Abstract
CDKN2A locus on chromosome 9p21 encodes two tumour suppressor proteins pl6INK4A, which is a regulator of the retinoblastoma (RB) protein, and p14ARF, which is involved in the ARF–Mdm2–p53 pathway. The aim of this study was to determine if CDKN2A gene products are implicated in differentiated thyroid carcinogenesis and progression. We used real-time quantitative RT–PCR and immunohistochemistry to assess both transcripts and proteins levels in 60 tumours specimens. Overexpression of p14ARF and pl6INK4A was observed in follicular adenomas, follicular carcinomas and papillary carcinomas, while downregulation was found in oncocytic adenomas compared to nontumoral paired thyroid tissues. These deregulations were statistically significant for pl6INK4a (P=0.006) in follicular adenomas and close to statistical significance for p14ARF in follicular adenomas (P=0.06) and in papillary carcinomas (P=0.05). In all histological types, except papillary carcinomas, we observed a statistically significant relationship between p14ARF and E2F1 (r=0.64 to 1, P<0.05). Our data are consistent with involvement of CDKN2A transcript upregulation in thyroid follicular tumorigenesis as an early event. However, these deregulations do not appear to be correlated to the clinical outcome and they could not be used as potential prognostic markers.
Keywords: P14ARF, P16INK4A, thyroid, differentiated, carcinomas
Tumours arising from the thyroid follicular epithelium are regarded as a unique model to study human carcinogenesis because, while they all derive from thyrocytes, they have different clinical and histological features (reviewed in Gimm, 2001). Papillary (PTC) and follicular thyroid (FTC) cancers are well-differentiated tumours, which maintain some morphological features of the normal thyroid, and are, most of the time, responsive to radioiodine treatment (Vini and Harmer, 2002). In contrast, anaplastic thyroid cancers are undifferentiated neoplasias with a more aggressive behaviour (Giuffrida and Gharib, 2000). They have lost any significant resemblance with the structural organisation of a normal thyroid follicle, and are unresponsive to both radioactive iodine treatment and chemotherapeutic agents (reviewed in Gimm, 2001). From a molecular standpoint, well-differentiated thyroid cancers are recognised to be initiated by genetic events that involved the improper activation of cellular proto-oncogenes (Pierotti et al, 1996). Up to 60% of papillary thyroid carcinomas present either BRAF mutations or rearrangements in the RET or NTRK1 tyrosine kinase genes with a plethora of activating genes that are considered to represent the first step in tumour development (Santoro et al, 1996; Greco et al, 1997, reviewed in Xing, 2005). In 35% of follicular thyroid carcinomas, neoplastic transformation is triggered by activating mutations of the RAS gene or by the fusion of the PAX8 transcription factor with the gene encoding for peroxisome proliferator-activated receptor (Fagin, 2000; Kroll et al, 2000). Some of the genetic events responsible for the development of well-differentiated thyroid cancers have been identified; loss of heterozygosity in several chromosomal regions (1p, 3p, 3q, 10q, 11p, 13q and 22q) has been reported in papillary and follicular carcinomas (Ward et al, 1998; Kitamura et al, 2000a, 2000b). The only established genetic alteration involved in the dedifferentiation process leading to anaplastic thyroid tumour development is the loss of the p53 tumour suppressor gene (Fagin et al, 1993).
CDKN2A locus on chromosome 9p21 (reviewed in Sherr, 2001) encodes two proteins. INK4A (also referred to as pl6INK4A) specially blocks the CDK4 and CDK6 cyclin-dependent kinases that control the phosphorylation status of the retinoblastoma (RB) protein (Serrano et al, 1993). ARF (known as p14ARF in man and p19ARF in mouse) has been designed as a positive regulator of p53 levels because, through its complexation with Mdm2 (HDM2 in human), it prevents mdm2-mediated cytoplasmic translocation and degradation of p53 (Kubbutat et al, 1997). For this reason, ARF is considered to be an important factor of the so-called ARF–Mdm2–p53 pathway, that is activated by potential oncogenic signals such as oncogenic ras protein, E1A and v-Abl oncoproteins and ectopic expression of both c-myc and E2F1 (Sherr, 1998). Several experimental evidences also unequivocally established that ARF could inhibit cell proliferation independently of p53. For example, introduction of ARF into cells devoid of any functional p53 and Mdm2 proteins results in cell proliferation arrest (Carnero et al, 2000; Weber et al, 2000). Moreover, p14ARF inhibits the growth of p53 nullizygous human tumours in nude mice and induces the regression of p53−/− established tumours (Eymin et al, 2003).
The ARF locus is frequently impaired (in conjunction with the INK4A gene) in a variety of human primary tumours, indicating that disruption of this locus is essential for deregulating cell proliferation. However, implication of the p16INK4A and p14ARF has not been clearly defined in thyroid cancer development and progression. The present study was aimed at evaluating possible relationship between the p16INK4A and p14ARF expression on the one hand, and both clinical and pathological features of thyroid carcinomas on the other hand, by using quantitative RT–PCR and immunohistochemistry approaches.
MATERIALS AND METHODS
Patients and samples
A total of 102 thyroid tissue samples from 60 patients were processed, including 31 papillary carcinomas, five follicular carcinomas, 10 follicular adenomas, 14 oncocytic adenomas and 42 nontumoral thyroid tissue samples adjacent to carcinomas or adenomas. Tissue samples were obtained from the frozen tissue bank of the pathology department of Poitiers University Hospital. Specimen collection was performed in accordance with the Declaration of Helsinki. Tumour specimens were snap frozen in liquid nitrogen and stored at −80°C until processed for RNA extraction.
