Abstract
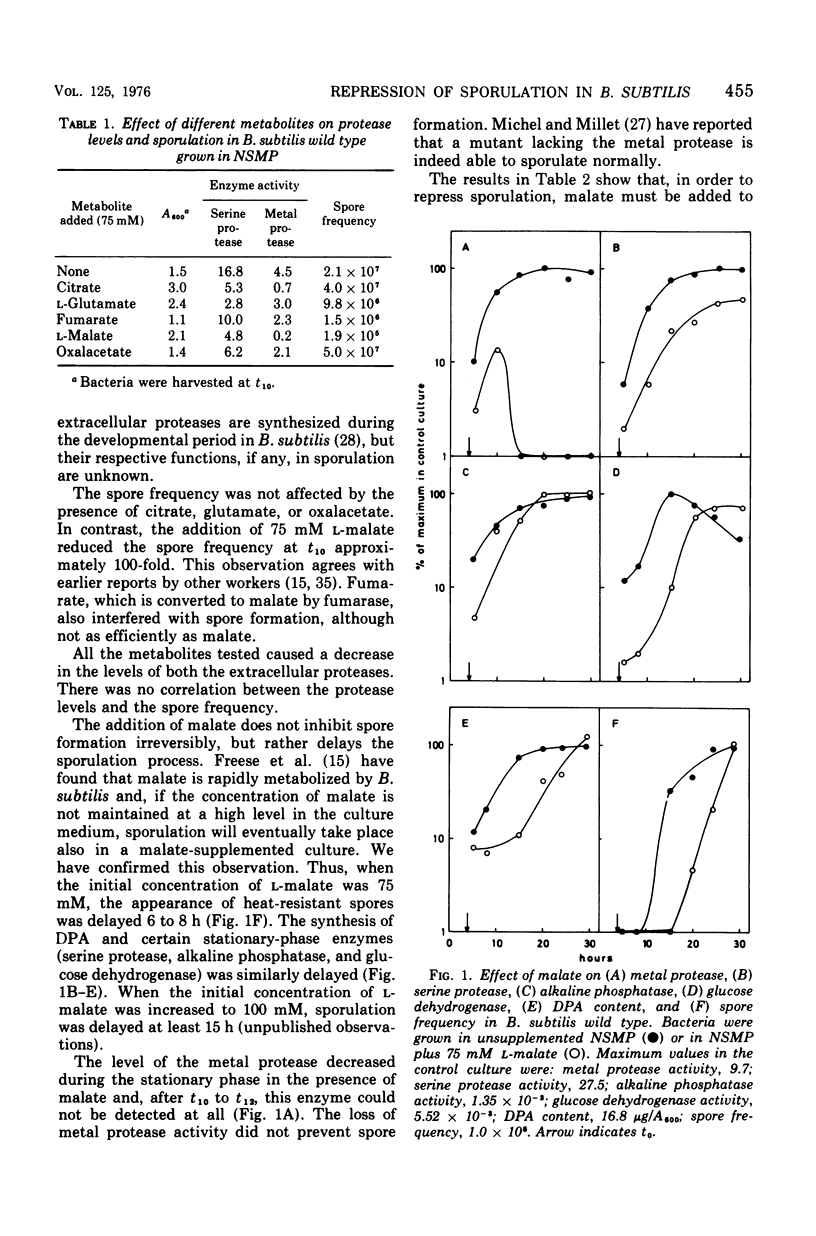
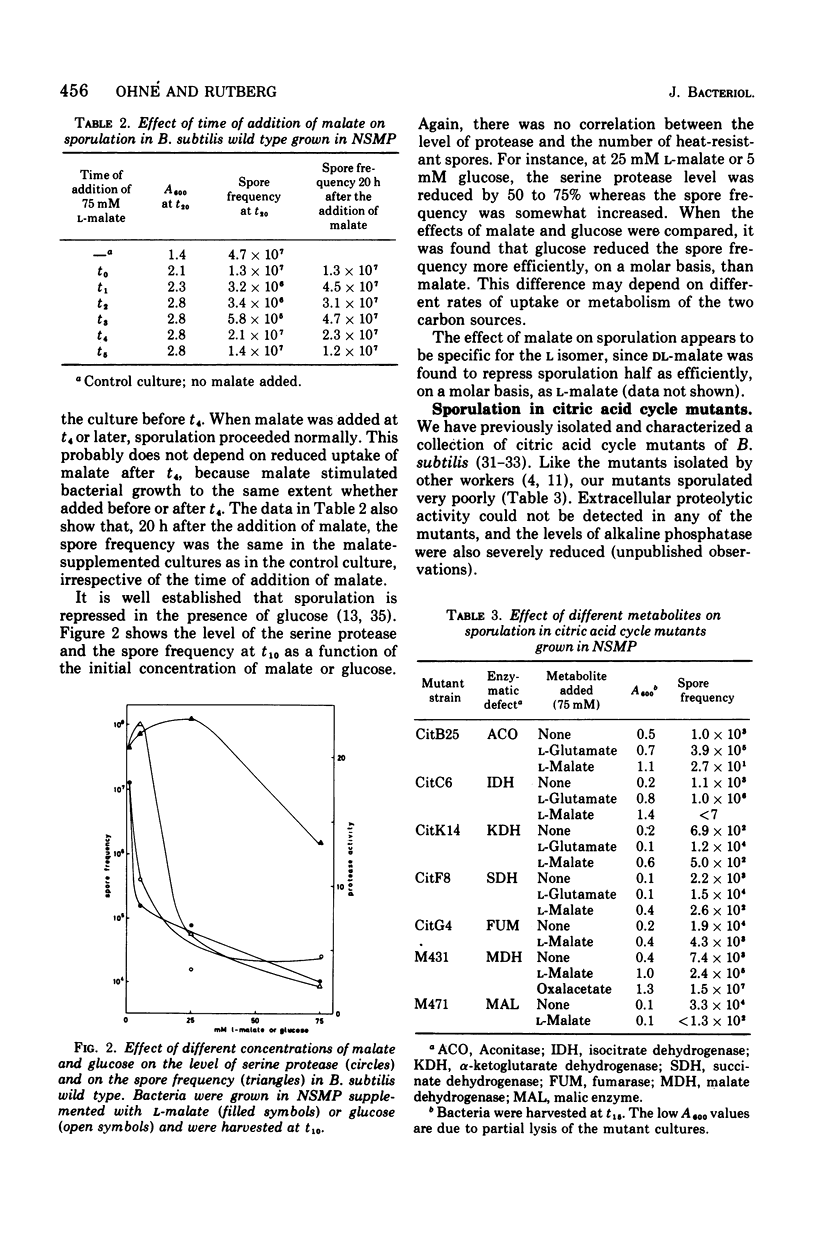
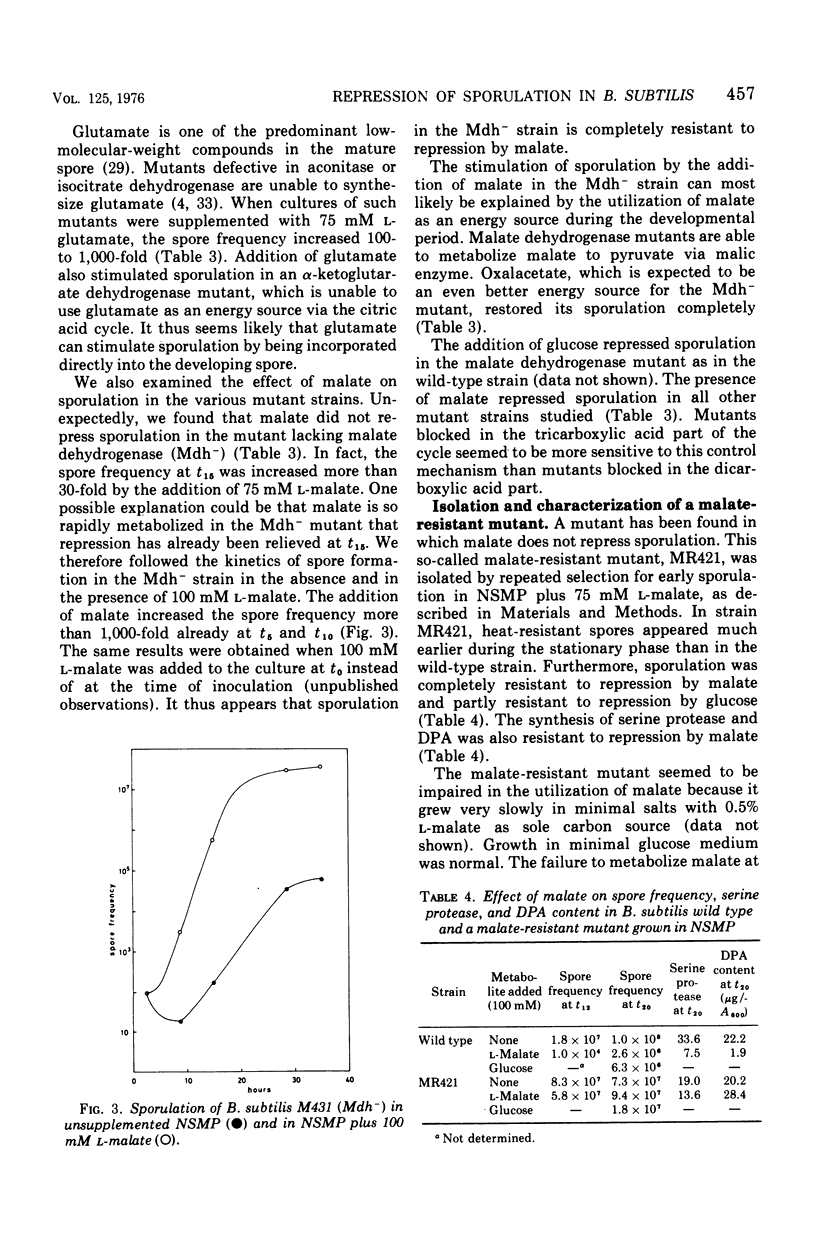
L-Malate repressed sporulation in the wild-type strain of Bacillus subtilis. When 75 mM L-malate was added to the growth medium at the time of inoculation, the appearance of heat-resistant spores was delayed 6 to 8 h. The synthesis of extracellular serine protease, alkaline phosphatase, glucose dehydrogenase, and dipicolinic acid was similarly delayed. Sporulation was not repressed when malate was added to the culture at t4 or later. A mutant was selected for ability to sporulate in the presence of malate. This strain could also sporulate in the presence of glucose. The malate-resistant mutant grew poorly with malate as sole carbon source, although it possessed an intact citric acid cycle, and it showed increased levels of malic enzyme. This indicates a defect in the metabolism of malate in the mutant. A mutant lacking malate dehydrogenase activity was also able to sporulate in the presence of malate. A model for the regulation of sporulation by malate is presented and discussed. Citric acid cycle intermediates other than malate did not affect sporulation. In contrast to previous results, sporulation of certain citric acid cycle mutants could be greatly increased or completely restored by the addition of intermediates after the enzymatic block. The results indicate that the failure of citric acid cycle mutants to sporulate can be adequately explained by lack of energy and lack of glutamate.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brehm S. P., Staal S. P., Hoch J. A. Phenotypes of pleiotropic-negative sporulation mutants of Bacillus subtilis. J Bacteriol. 1973 Sep;115(3):1063–1070. doi: 10.1128/jb.115.3.1063-1070.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHAIX P., PETIT J. F. Etude de différents spectres cytochromiques de Bacillus subtilis. Biochim Biophys Acta. 1956 Oct;22(1):66–71. doi: 10.1016/0006-3002(56)90224-4. [DOI] [PubMed] [Google Scholar]
