Abstract
Leptin is a 167-aa protein that is secreted from adipose tissue and is important in the regulation of energy balance. It also functions in hematopoiesis and reproduction. To assess whether leptin is involved in fetal growth and development we have examined the distribution of mRNAs encoding leptin and the leptin receptor (which has at least six splice variants) in the 14.5-day postcoitus mouse fetus and in the placenta using reverse transcription–PCR and in situ hybridization. High levels of gene expression for leptin, the leptin receptor, and the long splice variant of the leptin receptor with an intracellular signaling domain were observed in the placenta, fetal cartilage/bone, and hair follicles. Receptor expression also was detected in the lung, as well as the leptomeninges and choroid plexus of the fetal brain. Western blotting and immunocytochemistry, using specific antibodies, demonstrated the presence of leptin and leptin receptor protein in these tissues. These results suggest that leptin may play a role in the growth and development of the fetus, both through placental and fetal expression of the leptin and leptin receptor genes. In the fetus, leptin may be multifunctional and have both paracrine and endocrine effects.
Keywords: splice variants, in situ hybridization, immunocytochemistry
The mouse Obese (OB) gene was isolated using classical positional cloning techniques (1). The Obese gene product, leptin, is a 167-aa protein, and adipose tissue has been recognized as the major, if not exclusive, site of OB gene expression and leptin secretion (2). Leptin is deficient in the obese ob/ob mouse where daily administration of recombinant leptin causes a reduction in body weight, body fat, and food intake, as well as an increase in metabolic rate and physical activity (3–5). These studies demonstrate that leptin is important in the regulation of body weight and fat deposition; however, that does not appear to be leptin’s only function. In common with many other cytokines, leptin has a role in hematopoiesis (6–8) where it stimulates proliferation and differentiation (6, 7). Leptin treatment also has been demonstrated to restore fertility to mice that are genetically deficient in leptin (9, 10) and to accelerate the onset of puberty in female rodents (11, 12), indicating another role for this hormone in the control of reproductive status.
The leptin receptor (OB-R) gene has been shown to have at least six splice variants OB-R(a-e) (13, 14) and muB219 (8). The OB-Rb variant encodes a receptor with a long intracellular domain that is thought to be essential for intracellular signal transduction (15). Using in situ hybridization in the mouse, OB-Rs have been localized in the brain (16, 17) and other tissues (18). In the latter study, OB-R, in particular OB-Ra, was expressed in a wide range of tissues in agreement with earlier studies using reverse transcription–PCR (RT-PCR) (14, 15). In contrast, the long form of the receptor, OB-Rb, was expressed at detectable levels, outside of the brain, only in the kidney and adrenals (18). A study using RNase protection assays, however, reported the long form of the OB-R to be present in a range of tissues at between 3–5% of the total OB-R expression, with much higher levels in the hypothalamus (19).
Although the secretion of leptin by adipose tissue has been the major focus of research, the expression of leptin, by Northern blotting at low levels, in the human placenta (20) indicates a role in placental function or fetal growth and development. To assess a possible role of leptin in placental and fetal function we have examined leptin and OB-R splice variant gene expression in the mouse placenta and fetus (14.5 days postcoitus) using both RT-PCR and in situ hybridization. Western blotting and immunocytochemistry also were used to examine the presence of leptin and OB-R protein using specific antibodies.
EXPERIMENTAL PROCEDURES
Animals.
Pregnant lean Aston strain mice from a colony maintained at the Rowett Research Institute were killed by cervical dislocation. The fetuses were killed by lethal i.p. injection of sodium pentobarbitone. Six fetuses and six placentae were frozen immediately either in liquid nitrogen for RT-PCR and Western blotting or on dry ice for in situ hybridization and immunocytochemistry. Tissue also was obtained from rat (term), sheep (term), and human (term) placentae for Western blotting.
RT-PCR.