All patients were treated at Department of Endocrine Surgery of Poitiers, between 1998 and 2002. They underwent surgical treatment such as unilateral lobectomy, isthmolobectomy or total thyroidectomy according to clinical data. Clinicopathological data of the 60 patients was summarised in Table 1. AGES (age, grade (according to Broder's classification) tumour extent (local invasion and distant metastases) size of the primary tumour) prognostic scores were calculated and patients were included in minimal (score of 0–3.99) or higher risk groups (score of 4 and more) as described by Hay et al (1987).
Table 1. Characteristics of patients and tumours.
| All patients | Oncocytic adenomas | Follicular adenomas | Follicular carcinomas | Papillary carcinomas | |
|---|---|---|---|---|---|
| n patients | 60 | 14 | 10 | 5 | 31 |
| Mean age (years) | 48.2 (20–92) | 48.1 (26–69) | 41.6 (20–73) | 61.2 (23–92) | 48.3 (22–90) |
| Mean tumour size (mm) | 24 (4–100) | 30 (12–58) | 31 (13–72) | 27 (15–40) | 19 (4–100) |
| Multicentricity | 17 (23.8%) | 8 (57%) | 2 (20%) | 1 (20%) | 6 (19.35%) |
| Nodal metastasis | — | — | — | 1N+ (20%) | 3N+ (9.7%) |
| Juxtathyroid invasion | — | — | — | 2 (40%) | 6 (19.35%) |
| Viscéral metastasis | — | — | — | 0 | 0 |
| Tumour grade | |||||
| 1 | — | — | — | 2 (40%) | 29 (93.54%) |
| 2 | — | — | — | 2 (40%) | 1 (3.23%) |
| 3 | — | — | — | 1 (20%) | 1 (3.23%) |
| AGES score <4 | — | — | — | 2 (40%) | 26 (83.87%) |
| AGES score ⩾4 | — | — | — | 3 (60%) | 5 (16.13%) |
RNA extraction and cDNA preparation
Total RNAs were extracted from tumour specimens using Qiagen RNeasy® Mini Kit according to the manufacturer's instructions with minor modifications: for exclusion of contaminating genomic DNA, the spin–column membranes were treated with DNase (Qiagen, Courtaboeuf Cedex, France) for 15 min on the bench top before elution. DNase-treated total RNA (3 μg) was transcribed into cDNA using Superscript™ II RnaseH- and random hexamers (Invitrogen).
Quantitative real-time RT–PCR (QRT–PCR)
We assessed mRNA levels of p14ARF, p16INK4A, and E2F1 relative to GAPDH in 102 thyroid tissue by using quantitative real time PCR (QRT–PCR) in the ABIPRISM 7000 Sequence Detection System and the human p14ARF, p16INK4a, E2F1 and GAPDH Taqman Pre-Developed Taqman® Assay Reagents (Applied Biosystems, Foster city, CA, USA). The amplification of p14ARF, p16INK4A, E2F1 and GAPDH cDNA was carried out in 25 μl reaction volume consisting in 1 × Taqman Universal PCR Master-Mix and 1.25 μl of Pre-Developed Taqman® Assay Reagents (900 nm primers and 200 nM probe) (Applied Biosystems). The reaction was performed as follows: 50°C for 2 min, 95°C for 10 min followed by 40 cycles at 95°C × 15 s, 60°C × 1 min. Each sample was tested in duplicate and a negative control was included in every plate. The computed tomography value was defined as the cycle number (Ct) at which the fluorescence crossed the threshold. The results were expressed in ΔCt (CtGAPDH–Ctgene) or in 2−ΔCt*1000 for the amount of the target normalised and relative to the endogenous reference.
Immunohistochemistry
Immunostaining was performed on 15 papillary carcinomas, five follicular carcinomas, 10 follicular adenomas and nontumoral thyroid tissue samples adjacent to thyroid tumours for each patient. The surgical specimen were fixed in formalin, paraffin embedded and cut in 5 μm tissue sections placed on charged slides. Slides were deparaffinised, rehydrated and microwaved in 10 mM citrate buffer pH 6.0 for antigen retrieval. Immunohistochemistry was performed using mouse monoclonal antibodies directed against p14 ARF (Clone H-132) and p16 INK4A (Clone F12) (Santa Cruz Biotechnology, CA, USA) at 1 : 200 dilution. After blocking for endogenous peroxidase with 1% hydrogene peroxide, the primary antibodies were incubated at 4°C overnight. Immunostaining was performed using the Immunotech automated immunohistochemistry system (Microm Microtech, France). The automated procedure is based on an indirect biotin–avidin system with a universal biotinylated immunoglobulin secondary antibody, diaminobenzidin substrate and haemetoxylin counterstain. Negative controls were obtained by incubation with an immunoglobulin class-matched no immune antibody (mouse monoclonal immunoglobulin, Dakocytomation, Denmark). Positive controls were obtained by immunostaining on reactive lymph nodes, as previously described (Paik et al, 2005).
Scoring of antibody staining
Scoring of antibody staining was performed by pathologist G Fromont who was blind to gene expression status obtained by QRT–PCR. A specimen was considered positive for p14ARF or p16INK4A if there was nuclear staining above any cytoplasmic background. Cytoplasmic staining itself was noted but not included in the scoring (Geradts et al, 2001). The immunohistochemical results were evaluated using a semiquantitative analysis: no staining reaction=0, <10% positive-stained cells=+, 10–80% positive cells or focal staining=++, >80% positive cells with diffuse staining=+++.
