) | 31 (58.5) | 3 (23.1) | |
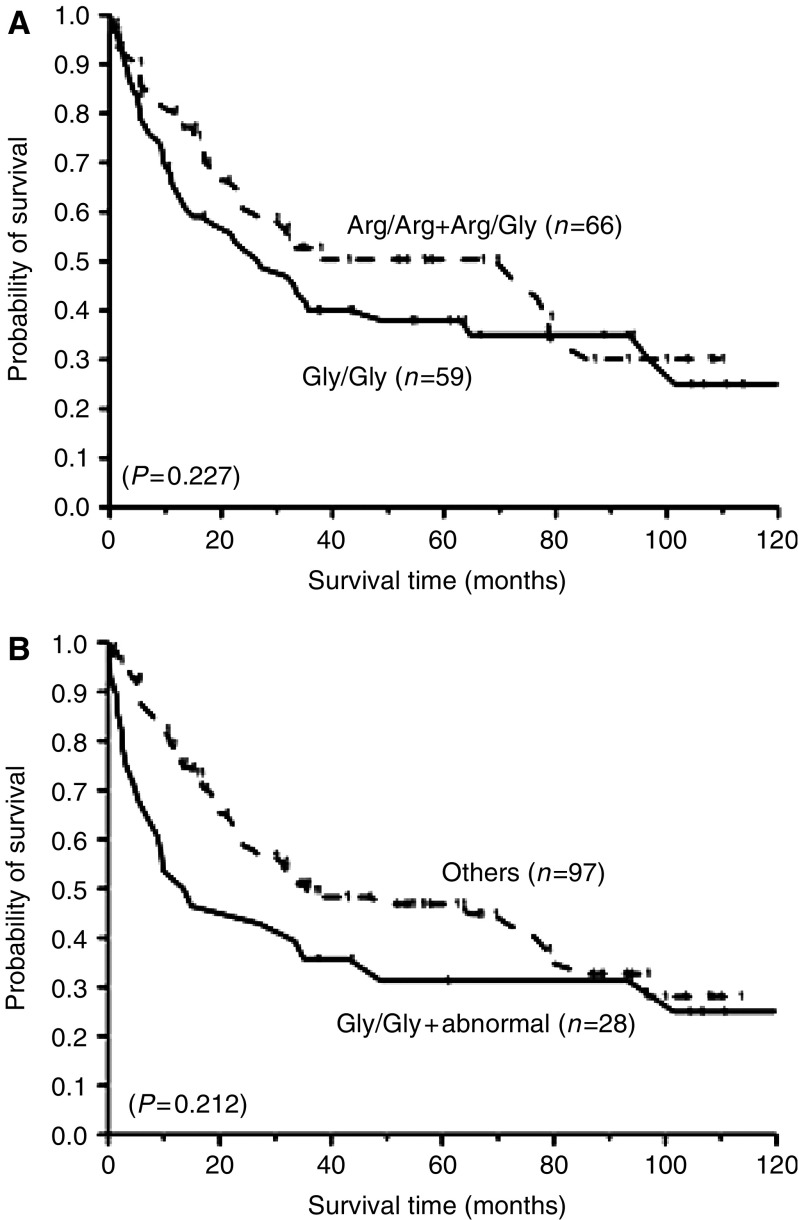
Of the 125 patients who were treated with radical cystectomy, 98 patients died during the 120-month follow-up period. Seventy three per cent of all deaths (n=72) were due to bladder cancer. No obvious difference was found between the FGFR4 genotype or TP53 mutations and the disease-specific survival (Table 2). The combination of both markers, however, was strongly associated with the disease-specific survival. FGFR4 Gly388 homozygous patients with TP53 mutation (median survival time=14.2 months) had a hazard ratio of 1.85 (95% CI, 1.01–3.39) for disease-specific survival compared with the reference group, which consisted of Gly388 homozygote without TP53 mutation and Arg388 allele carriers with or without TP53 mutation. (median survival time=23.3, 23.9 and 31.5 months, respectively) (Figure 1B).