Total RNA was extracted from the tissues using RNAzol (BRL) and cDNA generated by RT using the Superscript Pre-amplification system (GIBCO/BRL) as described (18). The cDNA primers to the leptin gene were 5′-TGCTGCAGATAGCCAATGAC-3′ (+277–296) and 5′-GAGTAGAGTGAGGCTTCCAGGA-3′ (+418–397), GenBank U18812; a 142-bp fragment was generated. The cDNA primers to the common OB-R sequence (which recognizes the splice variants OB-Ra, OB-Rb, OB-Rc, OB-Rd, OB-Re, and muB219; 473-bp product) and to the short form OB-Ra (237-bp product) were as described (18). The cDNA primers to the long form of the receptor, OB-Rb, were 5′-ACACTGTTAATTTCACACCAGAG-3′ (+2,568–2,591) and 5′-TGGATAAACCCTTGCTCTTCA-3′ (+3,015–2,994), GenBank U49106; a 447-bp fragment was generated (14). The quality of each cDNA and mock cDNA was determined by the relative level of amplification of the mouse β-actin gene (542-bp product) (18). PCR was performed on a Hybaid Touchdown thermal cycler using the same conditions as described (18). The leptin gene and OB-Rb used the same amplification conditions as OB-R (18). Agarose gel electrophoresis (2%) in the presence of ethidium bromide confirmed the presence of a single band of the expected size for each of the PCR primer pairs.
In Situ Hybridization.
Using the same specific primers used for RT-PCR, except for OB-Rb where the primers are described elsewhere (18), cDNAs were cloned for the generation of in situ hybridization probes. Probes to leptin, the common OB-R sequence, OB-R, and the long form of the OB-R, OB-Rb, were produced as described (16, 18). PCR products were purified using Wizard PCR preps (Promega) and cloned directly into pGEM-T (Promega). The sequence and orientation of the inserts were confirmed by automated sequencing. Plasmids were linearized with SacI or ApaI for transcription with T7 or SP6 RNA polymerase to generate antisense and sense riboprobes.
In situ hybridization techniques have been described in detail elsewhere (16, 21). Twenty-microgram thick cryostat sections were mounted on poly(l-lysine)-coated slides and stored at −70°C. After fixation in 4% paraformaldehyde in 0.1 M phosphate buffer, and acetylation in 0.1 M triethanolamine/0.25% acetic anhydride for 10 min, hybridization was performed using 35S-labeled cRNA probes at concentrations of 1.5–2 × 107 cpm/ml. Probes were prepared in a solution containing 50% formamide, 0.3 M NaCl, 10 mM Tris (pH 8), 1 mM EDTA, 0.05% tRNA, 10 mM DTT, 1× Denhardt’s solution, and 10% dextran sulfate, and they were hybridized to sections overnight at 58°C. After hybridization, slides were treated with RNase A, desalted, with a final high stringency wash (30 min) in 0.1× standard saline citrate at 60°C, and dehydrated. Dried slides were apposed to Hyperfilm β-max (Amersham) for film autoradiography or coated with LM-1 autoradiographic emulsion (Amersham).
Antisera.
The murine leptin antiserum is a rabbit anti-leptin antiserum that binds to the epitope corresponding to amino acids 145–156 (Genosys, Cambridge, U.K.). This antiserum was used for Western analysis. The human leptin antiserum is a rabbit anti-leptin antiserum that was raised against recombinant human leptin (a gift from SmithKline Beecham Pharmaceuticals, Great Burgh, Surrey, U.K.). This antiserum was used for immunocytochemistry. The murine OB-R antiserum is a goat anti-OB-R antiserum that binds to the epitope corresponding to amino acids 32–51 mapping at the amino terminus of the murine OB-R (Research Diagnostics, Flanders, NJ). This antiserum was used for both Western blotting and immunocytochemistry.
Western Analysis.