Statistical analysis
The data distribution of normalised expression levels of tissue samples is non-Gaussian. So, nonparametric tests were applied for data analysis. The Wilcoxon test was used for comparing tumour tissue to its own nontumoral paired tissue or to mean of the 42 paired tissue expression levels when no paired tissue was available. Upregulation or downregulation was defined by the ratio value between expression level in tumoral tissue and in nontumoral paired tissue (T/S= 2−ΔCt tumor/ 2−ΔCtnormal tissue). A normal interval was defined for this ratio by the 95% confidence interval of mean for normal tissues and was 0.71–1.32 for p14ARF, 0.7–1.40 for p16INK4A, 0.85–1.18 for E2F1. For correlations between expression levels and clinicopathological data, the Spearman test was used. P-values <0.05 were considered statistically significant.
RESULTS
p14ARF, p16INK4A expression in thyroid malignancies
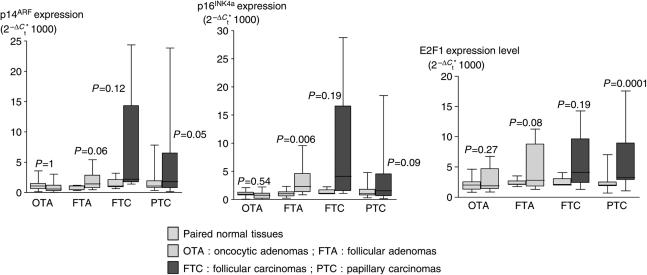
In this work we determined the expression of p14ARF and p16INK4A in 60 thyroid tumours and compared them to their nontumoral paired thyroid tissue control. In all, 18 thyroid tumours without nontumoral paired thyroid tissue control were compared to mean expression of the 42 nontumoral tissues available. We observed an overexpression of p16INK4A and p14ARF transcripts in follicular adenomas, follicular carcinomas and papillary carcinomas and a downregulation in oncocytic adenomas (Figure 1 and Table 2). This overexpression in follicular adenomas was statistically significant for p16INK4A (P=0.006) and almost reached statistical significance for p14ARF (P=0.06). In follicular carcinomas and oncocytic adenomas, we observed, respectively, an upregulation and a downregulation of p14ARF and p16INK4A expression. Also suggestive, these data were not statistically significant probably because of the too low number of tumour samples analysed (Figure 1 and Table 2). In papillary carcinomas, the expression of p14ARF and p16INK4A was heterogeneous with equal proportions of tumours overexpressing or downexpressing p14ARFor p16INK4A. The observed deregulation of these two transcripts was close to statistical significance (P=0.05 and P=0.09) for p14ARF and p16INK4A, respectively (Figure 1 and Table 2).
Figure 1.
Box plot diagrams showing the expression levels of p14ARF, p16INK4A and E2F1. After normalization of sample to their own GAPDH level, data are expressed in 2−2−ΔCt*1000. OA: oncocytic adenomas; FA: follicular adenomas; FC: follicular carcinomas; PC: papillary carcinomas.
Table 2. Expression levels of p14ARF, p16INK4A and E2F1 in nontumoral and in tumoral tissues according to histological type.
| Nontumoral tissue (n=42) | Oncocytic adenoma (n=14) | Follicular adenoma (n=10) | Follicular carcinom (n=5) | Papillary carcinoma (n=31) | ||
|---|---|---|---|---|---|---|
| mRNA expression levels | p14ARF | 1.14 (0.13–9.1) | 0.58 (0.025–3.02) (P=1) | 1.42 (0.45–5.41) (P=0.06) | 2.20 (1.39–24.35) (P=0.12) | 1.80 (0.14–23.85) (P=0.05) |
| p16INK4A | 1.11 (0.05–9.04) | 0.73 (0.009–2.21) (P=0.54) | 2.30 (0.83–9.55) (P=0.006) | 4.10 (1.07–28.75) (P=0.19) | 1.61 (0.12–52.92) (P=0.09) | |
| E2F1 | 2.15 (0.68–7.04) | 1.89 (0.86–6.75) (P=0.27) | 2.81 (1.30–11.28) (P=0.08) | 3.42 (1.03–6.39) (P=0.19) | 3.24 (1.06–17.58) (P=0.0001) |
The results (medians and minims–maxims) are expressed in 2−ΔCt*1000 where ΔCt=CtGAPDH–Ctgene. The Wilcoxon test was used to compare the expression levels between nontumoral and tumoral tissues. P<0.05 is considered statistically significant. Statistically relevant P-values are in bold.
We studied the protein expression level of p14ARF and p16INK4a by immunohistochemistry on papillary carcinomas (n=15), follicular adenomas (n=10) and follicular carcinomas (n=5). Results are summarized in Table 3.
Table 3. Immunohistochemical analysis for p14ARF and p16INK4A in normal tissue (N) and thyroid tumours (T) and comparison with their mRNA levels.