Table 2. Hazard ratios of death from bladder cancer for the FGFR4 Gly388 polymorphism and TP53 mutation status in 125 cancer patients.
| No. | Death | Death (%) | Hazard ratioa | 95% CI | P-value | |
|---|---|---|---|---|---|---|
| FGFR4 genotype | ||||||
| Gly/Gly | 59 | 38 | 64 | 1.42 | (0.83–2.40) | 0.198 |
| Arg/Arg+Arg/Gly | 66 | 34 | 52 | ref. | ||
| TP53 status | ||||||
| Abnormal | 62 | 38 | 61 | 1.53 | (0.88–2.65) | 0.133 |
| Normal | 63 | 34 | 54 | ref. | ||
| FGFR4/TP53 | ||||||
| Gly/Gly/abnormal | 28 | 21 | 75 | 2.25 | (1.04–4.88) | 0.039 |
| Gly/Gly/normal | 31 | 17 | 55 | 1.28 | (0.58–2.83) | 0.538 |
| Arg/Arg+Arg/Gly/ abnormal | 34 | 17 | 50 | 1.38 | (0.63–3.04) | 0.423 |
| Arg/Arg+Arg/Gly/ normal | 32 | 17 | 53 | ref. | ||
| Gly/Gly+abnormal | 28 | 21 | 75 | 1.85 | (1.01–3.39) | 0.046 |
| Other | 97 | 51 | 53 | ref. | ||
Hazard ratios were adjusted for age, gender, lymph node involvement, tumour stage and grade.
Figure 1.
Kaplan–Meier disease-specific survival curves of bladder cancer patients. (A) Comparison between patients with FGFR4 Gly/Gly genotype and patients with FGFR4 Arg/Arg or Arg/Gly genotypes. The curves of Gly/Gly (n=59) and Arg allele carrier (n=66) patients are shown as solid and dashed lines, respectively. (B) Based on the combination of the FGFR4 genotype and TP53 mutations status, comparison between Gly allele homozygotes with abnormal p53 status (n=28, solid) and other (n=97, dashed). Small dots indicate censored observations. P-values were calculated by the log-rank test.
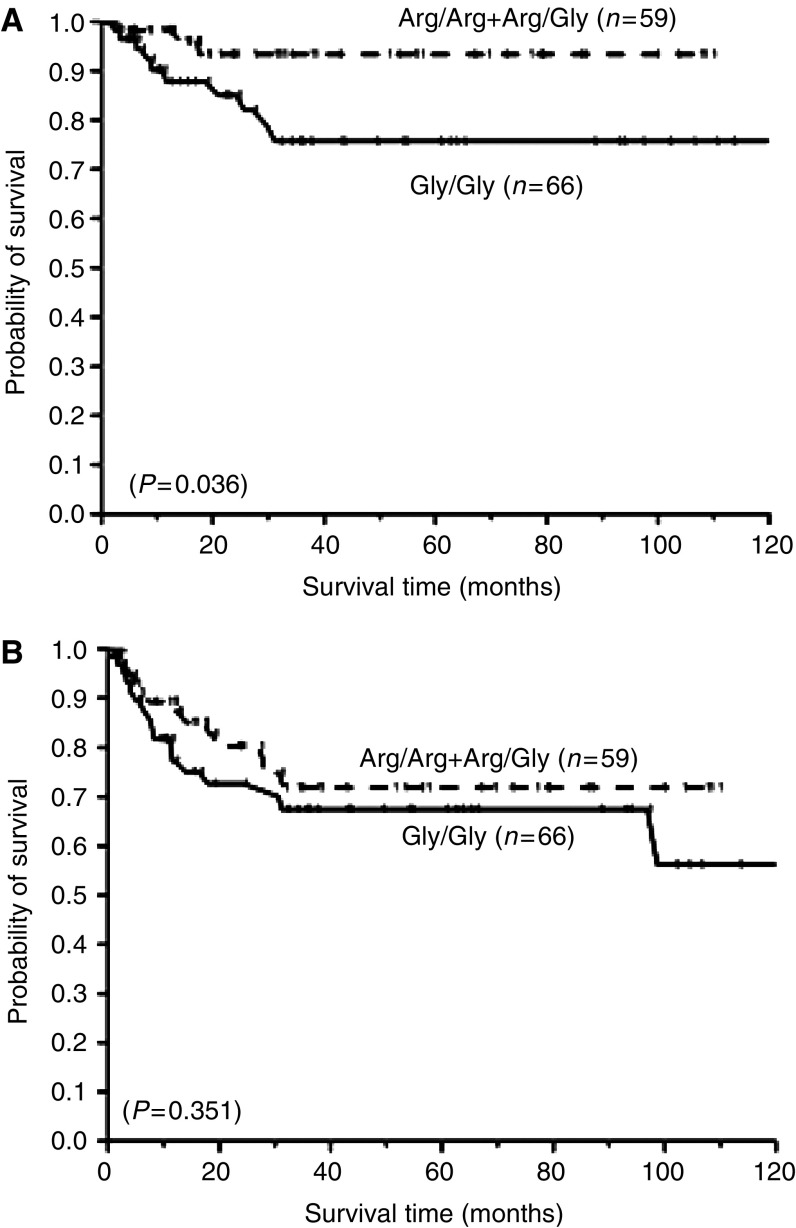
The FGFR4 Gly/Gly genotype was associated with poor recurrence-free survival (P=0.036, Figure 2A), but not metastasis-free survival (P=0.351, Figure 2B). Due to the small proportion of patients who suffered the recurrence of bladder cancer during the follow-up period, we combined recurrence and metastasis and calculated the disease-free survival time for each case. After adjusting for age, gender and pathologic stage, patients with the Gly/Gly genotype or TP53 mutation had a similar disease-free survival as Arg388 allele carriers (adjusted HR=0.99 and 1.01, respectively; data not shown).
Figure 2.
Survival of patients with bladder cancer according to variants of the FGFR Gly388Arg polymorphism. Log-rank analyses of the association between FGFR4 genotypes and (A) recurrence-free survival time and (B) metastasis-free survival time in bladder cancer.