- Carls R. A., Hanson R. S. Isolation and characterization of tricarboxylic acid cycle mutants of Bacillus subtilis. J Bacteriol. 1971 Jun;106(3):848–855. doi: 10.1128/jb.106.3.848-855.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrand S. K., Taber H. W. Physiological effects of menaquinone deficiency in Bacillus subtilis. J Bacteriol. 1973 Sep;115(3):1035–1044. doi: 10.1128/jb.115.3.1035-1044.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foerster H. F. Spore pool glutamic acid as a metabolite in germination. J Bacteriol. 1972 Aug;111(2):437–442. doi: 10.1128/jb.111.2.437-442.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortnagel P., Freese E. Analysis of sporulation mutants. II. Mutants blocked in the citric acid cycle. J Bacteriol. 1968 Apr;95(4):1431–1438. doi: 10.1128/jb.95.4.1431-1438.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortnagel P., Freese E. Inhibition of aconitase by chelation of transition metals causing inhibition of sporulation in Bacillus subtilis. J Biol Chem. 1968 Oct 25;243(20):5289–5295. [PubMed] [Google Scholar]
- Fortnagel P. The regulation of aconitase and isocitrate dehydrogenase in sporulation mutants of Bacillus subtilis. Biochim Biophys Acta. 1970 Nov 24;222(2):290–298. doi: 10.1016/0304-4165(70)90116-9. [DOI] [PubMed] [Google Scholar]