Placental tissue was homogenized in a buffer containing 250 mM sucrose, 0.2 mM EDTA, and 1 mM Hepes at pH 7.2 and quantified using BSA as standard. Ten micrograms of placental protein were loaded onto a gel, subjected to SDS/PAGE (3% stacking gel; 11% running gel), and then electroblotted onto nitrocellulose membranes (Hybond C-extra, Amersham) using a semidry electroblotter (Sartorius, GB-Belmont). Membranes were blocked in Tris-buffered saline (TBS)-Tween buffer, pH 7.5 (20 mM Tris/500 mM NaCl/0.05% Tween-20) containing 10% skimmed milk powder for 1 hr then exposed to anti-leptin or anti-OB-R serum at a dilution of 1/200 in TBS-Tween buffer, pH 7.5 containing 3% skimmed milk powder for 2 hr. Membranes then were washed and incubated with anti-rabbit Ig or anti-goat Ig conjugated to horseradish peroxidase diluted 1/5,000 in the same buffer for 1 hr. After a series of washes in TBS-Tween buffer, protein bands were visualized by chemiluminescence with an ECL luminescence kit (Amersham) and exposure to Hyper-film ECL (Amersham). The size of the protein bands was determined using electrophoresis color markers.
Immunocytochemistry.
Sections were prepared as described for in situ hybridization. Nonspecific binding was blocked by incubating in normal serum for 20 min. Tissue sections were incubated overnight at 4°C with primary antiserum directed against either human leptin or the murine OB-R. The leptin-antiserum was diluted 1/100, and the OB-R antiserum was diluted 1/200. Sections were washed for 15 min in PBS buffer and then incubated for 30 min with biotinylated secondary antibody and either anti-rabbit or anti-goat conjugated to biotin according to the manufacturer’s instructions (Vectastain Elite ABC kit, Vector Laboratories). Colored end-product was developed with Sigma Fast DAB peroxidase substrate kit. Sections were counterstained with either neutral red or toluidine blue. The specificity of the immunoreaction was confirmed by (i) omission of the primary antibody for both leptin and the OB-R, (ii) incubation with human IgG antiserum (Vector Laboratories) instead of the primary antibody in the case of the OB-R, or (iii) preabsorption of the leptin antiserum with synthetic leptin.
RESULTS
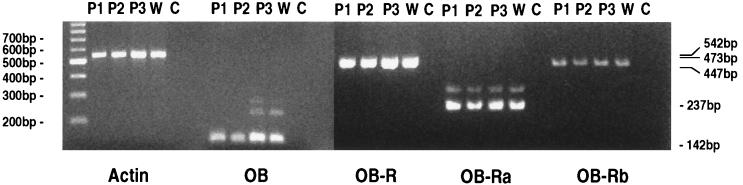
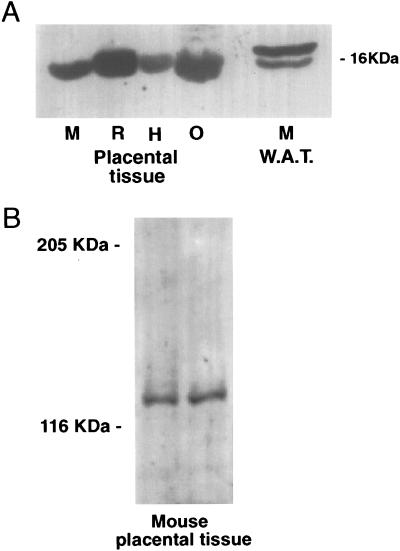
Leptin mRNA was detected in the murine placenta at an apparently similar level of expression to white adipose tissue as determined by RT-PCR (Fig. 1). The receptor splice variants, OB-R, OB-Ra and OB-Rb, all also were expressed in placentae (Fig. 1). RT-PCR is a highly sensitive technique, and it is important from a functional perspective to demonstrate both mRNA and associated protein expression. Western analysis showed that leptin protein was present in the murine placenta (approx. 16 kDa); leptin protein also was detected in rat, human, and ovine placentae (Fig. 2A). The other band detected in the white adipose tissue may represent a degradation product (Fig. 2A). In addition to leptin, the OB-R protein also was detected in murine placenta with a molecular mass of approximately 120 kDa (Fig. 2B).
Figure 1.