| mRNA levels |
IHC
|
MRNA levels |
IHC
|
|||
|---|---|---|---|---|---|---|
| p14ARF T/N | p14ARF N | p14ARF T | p16INK4 T/N | p16INK4 N | p16INK4 T | |
| Follicular adenomas | ||||||
| Case 1 | 2.5 | + | +++ | 2.3 | ++ | +++ |
| Case 2 | 2.5 | ++ | +++ | 1.4 | +++ | +++ |
| Case 3 | 7.5 | + | ++ | 9.1 | + | ++ |
| Case 4 | 15.5 | 0 | ++ | 11.5 | +++ | +++ |
| Case 5 | 0.8 | + | + | 1.2 | +++ | +++ |
| Case 6 | 1.3 | ++ | ++ | 4.2 | ++ | ++ |
| Case 7 | 0.5 | ++ | ++ | 1.3 | ++ | ++ |
| Case 8 | 1.2 | ++ | +++ | 2.1 | ++ | +++ |
| Case 9 | 0.7 | ++ | ++ | 0.7 | +++ | +++ |
| Case 10 | 3.3 | + | ++ | 7.4 | + | ++ |
| Follicular carcinomas | ||||||
| Case 11 | 1 | ++ | ++ | 0.85 | ++ | ++ |
| Case 12 | 4.2 | +++ | +++ | 3.8 | +++ | +++ |
| Case 13 | 3.9 | ++ | +++ | 4 | ++ | +++ |
| Case 14 | 1.9 | +++ | +++ | 1.1 | +++ | +++ |
| Case 15 | 22.6 | +++ | +++ | 28.3 | +++ | +++ |
| Papillary carcinomas | ||||||
| Case 16 | 24.3 | ++ | +++ | 17.2 | NA | NA |
| Case 17 | 2.6 | + | +++ | 1.2 | ++ | ++ |
| Case 18 | 6.3 | + | 0 (+C) | 2.7 | ++ | 0 (+C) |
| Case 19 | 3.1 | + | + (+C) | 2.1 | + | + (+C) |
| Case 20 | 0.5 | ++ | ++ | 0.7 | ++ | ++ |
| Case 21 | 0.1 | +++ | ++ | 0.1 | ++ | 0 |
| Case 22 | 0.6 | ++ | + | 0.2 | NA | NA |
| Case 23 | 2.2 | +++ | + (+C) | 1.6 | ++ | + (+C) |
| Case 24 | 0.3 | ++ | +++ | 0.3 | ++ | +++ |
| Case 25 | 1.2 | ++ | ++ | 1.7 | ++ | ++ (+C) |
| Case 26 | 0.3 | ++ | +++ | 0.5 | ++ | +++ |
| Case 27 | 9.4 | ++ | +++ | 25.9 | +++ | +++ |
| Case 28 | 5.5 | ++ | ++ (+C) | 4.8 | ++ | ++ (+C) |
| Case 29 | 19.3 | ++ | ++ (+C) | 21.7 | ++ | ++ (+C) |
| Case 30 | 11.3 | 0 | +++ | 7.4 | ++ | +++ |
The immunohistochemical results were evaluated using a semiquantitative analysis: no staining reaction=0, <10% positive-stained cells=+, 10–80% positive cells or focal staining=++, >80% positive cells with diffuse staining=+++. Increased expression appeared in red, decreased expression in green and cytoplasmic localisation in blue. Transcriptional upregulation or downregulation was defined by the ratio value between expression level in tumoral tissue and in normal paired tissue (T/S= 2−ΔCttumour/ 2−ΔCtnormal tissue). A normal interval was defined for this ratio by the 95% confidence interval of mean for normal tissues and was 0.71–1.32 for p14ARF, 0.7–1.40 for p16INK4A (NA=not analysed).
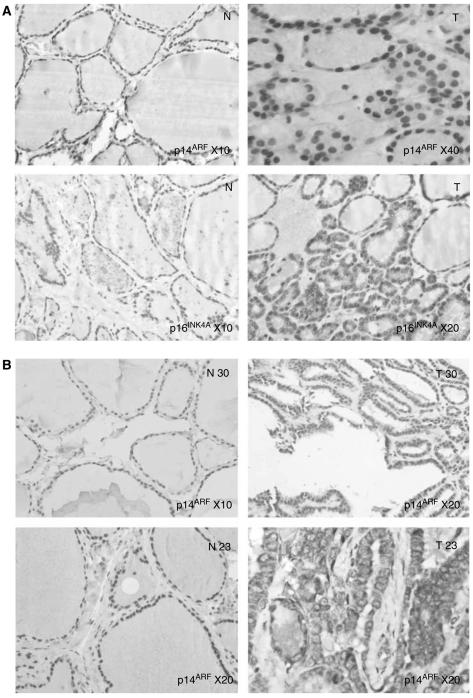
In follicular adenomas, p14ARF protein was found to be overexpressed in six cases when compared to nontumoral tissue and p16INK4A in four cases according to QRT–PCR data, (Figure 2A and Table 3). The other cases show identical immunostaining compared to nontumoral tissue. Overexpression of p14ARF and p16INK4A was identified in only one follicular carcinoma, case 13 (Table 3). As in follicular adenomas, the other cases shows identical immunostaining compared to nontumoral tissue. No underexpression of both p14ARF and p16INK4A was observed by immunohistochemistry in follicular tumours.
Figure 2.
Immunohistochemical detection for p14ARF and p16INK4A. (A) Follicular adenoma, case 1: Increased p14ARF and p16INK4A expression in tumour (T) when compared to nontumoral tissue (N). (B) Papillary carcinoma, case 30: increased nuclear p14ARF expression in tumor (T30) when compared to nontumoral tissue (N30). Case 23: cytoplasmic delocalisation of p14ARF in tumour (T23), when compared to nontumoral (N23). N: nontumoral adjacent tissue, T: Tumour.
Among the nine papillary carcinomas that overexpressed p14ARF by QRT–PCR, four showed increased nuclear expression of p14ARF by immunohistochemistry, and interestingly five displayed a cytoplasmic delocalization of the protein (Figure 2B and Table 3). When p16INK4A expression increased by QRT–PCR, immunohistochemical nuclear staining was increased in one case, unchanged in one case, and was localised to the cytoplasm in six cases. Decreased expression of both p14ARF and p16INK4A by QRT–PCR in papillary carcinoma was associated in majority either with decreased or identical immunostaining when compared to normal tissue, or with increased staining in two cases.
In some cases, nodular lymphoid infiltrate was present, mainly in nontumoral tissues, and expressed both p14ARF and p16INK4A.