- Freese E. B., Marks C. L. Developmental block in citric acid cycle mutants of Bacillus subtilis. J Bacteriol. 1973 Dec;116(3):1466–1468. doi: 10.1128/jb.116.3.1466-1468.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freese E., Fortnagel P. Analysis of sporulation mutants. I. Response of uracil incorporation to carbon sources, and other mutant properties. J Bacteriol. 1967 Dec;94(6):1957–1969. doi: 10.1128/jb.94.6.1957-1969.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freese E., Klofat W., Galliers E. Commitment to sporulation and induction of glucose-phosphoenolpyruvate-transferase. Biochim Biophys Acta. 1970 Nov 24;222(2):265–289. doi: 10.1016/0304-4165(70)90115-7. [DOI] [PubMed] [Google Scholar]
- HANSON H. M., WITOSLAWSKI J. J., CAMPBELL E. H. REVERSIBLE DISRUPTION OF A WAVELENGTH DISCRIMINATION IN PIGEONS FOLLOWING ADMINISTRATION OF PHENIPRAZINE. Toxicol Appl Pharmacol. 1964 Nov;6:690–695. doi: 10.1016/0041-008x(64)90119-x. [DOI] [PubMed] [Google Scholar]
- Hanson R. S., Cox D. P. Effect of different nutritional conditions on the synthesis of tricarboxylic acid cycle enzymes. J Bacteriol. 1967 Jun;93(6):1777–1787. doi: 10.1128/jb.93.6.1777-1787.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson R. S., Peterson J. A., Yousten A. A. Unique biochemical events in bacterial sporulation. Annu Rev Microbiol. 1970;24:53–90. doi: 10.1146/annurev.mi.24.100170.000413. [DOI] [PubMed] [Google Scholar]
- Higerd T. B., Hoch J. A., Spizizen J. Hyperprotease-producing mutants of Bacillus subtilis. J Bacteriol. 1972 Nov;112(2):1026–1028. doi: 10.1128/jb.112.2.1026-1028.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JANSSEN F. W., LUND A. J., ANDERSON L. E. Colorimetric assay for dipicolinic acid in bacterial spores. Science. 1958 Jan 3;127(3288):26–27. doi: 10.1126/science.127.3288.26. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Le Hégarat J. C., Anagnostopoulos C. Purification, subunit structure and properties of two repressible phosphohydrolases of Bacillus subtilis. Eur J Biochem. 1973 Nov 15;39(2):525–539. doi: 10.1111/j.1432-1033.1973.tb03151.x. [DOI] [PubMed] [Google Scholar]