RT-PCR expression analysis of leptin and alternatively spliced OB-Rs in mouse placentae (P1-P3) and mouse adipose tissue (W). The expression of leptin (OB) was compared with the common extracellular domain of the OB-R, the short splice variant (OB-Ra), and the long form of the OB-R (OB-Rb). β-actin amplification was used as a control. The tissues all were extracted and reverse-transcribed at the same time. No bands were observed with the mock cDNA (data not shown) or in the absence of cDNA (C). Molecular markers (100-bp ladder) and size of RT-PCR products are shown.
Figure 2.
(A) Western blot of leptin protein in mouse (M), rat (R), human (H), and ovine (O) placentae compared with mouse white adipose tissue (WAT). A major band was observed at 16 kDa. (B) Western blot of OB-R protein in two mouse placentae. Markers are as shown. Ten micrograms of protein was loaded onto each lane, and Western blotting was performed as described in Experimental Procedures.
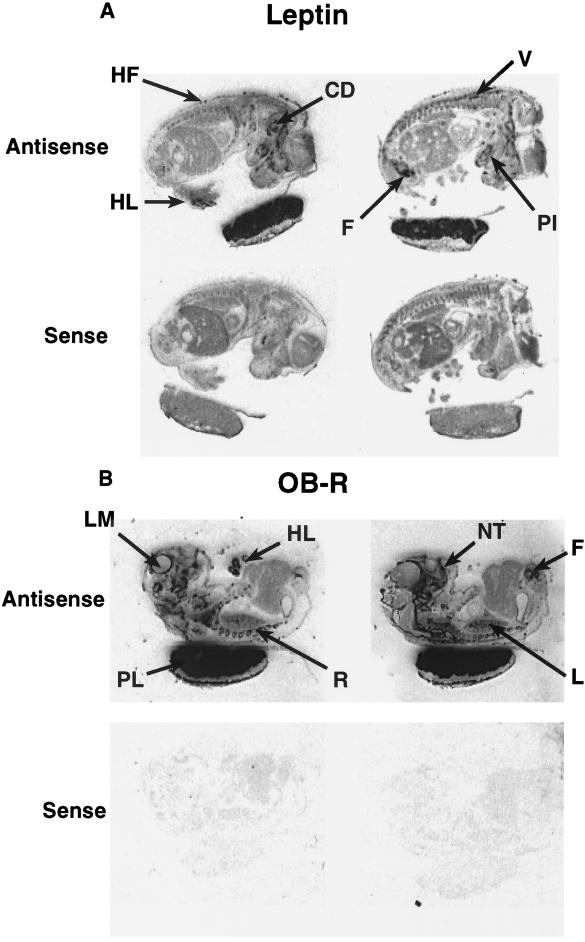
Leptin mRNA was localized by in situ hybridization to the placenta and specific sites in the mouse fetus. High levels of leptin gene expression were observed in the placenta and in fetal cartilage/bone, in particular, the vertebrae, ribs, scapula, clavicle, humerous, ulna, radius, mandible, femur, digits of the hindlimb, and the primordium of incisor teeth. Hybridization of the leptin probe also was observed over the cochlear duct, nasal turbinate, and hair follicles including the verbrisae (Figs. 3 and 4). Hybridization with the sense probe to leptin resulted in a weak, general, or undetectable signal (Figs. 3 and 4).
Figure 3.
In situ hybridization to sections of murine fetus and placenta hybridized with a [35S] antisense (Upper) and sense (Lower) riboprobes to (A) leptin and (B) common extracellular domain of the OB-R mRNA. PL, placenta; L, lung; LM, leptomeninges; V, vertebrae; R, ribs; F, femur; HL, hindlimb; PI, primordium of incisor teeth; NT, nasal turbinate; CD, cochlear duct and HF, hair follicle. (×2.7.)
Figure 4.