Finally, no significant correlation has been found between p14ARF and p16INK4A mRNA levels and clinicopathological prognostic markers, such as stage, grade, histological subtype, age, node metastasis or AGES score.
p14ARF regulation by E2F1 is lost in papillary carcinomas
We examined the relationships between mRNA levels p14ARF, p16INK4A and E2F1 expression. For this we determined first the mRNA expression levels of E2F1 in the same tumours. Consistent with others studies, E2F1 levels were upregulated in all tumour types (Onda et al, 2004). This upregulation was statistically significant in PTC (P=0.0001) (Table 2 and Figure 1). A strong correlation occurred between p14ARF and p16INK4A in normal tissue (r=0.65, P<0.0001) as well as in tumoral tissues (r=0.65–0.89 depending on tumour type, P<0.05). E2F1 is known to positively regulate p14ARF expression (Bates et al, 1998). Indeed, such a relationship was observed between p14ARF and E2F1 expression in normal (r=0.41, P=0.01) and tumour tissues (r=0.64 to 1, P<0.05) except for papillary carcinomas (r=0.04, P=0.86) (Table 4).
Table 4. mRNA levels correlations between p14ARF, p16INK4A and E2F1 genes in nontumoral and in tumour tissues according to histological type.
| Normal tissue | Oncocytic adenomas | Follicular adenomas | Follicular carcinomas | Papillary carcinomas | ||
|---|---|---|---|---|---|---|
| mRNA level correlations | p14ARF and p16INK4A | r=0.65 | r=0.78 | r=0.65 | r=0.80 | r=0.89 |
| P<0.0001 | P=0.001 | P=0.04 | P=0.01 | P<0.0001 | ||
| p14ARF and E2F1 | r=0.41 | r=0.63 | r=0.68 | R=1 | r=0.04 | |
| P=0.01 | P=0.015 | P=0.029 | P<0.0001 | P=0.86 | ||
| p16INK4A and E2F1 | r=0.21 | r=0.7 | r=0.48 | r=0.80 | r=−0.09 | |
| P=0.20 | P=0.005 | P=0.16 | P=0.1 | P=0.66 |
The Spearman rank test was used. The correlation coefficient (r) is given with P. P<0.05 is considered statistically significant. Statistically relevant P-values are in bold.
DISCUSSION
The purpose of this study was to analyse p14ARF and p16INK4A mRNA and protein levels in various thyroid tumours including oncocytic adenomas, follicular adenomas, follicular carcinomas and papillary carcinomas. A high prevalence of upregulation for both p14ARF and p16INK4A transcripts was found in follicular adenomas and carcinomas. We observed an heterogenous profiling papillary carcinomas (45% overexpress p14ARF and p16INK4A transcripts and 45% downexpress these transcripts). In the majority of oncocytic adenomas, we observed a downregulation of p14ARF and p16INK4A.
We observed a concordance between mRNA and protein levels in most of tumours tested: 80% (24 out of 30) cases for p14ARF and 78.5% (22 out of 28) cases for p16INK4A when cytoplasmic staining observed in papillary carcinomas is taken into account. In 20% of tumors, protein expression did not reflect mRNA status for both p14ARF and p16INK4A. This apparent uncoupling have been previously reported on haematopoietic human cell lines (Della Valle et al, 1997) and on small-cell lung tumours (Gazzeri et al, 1998).
In the majority of the previously documented cases, the discrepancies between mRNA and protein expression corresponds to overexpression of mRNA and normoexpression of the protein. As some patients (particularly cases 4, 12, 15 and 27) showed a high expression of p14ARF and/or p16INK4A in nontumoral tissue, it was difficult to evidence any increase in tumoral tissue. Moreover, the heterogeneity of gene expression within the normal or tumoral tissue must also be taken into account. The presence of a lymphoid infiltrate, which expressed both p14ARF and/or p16INK4A, mainly in nontumoral tissue, can also explain some discordant results because positive lymphocytes were not included in IHC scoring. At the end, we should also take into account that accumulation or depletion of transcripts could result from an increased or decreased stability. Regarding this point, impaired mRNA turnover and stability are shown to play a critical role in the activation of specific genes during the cellular response to mitogens, stressful stimuli and differentiation agents (Bashirullah et al, 2001; Fan et al, 2002). Interestingly, we observed in six cases of papillary carcinoma a cytoplasmic localisation of both p14ARF and p16INK4A. This localisation is unusual for these proteins and suggest that in these cases p14ARF and p16INK4A could not be efficients.
Our results are consistent with others studies. Ferenc et al (2004) reported such an upregulation by use of a tissue array technology. Intensity of p16INK4A staining tended to increase from follicular adenoma to follicular carcinoma compared to normal tissue. Moreover, others found no mutation and no loss of heterozygosity for the MTS1 locus (that encodes both p16INK4A and p14AR) in differentiated carcinomas (Calabro et al, 1996; Schulte et al, 1998; Ward et al, 1998; Kitamura et al, 2000a, 2000b). In contrast, Boltze et al (2003) found hypermethylation of p16INK4a in 33% of follicular adenomas, in 44% of papillary carcinomas, in 50% of follicular carcinomas that correlated with loss of p16INK4a.
Taken together, these data, including our own results, suggest that the inactivation of p16INK4A/p14ARF locus in these carcinomas is rare and that in contrast, the upregulation of these genes could be associated with thyroid cancer progression.
Both p16INK4A and p14ARF proteins are tumour suppressors and it is their loss not their gain that should contribute to tumorigenesis. However, the increased expression of CDKN2a gene products alpha (p16INK4A) and beta (p14ARF) has been described to be associated with progression and unfavourable prognosis in different tumour types. For instance, the increased expression of p16INK4A has been described to be associated with progression and unfavourable prognosis in ovarian cancer (Dong et al, 1997), in high-grade prostatic intraepithelial neoplasia (Henshall et al, 2001) and in primary breast cancer (Hui et al, 2000). Also, the upregulation of p14ARF is mainly seen in haematological malignancies (Lee et al, 2003) and in aggressive B-cell lymphomas, and it predicts a shortened survival time (Sanchez-Aguilera et al, 2002).