DISCUSSION
We observed no clear association between the Gly388Arg genotype and risk of bladder cancer, indicating that this SNP may not be involved in the early development of bladder cancer. Our finding is in agreement with several previous studies on other types of cancer (Bange et al, 2002; Morimoto et al, 2003; Spinola et al, 2005a), except for one study on prostate cancer (Wang et al, 2004).
The present study found no clear correlation between the FGFR4 Gly388Arg polymorphism and pathological parameters. Furthermore, our results did not directly indicate that either the FGFR4 genotype or TP53 mutation status was an independent predictor of prognosis for bladder cancer but that they might act jointly on the disease-specific survival of patients, which supports the hypothesis that the FGFR3 and TP53 gene mutations may represent two alternative genetic pathways in the progression of bladder cancer (van Rhijn et al, 2004). A trend showed a better disease-specific survival rate for bladder cancer patients with the Arg/Arg genotype. This is contrary to the findings of previous studies on other types of cancer that the presence of the Arg allele is associated with poor survival (Bange et al, 2002; Morimoto et al, 2003; Streit et al, 2004; Wang et al, 2004; Spinola et al, 2005a). The reason for the conflicting results is unclear, but may reflect a tissue-specific effect of this polymorphism. For example, the activating somatic mutations in the FGFR3 gene appear to be bladder specific, and a much lower frequency of the mutations of the FGFR3 has been observed in other cancer sites. (Sibley et al, 2001; van Rhijn et al, 2001). The FGFR4 Gly388Arg polymorphism results in an amino-acid change in the transmembrane domain, which is a highly conserved region for receptor tyrosine kinase. Analogous missense mutations in the transmembrane domain in the FGFR3 gene, resulting from a Gly to Arg substitution at codon 380, were proposed to result in constitutive activation of FGFR3 signaling (Webster and Donoghue, 1996). In general, activating FGFR3 mutations are associated with favourable disease characteristics such as low stage and grade, low recurrence rate, and a lower mortality rate (Sibley et al, 2001; van Rhijn et al, 2001). Therefore, the FGFR4 Gly388 allele, but not the Arg388 allele, could exert an effect on the aggressive behaviors of the cancer cells in bladder.