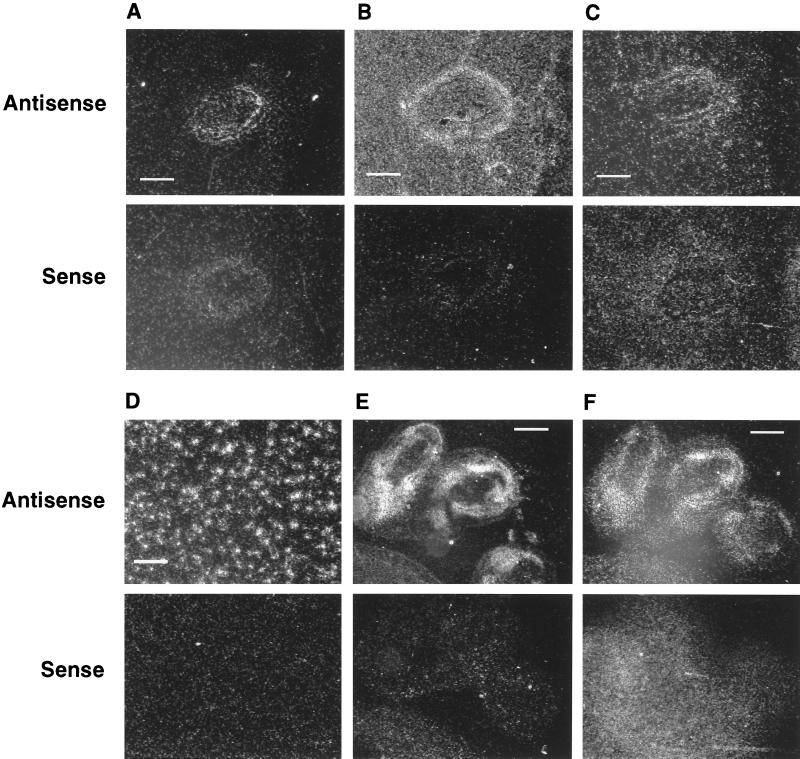
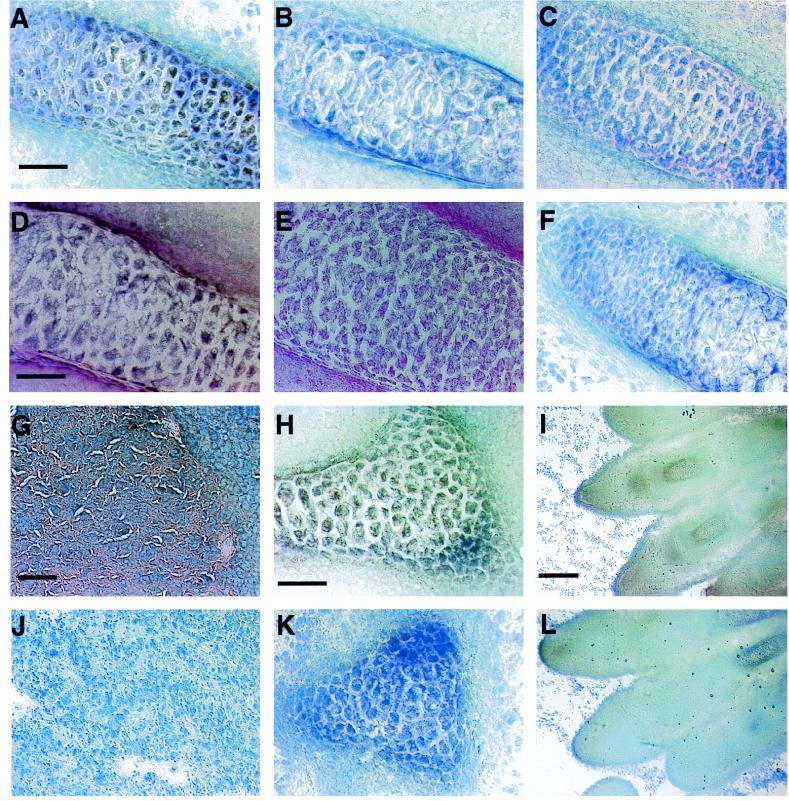
Dark-field high-power images of in situ hybridization to adjacent sections of the murine fetal fifth rib with antisense or sense riboprobes to (A) leptin (B) OB-R, and (C) OB-Rb mRNA. (Bar = 100 μm.) Dark-field high-power images of in situ hybridization to sections of (D) murine placenta hybridized with antisense or sense riboprobes to OB-Rb mRNA and murine fetal hindlimb digits hybridized with riboprobes to OB-R (E) and OB-Rb (F) mRNA. (Bar = 100 μm in D and 250 μm in E and F.)