The overexpression of p14ARF/p16INK4A tumour suppressor genes could be explained by several known data. Regarding the expression of p16INK4A, the most well-defined mechanism of p16INK4A overexpression is the loss of transcriptional repression in the presence of inactivating mutations in the RB gene (Ruas and Peters, 1998). It has been suggested that p16INK4A-mediated growth inhibition may occur only when cyclin E/Cdk2 complexes are inactivated concurrently by the cell cycle inhibitor p27KIP1(Lukas et al, 1997; Jiang et al, 1998). In this regard, the MDA-MB-157 breast cancer cell line retains functional pRB but can proliferate in the presence of p16INK4A overexpression (Sweeney et al, 1998). Furthermore, others have observed RAS and Rb mutations in 40–50% of follicular adenomas and carcinomas (Suarez et al, 1990; Capella et al, 1996; Zou et al, 1998) and a downregulation of p27KIP1, which was correlated with tumoral progression (Erickson et al, 2000; Troncone et al, 2000). Although we did not examine RB or Ras mutations, neither p27Kip1 status in our tumour cohort, these data could explain the p16INK4A upregulation we observed.
Regarding the expression of p14ARF, its expression is induced by oncogenes like myc, ras or viral genes (Palmero et al, 1998). E2F1 is a well-known transcriptional regulator of p14ARF as p14ARF promoter has an E2F1-binding site (Bates et al, 1998). Every cancer-related defect in these pathways should result in p14ARF upregulation. We found an upregulation of E2F1 in all tumour types which is consistent with others studies (Saiz et al, 2002; Onda et al, 2004), and we observed a link between E2F1 and p14ARF expression in nontumoral thyroid tissue as well as in follicular adenomas and carcinomas. These results suggest the involvement of E2F1 in the p14ARF upregulation in these tumours. The link between E2F1 and p14ARF expression found in nontumoral thyroid tissue was no more present in papillary carcinomas. Strikingly, E2F1 mRNA level was upregulated in all papillary carcinomas, while 45% of them showed a downregulation of p14ARF suggesting that transcriptional regulation of p14ARF in these tumours is independent of E2F1.
Because of their cytoplasmic localisation, these overexpressions of the two proteins are probably nonfunctional. When they are, at first glance, nuclear, their overexpression seems, however, not sufficient to inhibit cell proliferation during the process of thyroid tumorigenesis.
In contrast, in majority of oncocytic adenomas we observed a downregulation of p14ARF. As these tumours showed also more frequently decreased expression of E2F1 than other histological types and as a correlation between E2F1 and p14ARF was noted, this suggests that downregulation of p14ARF could be linked to the downregulation of E2F1 in this tumour type.
To conclude, we observed that p14ARF and p16INK4A expression levels are different according to histological type of thyroid tumours. Upregulation seems to be implicated in thyroid follicular carcinogenesis as an early event and could be considered as tumourprogression marker. Papillary carcinomas displayed a more complex pattern with a great heterogeneity for p14ARF, p16INK4A expression and an E2F1-independent transcriptional regulation. However, these deregulations are not correlated to the clinical outcome and they could not be used as potential prognostic markers alone.
Acknowledgments
This work was supported by a grant from Canceropôle Grand ouest.
References
- Bashirullah A, Cooperstock RL, Lipshitz HD (2001) Spatial and temporal control of RNA stability. Proc Natl Acad Sci USA 98: 7025–7028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates S, Phillips AC, Clark PA, Stott F, Peters G, Ludwig RL, Vousden KH (1998) p14ARF links the tumour suppressors RB and p53. Nature 395: 124–125 [DOI] [PubMed] [Google Scholar]
- Boltze C, Zack S, Quednow C, Bettge S, Roessner A, Schneider-Stock R (2003) Hypermethylation of the CDKN2/p16INK4A promotor in thyroid carcinogenesis. Pathol Res Pract 199: 399–404 [DOI] [PubMed] [Google Scholar]
- Calabro V, Strazzullo M, La Mantia G, Fedele M, Paulin C, Fusco A, Lania L (1996) Status and expression of the p16INK4 gene in human thyroid tumors and thyroid-tumor cell lines. Int J Cancer 67: 29–34 [DOI] [PubMed] [Google Scholar]
- Capella G, Matias-Guiu X, Ampudia X, de Leiva A, Perucho M, Prat J (1996) Ras oncogene mutations in thyroid tumors: polymerase chain reaction-restriction-fragment-length polymorphism analysis form paraffin-embedded tissues. Diagn Mol Pathol 5: 45–52 [DOI] [PubMed] [Google Scholar]
- Carnero A, Hudson JD, Price CM, Beach DH (2000) p16INK4A and p19ARF act in overlapping pathways in cellular immortalization. Nat Cell Biol 2: 148–155 [DOI] [PubMed] [Google Scholar]