We also found that the patients with the Gly/Gly genotype were associated with a greater risk of recurrence, but were not associated with metastasis compared with Arg allele carriers. Our results are in accordance with a study of melanoma patients, which showed no correlation between the FGFR4 genotype and metastasis (Streit et al, 2006). One possible reason for positive result in recurrence could possibly be due to false positives based on a relatively small number of recurrence cases (less than 10%).
Although the finding of differences in survival rates according to the combination of the FGFR4 genotype and TP53 mutation status is interesting, the results should be interpreted with caution. Because our study was limited to patients who underwent radical cystectomy, which is the standard treatment for muscle invasive bladder cancer (T2–T4), there are more patients with higher staging and grading tumours that have been recruited. However, no strong association between the FGFR4 genotype and indicators for the aggressive tumour was found in our study population because of potential selection bias.
In summary, our results suggest that the variations in neoplastic progression not only depends on somatic mutations occurring in the tumour itself but also on the patient's genetic characteristics. However, the molecular mechanisms of the FGFR4 Gly388Arg polymorphism has not been examined in human bladder cancer. Information on the function of this polymorphism or its potential biological interaction with TP53 is needed and may add information to optimise the treatment of patients with bladder cancer.
Acknowledgments
This study is partially supported in part by the NIH National Institute of Environmental Health Sciences, National Cancer Institute, Department of Health and Human Services, Grants ES06718, ES 011667, CA77954, CA09142, CA16042, CA42710, and CA96116.
References
- American Cancer Society (2006) Cancer facts and figures 2006 Report. Available from: http://www.cancer.org/
- Bange J, Prechtl D, Cheburkin Y, Specht K, Harbeck N, Schmitt M, Knyazeva T, Muller S, Gartner S, Sures I, Wang H, Imyanitov E, Haring HU, Knayzev P, Iacobelli S, Hofler H, Ullrich A (2002) Cancer progression and tumor cell motility are associated with the FGFR4 Arg(388) allele. Cancer Res 62: 840–847 [PubMed] [Google Scholar]
- Becker N, Nieters A, Chang-Claude J (2003) The fibroblast growth factor receptor gene Arg388 allele is not associated with early lymph node metastasis of breast cancer. Cancer Epidemiol Biomarkers Prev 12: 582–583 [PubMed] [Google Scholar]
- Cappellen D, De Oliveira C, Ricol D, de Medina S, Bourdin J, Sastre-Garau X, Chopin D, Thiery JP, Radvanyi F (1999) Frequent activating mutations of FGFR3 in human bladder and cervix carcinomas. Nat Genet 23: 18–20 [DOI] [PubMed] [Google Scholar]
- Jezequel P, Campion L, Joalland MP, Millour M, Dravet F, Classe JM, Delecroix V, Deporte R, Fumoleau P, Ricolleau G (2004) G388R mutation of the FGFR4 gene is not relevant to breast cancer prognosis. Br J Cancer 90: 189–193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karoui M, Hofmann-Radvanyi H, Zimmermann U, Couvelard A, Degott C, Faridoni-Laurens L, Ahomadegbe JC, Gazzeri S, Brambilla E, Clerici T, Charbonnier P, Tresallet C, Mitry E, Penna C, Rougier P, Boileau C, Thiery JP, Nordlinger B, Franc B, Radvanyi F (2001) No evidence of somatic FGFR3 mutation in various types of carcinoma. Oncogene 20: 5059–5061 [DOI] [PubMed] [Google Scholar]