Using the OB-R probe to the common OB-R sequence for in situ hybridization, high levels of expression were observed in the placenta, the same cartilage structures expressing leptin mRNA, as well as the lung, cochlear duct, nasal turbinate, and hair follicles (Figs. 3 and 4). OB-R gene expression also was observed in the lung as well as the leptomeninges and choroid plexus in the fetal brain, though not in the hypothalamus (Figs. 3 and 4). In situ hybridization with the probe for the long splice variant, OB-Rb, revealed a similar, but weaker, specific hybridization pattern to OB-R; however, no expression was observed in the lung (Fig. 3). At the light microscope level, dark-field images of serial sections illustrated that the leptin, OB-R, and OB-Rb probes hybridized to different cell populations in the rib (Figs. 3 and 4; fifth rib given as an example). It is noteworthy that leptin, OB-R, and OB-Rb mRNAs were expressed more densely in the placenta than in fetal tissues.
Immunostaining for leptin and the OB-R was observed in all tissues examined where leptin and OB-R mRNA previously had been detected by in situ hybridization (Fig. 5). The specificity of the leptin antiserum was confirmed by the absence of immunostaining in sections of adult tissues where leptin mRNA has been shown not to be expressed.
Figure 5.
Murine fetal tissue sections of rib (A–F), placenta (G and J), vertebrae (H and K), and hind limb digits (I and L) were incubated with leptin (A and G–I) and OB-R (D) antiserum. The immunoreaction is visualized by diaminobenzidine, positive cells giving a brown color at the site of reaction. The specificity of the immunoreaction of leptin (B and J–L) and OB-R (E) was confirmed by omission of the primary antibody. In the case of leptin, specificity was further confirmed by preabsorption of the leptin antiserum with synthetic leptin (C) and for the OB-R by incubation with human Ig G antiserum instead of the primary antibody (F). Sections were counterstained with neutral red (D and E) and toluidine blue (all other sections). (Bar = 50 μm in A, D, and H, 100 μm in (G), and 250 μm in I.)
DISCUSSION
The present study demonstrates that leptin gene expression and mature leptin protein are present in a number of tissues in the fetal mouse (Fig. 3A). This contrasts to previously published data, indicating that in adults leptin is almost exclusively synthesized and secreted by white adipose tissue (2). The expression of OB-R mRNA and protein in the same tissues, but different cell populations, indicates that leptin may function in an autocrine or paracrine manner in the fetus (Fig. 4). This expression of leptin in fetal tissues suggests that the hormone may be involved in the growth and development of the fetus.
High levels of leptin and its receptor (both mRNA and protein) were expressed in the placenta (Figs. 1, 2, 3, 4, 5). Further, the combination of RT-PCR and in situ hybridization demonstrated that both the short and long splice variants of the OB-R were present in the placenta. This suggests that leptin has an important function in this tissue. The presence of the OB-Ra mRNA, which previously has been linked to the transport of leptin (22), suggests a role in the transport of maternal leptin to the fetus or the removal of leptin from the maternal-fetal circulation. In addition, leptin apparently was differentially expressed in a number of species with different placentation. The specific site of leptin and OB-R expression in the placenta is under further investigation.
Although some areas of cartilage can be identified in the 14.5-day postcoitus fetus, it is not possible to differentiate clearly between bone and cartilage in the skeleton at this stage of development. High levels of leptin and its receptor (both mRNA and protein) are expressed in the fetal bone/cartilage (Figs. 4 and 5). This implies a role for leptin in fetal bone and/or cartilage development that may be linked to its influence on hematopoiesis in the adult (6–8), although at this stage of development hematopoiesis is not underway in fetal bone (23). However, it is noteworthy that expression of leptin and its receptor (both mRNA and protein) occurs in structures undergoing ossification at this stage of development, including the ribs, scapula, clavicle, humerus, ulna, radius, femur, and mandible.