- Della Valle V, Duro D, Bernard O, Larsen CJ (1997) The human protein p19ARF is not detected in hemopoietic human cell lines that abundantly express the alternative beta transcript of the p16INK4a/MTS1 gene. Oncogene 15: 2475–2481 [DOI] [PubMed] [Google Scholar]
- Dong Y, Walsh MD, McGuckin MA, Gabrielli BG, Cummings MC, Wright RG, Hurst T, Khoo SK, Parsons PG (1997) Increased expression of cyclin-dependent kinase inhibitor 2 (CDKN2A) gene product P16INK4A in ovarian cancer is associated with progression and unfavourable prognosis. Int J Cancer 74: 57–63 [DOI] [PubMed] [Google Scholar]
- Erickson LA, Yousef OM, Jin L, Lohse CM, Pankratz VS, Lloyd RV (2000) p27kip1 expression distinguishes papillary hyperplasia in Graves' disease from papillary thyroid carcinoma. Mod Pathol 13: 1014–1019 [DOI] [PubMed] [Google Scholar]
- Eymin B, Leduc C, Coll JL, Brambilla E, Gazzeri S (2003) p14ARF induces G2 arrest and apoptosis independently of p53 leading to regression of tumours established in nude mice. Oncogene 22: 1822–1835 [DOI] [PubMed] [Google Scholar]
- Fagin JA (2000) Molecular genetics of tumors of thyroid follicular cells. In The Thyroid Braverman LE, Utiger RD (eds) 8th edn, New York: Lippincott Williams &Wilkins, pp 886–898 [Google Scholar]
- Fagin JA, Matsuo K, Karmakar A, Chen DL, Tang SH, Koeffler HP (1993) High prevalence of mutations of the p53 gene in poorly differentiated human thyroid carcinomas. J Clin Invest 91: 179–184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan J, Yang X, Wang W, Wood III WH, Becker KG, Gorospe M (2002) Global analysis of stress-regulated mRNA turnover by using cDNA arrays. Proc Natl Acad Sci USA 99: 10611–10616, Epub July 29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferenc T, Lewinski A, Lange D, Niewiadomska H, Sygut J, Sporny S, Jarzab B, Salacinska-Los E, Kulig A, Wloch J (2004) Analysis of P161NK4A protein expression in follicular thyroid tumors. Pol J Pathol 55: 143–148 [PubMed] [Google Scholar]
- Gazzeri S, Della Valle V, Chaussade L, Brambilla C, Larsen CJ, Brambilla E (1998) The human p19ARF protein encoded by the beta transcript of the p16INK4a gene is frequently lost in small cell lung cancer. Cancer Res 58: 3926–3931 [PubMed] [Google Scholar]
- Geradts J, Wilentz RE, Roberts H (2001) Immunohistochemical detection of the alternate INK4a-encoded tumor suppressor protein p14ARF in archival human cancers and cell lines using commercial antibodies: correlation with p16INK4a expression. Mod Pathol 14: 162–168 [DOI] [PubMed] [Google Scholar]
- Gimm O (2001) Thyroid cancer. Cancer Lett 163: 143–156, Review [DOI] [PubMed] [Google Scholar]
- Giuffrida D, Gharib H (2000) Anaplastic thyroid carcinoma: current diagnosis and treatment. Ann Oncol 11: 1083–1089, Review [DOI] [PubMed] [Google Scholar]
- Greco A, Miranda C, Pagliardini S, Fusetti L, Bongarzone I, Pierotti MA (1997) Chromosome 1 rearrangements involving the genes TPR and NTRK1 produce structurally different thyroid-specific TRK oncogenes. Genes Chromosomes Cancer 19: 112–123 [PubMed] [Google Scholar]
- Hay ID, Grant CS, Taylor WF, McConahey WM (1987) Ipsilateral lobectomy versus bilateral lobar resection in papillary thyroid carcinoma: a retrospective analysis of surgical outcome using a novel prognostic scoring system. Surgery 102: 1088–1095 [PubMed] [Google Scholar]
- Henshall SM, Quinn DI, Lee CS, Head DR, Golovsky D, Brenner PC, Delprado W, Stricker PD, Grygiel JJ, Sutherland RL (2001) Overexpression of the cell cycle inhibitor p16INK4A in high-grade prostatic intraepithelial neoplasia predicts early relapse in prostate cancer patients. Clin Cancer Res 7: 544–550 [PubMed] [Google Scholar]
- Hui R, Macmillan RD, Kenny FS, Musgrove EA, Blamey RW, Nicholson RI, Robertson JF, Sutherland RL (2000) INK4a gene expression and methylation in primary breast cancer: overexpression of p16INK4a messenger RNA is a marker of poor prognosis. Clin Cancer Res 6: 2777–2787 [PubMed] [Google Scholar]
- Jiang H, Chou HS, Zhu L (1998) Requirement of cyclin E-Cdk2 inhibition in p16 (INK4a)-mediated growth suppression. Mol Cell Biol 18: 5284–5290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura Y, Shimizu K, Tanaka S, Ito K, Emi M (2000a) Allelotyping of anaplastic thyroid carcinoma: frequent allelic losses on 1q, 9p, 11, 17, 19p, and 22q. Genes Chromosomes Cancer 27: 244–251 [PubMed] [Google Scholar]
- Kitamura Y, Shimizu K, Tanaka S, Ito K, Emi M (2000b) Association of allelic loss on 1q, 4p, 7q, 9p, 9q, and 16q with postoperative death in papillary thyroid carcinoma. Clin Cancer Res 6: 1819–1825 [PubMed] [Google Scholar]
- Kroll TG, Sarraf P, Pecciarini L, Chen CJ, Mueller E, Spiegelman BM, Fletcher JA (2000) PAX8-PPARgamma1 fusion oncogene in human thyroid carcinoma. Science 289: 1357–1360 [DOI] [PubMed] [Google Scholar]
