Abstract
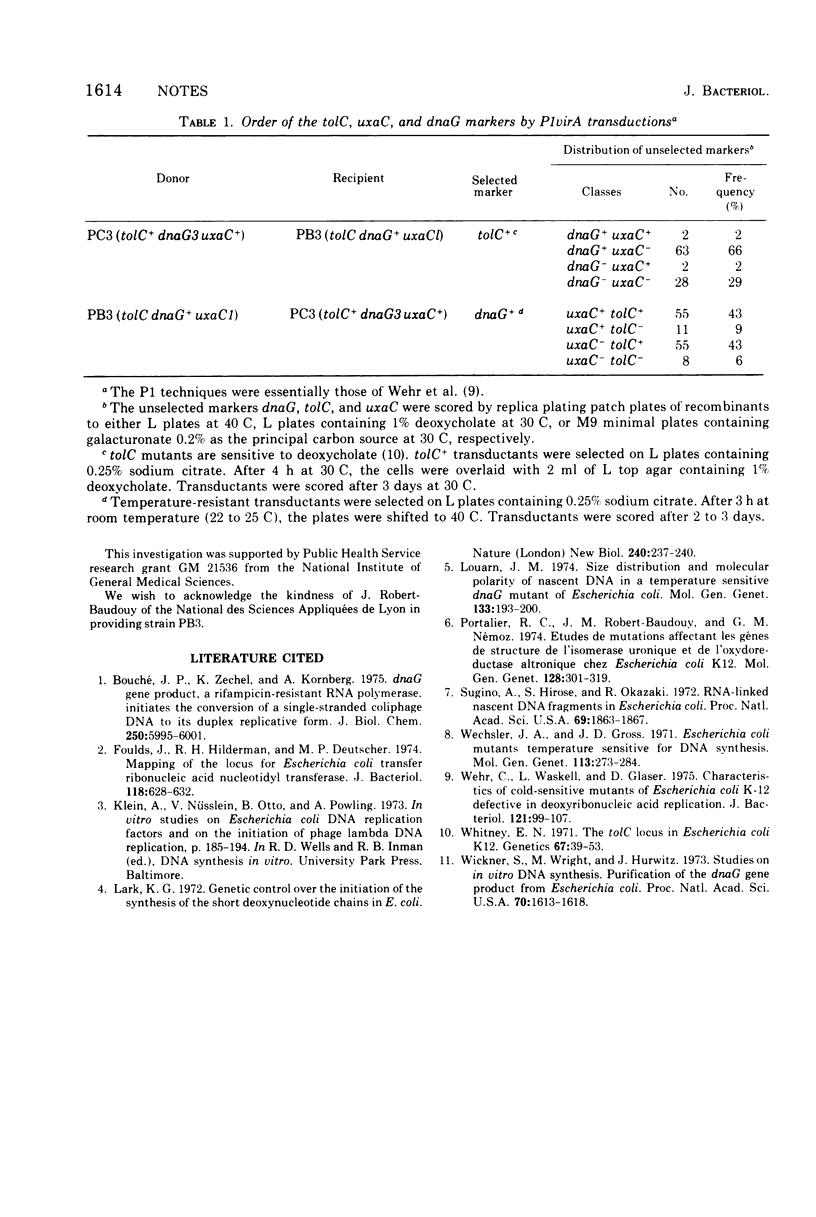
The dnaG locus of Escherichia coli K-12 has been mapped at about 60 min on the genetic map by three-factor crosses using P1 transduction. In crosses selecting for dnaG+, the cotransduction frequency with the tolC marker is 15% and that with the uxaC marker is 49%. The gene order is tolC dnaG uxaC.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bouché J. P., Zechel K., Kornberg A. dnaG gene product, a rifampicin-resistant RNA polymerase, initiates the conversion of a single-stranded coliphage DNA to its duplex replicative form. J Biol Chem. 1975 Aug 10;250(15):5995–6001. [PubMed] [Google Scholar]
- Foulds J., Hilderman R. H., Deutscher M. P. Mapping of the locus for Escherichia coli transfer ribonucleic acid nucleotidyltransferase. J Bacteriol. 1974 May;118(2):628–632. doi: 10.1128/jb.118.2.628-632.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lark K. G. Genetic control over the initiation of the synthesis of the short deoxynucleotide chains in E. coli. Nat New Biol. 1972 Dec 20;240(103):237–240. doi: 10.1038/newbio240237a0. [DOI] [PubMed] [Google Scholar]
- Louarn J. M. Size distribution and molecular polarity of nascent DNA in a temperature-sensitive dna G mutant of Escherichia coli. Mol Gen Genet. 1974;133(3):193–200. doi: 10.1007/BF00267668. [DOI] [PubMed] [Google Scholar]
- Portalier R. C., Robert-Baudouy J. M., Némoz G. M. Etudes de mutations affectant les gènes de structure de l'isomerase uronique et de l'oxydoreductase altronique chez Escherichia coli K 12. Mol Gen Genet. 1974;128(4):301–319. doi: 10.1007/BF00268518. [DOI] [PubMed] [Google Scholar]
- Sugino A., Hirose S., Okazaki R. RNA-linked nascent DNA fragments in Escherichia coli. Proc Natl Acad Sci U S A. 1972 Jul;69(7):1863–1867. doi: 10.1073/pnas.69.7.1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler J. A., Gross J. D. Escherichia coli mutants temperature-sensitive for DNA synthesis. Mol Gen Genet. 1971;113(3):273–284. doi: 10.1007/BF00339547. [DOI] [PubMed] [Google Scholar]
- Wehr C. T., Waskell L., Glaser D. A. Characteristics of cold-sensitive mutants of Escherichia coli K-12 defective in deoxyribonucleic acid replication. J Bacteriol. 1975 Jan;121(1):99–107. doi: 10.1128/jb.121.1.99-107.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitney E. N. The tolC locus in Escherichia coli K12. Genetics. 1971 Jan;67(1):39–53. doi: 10.1093/genetics/67.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickner S., Wright M., Hurwitz J. Studies on in vitro DNA synthesis. Purification of the dna G gene product from Escherichia coli. Proc Natl Acad Sci U S A. 1973 May;70(5):1613–1618. doi: 10.1073/pnas.70.5.1613. [DOI] [PMC free article] [PubMed] [Google Scholar]