OB-R and OB-Rb mRNA and OB-R protein were identified in the leptomeninges and choroid plexus of the fetal brain (Fig. 3). This demonstration of OB-R protein in the fetal choroid plexus and leptomeninges by immunocytochemistry extends earlier studies in the adult rodent (16, 17). We found no evidence of mRNA or immunoreactive protein in the hypothalamus at this stage of fetal development. This contrasts with the studies in the adult rodent, although low levels of OB-R gene expression in the fetal hypothalamus cannot be discounted.
The OB-R mRNA is widely expressed in adult rodent tissues (14, 15, 18, 19) in contrast to the murine fetus where, with the exception of brain tissues, only the lung expresses OB-R mRNA (Fig. 3). No OB-R mRNA or protein expression was identified in the heart, kidney, liver, adrenal, or pancreatic primordium of the murine fetus. The fetal lung contains OB-R protein and OB-R mRNA, but not OB-Rb mRNA. The absence of OB-Rb mRNA in the fetal lung was in agreement with our previous data in the adult rodent (18), but it is of note that this is the only fetal tissue that expresses OB-R, but not OB-Rb mRNA.
In conclusion, we have demonstrated that leptin and its receptor are expressed in the murine placenta and have described sites of leptin expression in the murine fetus that differ from the adult. This may reflect different roles for leptin in the adult, which have not developed yet, and/or other roles in the fetus that are redundant in the adult, as has been reported for other factors (24). Expression of leptin and its receptor occur in different cell populations in the ribs, suggesting a paracrine function. The high levels of expression of both leptin and OB-Rs in the placenta and in the fetus, suggests that this cytokine plays a key role in fetal development. One possible role is that of a fetal growth factor or a signal to the fetus of maternal energy status. Alternatively, fetal leptin could provide a signal to the mother of fetal growth and development. In the fetus, leptin appears to be multifunctional with both paracrine and endocrine effects.
Acknowledgments
We are grateful to Mr. R. A. Duthie for graphics expertise. We thank Drs. Jon Arch and Steve Holmes of SmithKline Beecham Pharmaceuticals for the human leptin antiserum. This work is supported by the Scottish Office Agriculture, Environment, and Fisheries Department.
ABBREVIATIONS
- OB-R
leptin receptor
- RT-PCR
reverse transcription–PCR
References
- 1.Zhang Y Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman J M. Nature (London) 1994;372:425–432. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- 2.Campfield L A, Smith F J, Burn P. Horm Metab Res. 1996;28:619–632. doi: 10.1055/s-2007-979867. [DOI] [PubMed] [Google Scholar]
- 3.Halaas J L, Gajiwala K S, Maffei M, Cohen S L, Chait B T, Rabinowitz D, Lallone R L, Burley S K, Friedman J M. Science. 1995;269:543–546. doi: 10.1126/science.7624777. [DOI] [PubMed] [Google Scholar]
- 4.Pelleymounter M A, Cullen M J, Baker M B, Hecht R, Winters D, Boone T, Collins F. Science. 1995;269:540–543. doi: 10.1126/science.7624776. [DOI] [PubMed] [Google Scholar]
- 5.Campfield L A, Smith F J, Guisez Y, Devos R, Burn P. Science. 1995;269:546–549. doi: 10.1126/science.7624778. [DOI] [PubMed] [Google Scholar]
- 6.Bennett B D, Solar G P, Yuan J Q, Mathias J, Thomas G R, Matthews W. Curr Biol. 1996;6:1170–1180. doi: 10.1016/s0960-9822(02)70684-2. [DOI] [PubMed] [Google Scholar]