- Kubbutat MH, Jones SN, Vousden KH (1997) Regulation of p53 stability by Mdm2. Nature 387: 299–303 [DOI] [PubMed] [Google Scholar]
- Lee YK, Park JY, Kang HJ, Cho HC (2003) Overexpression of p16INK4A and p14ARF in haematological malignancies. Clin Lab Haematol 25: 233–237 [DOI] [PubMed] [Google Scholar]
- Lukas J, Herzinger T, Hansen K, Moroni MC, Resnitzky D, Helin K, Reed SI, Bartek J (1997) Cyclin E-induced S phase without activation of the pRb/E2F pathway. Genes Dev 11: 1479–1492 [DOI] [PubMed] [Google Scholar]
- Onda M, Nagai H, Yoshida A, Miyamoto S, Asaka S, Akaishi J, Takatsu K, Nagahama M, Ito K, Shimizu K, Emi M (2004) Up-regulation of transcriptional factor E2F1 in papillary and anaplastic thyroid cancers. J Hum Genet 49: 312–318 [DOI] [PubMed] [Google Scholar]
- Paik JH, Jeon YK, Park SS, Kim YA, Kim JE, Huh J, Lee SS, Kim WH, Kim CW (2005) Expression and prognostic implications of cell cycle regulatory molecules, p16, p21, p27, p14 and p53 in germinal centre and non-germinal centre B-like diffuse large B-cell lymphomas. Histopathology 47: 281–291 [DOI] [PubMed] [Google Scholar]
- Palmero I, Pantoja C, Serrano M (1998) p19ARF links the tumour suppressor p53 to Ras. Nature 395: 125–126 [DOI] [PubMed] [Google Scholar]
- Pierotti MA, Bongarzone I, Borello MG, Greco A, Pilotti S, Sozzi G (1996) Cytogenetics and molecular genetics of carcinomas arising from thyroid epithelial follicular cells. Genes Chromosomes Cancer 16: 1–14, Review [DOI] [PubMed] [Google Scholar]
- Ruas M, Peters G (1998) The p16INK4a/CDKN2A tumor suppressor and its relatives. Biochim Biophys Acta 1378: 115–177, Review [DOI] [PubMed] [Google Scholar]
- Saiz AD, Olvera M, Rezk S, Florentine BA, McCourty A, Brynes RK (2002) Immunohistochemical expression of cyclin D1, E2F-1, and Ki-67 in benign and malignant thyroid lesions. J Pathol 198: 157–162 [DOI] [PubMed] [Google Scholar]
- Sanchez-Aguilera A, Sanchez-Beato M, Garcia JF, Prieto I, Pollan M, Piris MA (2002) p14(ARF) nuclear overexpression in aggressive B-cell lymphomas is a sensor of malfunction of the common tumor suppressor pathways. Blood 99: 1411–1418 [DOI] [PubMed] [Google Scholar]
- Santoro M, Chiappetta G, Cerrato A, Salvatore D, Zhang L, Manzo G, Picone A, Portella G, Santelli G, Vecchio G, Fusco A (1996) Development of thyroid papillary carcinomas secondary to tissue-specific expression of the RET/PTC1 oncogene in transgenic mice. Oncogene 12: 1821–1826 [PubMed] [Google Scholar]
- Schulte KM, Staudt S, Niederacher D, Finken-Eigen M, Kohrer K, Goretzki PE, Roher HD (1998) Rare loss of heterozygosity of the MTS1 and MTS2 tumor suppressor genes in differentiated human thyroid cancer. Horm Metab Res 30: 549–554 [DOI] [PubMed] [Google Scholar]
- Serrano M, Hannon GJ, Beach D (1993) A new regulatory motif in cell-cycle control causing specific inhibition of cyclin D/CDK4. Nature 366: 704–707 [DOI] [PubMed] [Google Scholar]
- Sherr CJ (1998) Tumor surveillance via the ARF-p53 pathway. Genes Dev 12: 2984–2991, Review [DOI] [PubMed] [Google Scholar]
- Sherr CJ (2001) The INK4a/ARF network in tumour suppression. Nat Rev Mol Cell Biol 2: 731–737, Review [DOI] [PubMed] [Google Scholar]
- Suarez HG, du Villard JA, Severino M, Caillou B, Schlumberger M, Tubiana M, Parmentier C, Monier R (1990) Presence of mutations in all three ras genes in human thyroid tumors. Oncogene 5: 557–565 [PubMed] [Google Scholar]
- Sweeney KJ, Swarbrick A, Sutherland RL, Musgrove EA (1998) Lack of relationship between CDK activity and G1 cyclin expression in breast cancer cells. Oncogene 16: 2865–2878 [DOI] [PubMed] [Google Scholar]
- Troncone G, Fulciniti F, Zeppa P, Vetrani A, Caleo A, Palombini L (2000) Cyclin-dependent kinase inhibitor p27(Kip1) expression in thyroid cells obtained by fine-needle aspiration biopsy: a preliminary report. Diagn Cytopathol 23: 77–81 [DOI] [PubMed] [Google Scholar]
- Vini L, Harmer C (2002) Management of thyroid cancer. Lancet Oncol 3: 407–414, Review [DOI] [PubMed] [Google Scholar]
- Ward LS, Brenta G, Medvedovic M, Fagin JA (1998) Studies of allelic loss in thyroid tumors reveal major differences in chromosomal instability between papillary and follicular carcinomas. J Clin Endocrinol Metab 83(2): 525–530 [DOI] [PubMed] [Google Scholar]
- Weber JD, Jeffers JR, Rehg JE, Randle DH, Lozano G, Roussel MF, Sherr CJ, Zambetti GP (2000) p53-independent functions of the p19(ARF) tumor suppressor. Genes Dev 14: 2358–2365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing M (2005) BRAF mutation in thyroid cancer. Endocr Relat Cancer 12: 245–262 [DOI] [PubMed] [Google Scholar]
- Zou M, Shi Y, Farid NR, al-Sedairy ST (1998) Inverse association between cyclin D1 overexpression and retinoblastoma gene mutation in thyroid carcinomas. Endocrine 8: 61–64 [DOI] [PubMed] [Google Scholar]