- Lu ML, Wikman F, Orntoft TF, Charytonowicz E, Rabbani F, Zhang Z, Dalbagni G, Pohar KS, Yu G, Cordon-Cardo C (2002) Impact of alterations affecting the p53 pathway in bladder cancer on clinical outcome, assessed by conventional and array-based methods. Clin Cancer Res 8: 171–179 [PubMed] [Google Scholar]
- Morimoto Y, Ozaki T, Ouchida M, Umehara N, Ohata N, Yoshida A, Shimizu K, Inoue H (2003) Single nucleotide polymorphism in fibroblast growth factor receptor 4 at codon 388 is associated with prognosis in high-grade soft tissue sarcoma. Cancer 98: 2245–2250 [DOI] [PubMed] [Google Scholar]
- Powers CJ, McLeskey SW, Wellstein A (2000) Fibroblast growth factors, their receptors and signaling. Endocr Relat Cancer 7: 165–197 [DOI] [PubMed] [Google Scholar]
- Sibley K, Stern P, Knowles MA (2001) Frequency of fibroblast growth factor receptor 3 mutations in sporadic tumours. Oncogene 20: 4416–4418 [DOI] [PubMed] [Google Scholar]
- Spinola M, Leoni V, Pignatiello C, Conti B, Ravagnani F, Pastorino U, Dragani TA (2005a) Functional FGFR4 Gly388Arg polymorphism predicts prognosis in lung adenocarcinoma patients. J Clin Oncol 23: 7307–7311 [DOI] [PubMed] [Google Scholar]
- Spinola M, Leoni VP, Tanuma J, Pettinicchio A, Frattini M, Signoroni S, Agresti R, Giovanazzi R, Pilotti S, Bertario L, Ravagnani F, Dragani TA (2005b) FGFR4 Gly388Arg polymorphism and prognosis of breast and colorectal cancer. Oncol Rep 14: 415–419 [PubMed] [Google Scholar]
- Streit S, Bange J, Fichtner A, Ihrler S, Issing W, Ullrich A (2004) Involvement of the FGFR4 Arg388 allele in head and neck squamous cell carcinoma. Int J Cancer 111: 213–217 [DOI] [PubMed] [Google Scholar]
- Streit S, Mestel DS, Schmidt M, Ullrich A, Berking C (2006) FGFR4 Arg388 allele correlates with tumour thickness and FGFR4 protein expression with survival of melanoma patients. Br J Cancer 94: 1879–1886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Rhijn BW, Lurkin I, Radvanyi F, Kirkels WJ, van der Kwast TH, Zwarthoff EC (2001) The fibroblast growth factor receptor 3 (FGFR3) mutation is a strong indicator of superficial bladder cancer with low recurrence rate. Cancer Res 61: 1265–1268 [PubMed] [Google Scholar]
- van Rhijn BW, van der Kwast TH, Vis AN, Kirkels WJ, Boeve ER, Jobsis AC, Zwarthoff EC (2004) FGFR3 and P53 characterise alternative genetic pathways in the pathogenesis of urothelial cell carcinoma. Cancer Res 64: 1911–1914 [DOI] [PubMed] [Google Scholar]
- Wang J, Stockton DW, Ittmann M (2004) The fibroblast growth factor receptor-4 Arg388 allele is associated with prostate cancer initiation and progression. Clin Cancer Res 10: 6169–6178 [DOI] [PubMed] [Google Scholar]
- Webster MK, Donoghue DJ (1996) Constitutive activation of fibroblast growth factor receptor 3 by the transmembrane domain point mutation found in achondroplasia. EMBO J 15: 520–527 [PMC free article] [PubMed] [Google Scholar]