- 7.Gainsford T, Willson T A, Metcalf D, Handman E, McFarlane C, Ng A, Nicola N A, Alexander W S, Hilton D J. Proc Natl Acad Sci USA. 1996;93:14564–14568. doi: 10.1073/pnas.93.25.14564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cioffi J A, Shafer A W, Zupancic T J, Smith-Gbur J, Mikhail A, Platika D, Snodgrass H R. Nat Med. 1996;2:585–589. doi: 10.1038/nm0596-585. [DOI] [PubMed] [Google Scholar]
- 9.Chehab F E, Lim M E, Lu R H. Nat Genet. 1996;12:318–320. doi: 10.1038/ng0396-318. [DOI] [PubMed] [Google Scholar]
- 10.Barash I A, Cheung C C, Weigle D S, Ren H P, Kabigting E B, Kuijper J L, Clifton D K, Steiner R A. Endocrinology. 1996;137:3144–3147. doi: 10.1210/endo.137.7.8770941. [DOI] [PubMed] [Google Scholar]
- 11.Chehab F F, Mounzih K, Lu R H, Lim M E. Science. 1997;275:88–90. doi: 10.1126/science.275.5296.88. [DOI] [PubMed] [Google Scholar]
- 12.Ahima R S, Dushay J, Flier S N, Prabakaran D, Flier J S. J Clin Invest. 1997;99:391–395. doi: 10.1172/JCI119172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen H, Charlat O, Tartaglia L A, Woolf E A, Weng X, Ellis S J, Lakey N D, Culpepper J, Moore K J, Breitbart R E, Duyk G M, Tepper R I, Morgenstern J P. Cell. 1996;84:491–495. doi: 10.1016/s0092-8674(00)81294-5. [DOI] [PubMed] [Google Scholar]
- 14.Lee G H, Proenca R, Montez J M, Carroll K M, Darvishzadeh J G, Lee J I, Friedman J M. Nature (London) 1996;379:632–635. doi: 10.1038/379632a0. [DOI] [PubMed] [Google Scholar]
- 15.Tartaglia L A, Dembski M, Weng X, Deng N H, Culpepper J, Devos R, Richards G J, Campfield L A, Clark F T, Deeds J, Muir C, Sanker S, Moriarty A, Moore K J, Smutko J S, Mays G G, Woolf E A, Monroe C A, Tepper R I. Cell. 1995;83:1263–1271. doi: 10.1016/0092-8674(95)90151-5. [DOI] [PubMed] [Google Scholar]
- 16.Mercer J G, Hoggard N, Williams L M, Lawrence C B, Hannah L T, Trayhurn P. FEBS Lett. 1996;387:113–116. doi: 10.1016/0014-5793(96)00473-5. [DOI] [PubMed] [Google Scholar]
- 17.Schwartz M W, Seeley R J, Campfield L A, Burn P, Baskin D G. J Clin Invest. 1996;98:1101–1106. doi: 10.1172/JCI118891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hoggard N, Mercer J G, Rayner D V, Moar K, Trayhurn P, Williams L M. Biochem Biophys Res Commun. 1997;232:383–387. doi: 10.1006/bbrc.1997.6245. [DOI] [PubMed] [Google Scholar]
- 19.Ghilardi N, Ziegler S, Wiestner A, Stoffel R, Heim M H, Skoda R C. Proc Natl Acad Sci USA. 1996;93:6231–6235. doi: 10.1073/pnas.93.13.6231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Green E D, Maffei M, Braden V V, Proenca R, Desilva U, Zhang Y Y, Chua S C, Leibel R L, Weissenbach J, Friedman J M. Genome Res. 1995;5:5–12. doi: 10.1101/gr.5.1.5. [DOI] [PubMed] [Google Scholar]
- 21.Mercer J G, Lawrence C B, Beck B, Burlet A, Atkinson T, Barrett P. Am J Physiol Regul Integr Comp Physiol. 1995;38:R1099–R1106. doi: 10.1152/ajpregu.1995.269.5.R1099. [DOI] [PubMed] [Google Scholar]
- 22.Banks W A, Kastin A J, Huang W T, Jaspan J B, Maness L M. Peptides. 1996;17:305–311. doi: 10.1016/0196-9781(96)00025-3. [DOI] [PubMed] [Google Scholar]
- 23.Rugh R. The Mouse: Its Reproduction and Development. London: Oxford Science; 1994. [Google Scholar]
- 24.Millan M A, Carvallo P, Izumi S, Zemel S, Catt K J, Aguilera G. Science. 1989;244:1340–1342. doi: 10.1126/science.2734613. [DOI] [PubMed] [Google Scholar]