- Michel J. F., Millet J. Physiological studies on early-blocked sporulation mutants of Bacillus subtilis. J Appl Bacteriol. 1970 Mar;33(1):220–227. doi: 10.1111/j.1365-2672.1970.tb05246.x. [DOI] [PubMed] [Google Scholar]
- Millet J. Characterization of proteinases excreted by Bacillus subtilis Marburg strain during sporulation. J Appl Bacteriol. 1970 Mar;33(1):207–219. doi: 10.1111/j.1365-2672.1970.tb05245.x. [DOI] [PubMed] [Google Scholar]
- Nelson D. L., Kornberg A. Biochemical studies of bacterial sporulation and germination. 18. Free amino acids in spores. J Biol Chem. 1970 Mar 10;245(5):1128–1136. [PubMed] [Google Scholar]
- Ohné M. Regulation of the dicarboxylic acid part of the citric acid cycle in Bacillus subtilis. J Bacteriol. 1975 Apr;122(1):224–234. doi: 10.1128/jb.122.1.224-234.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohné M., Rutberg B., Hoch J. A. Genetic and biochemical characterization of mutants of Bacillus subtilis defective in succinate dehydrogenase. J Bacteriol. 1973 Sep;115(3):738–745. doi: 10.1128/jb.115.3.738-745.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutberg B., Hoch J. A. Citric acid cycle: gene-enzyme relationships in Bacillus subtilis. J Bacteriol. 1970 Nov;104(2):826–833. doi: 10.1128/jb.104.2.826-833.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaeffer P., Millet J., Aubert J. P. Catabolic repression of bacterial sporulation. Proc Natl Acad Sci U S A. 1965 Sep;54(3):704–711. doi: 10.1073/pnas.54.3.704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spizizen J. TRANSFORMATION OF BIOCHEMICALLY DEFICIENT STRAINS OF BACILLUS SUBTILIS BY DEOXYRIBONUCLEATE. Proc Natl Acad Sci U S A. 1958 Oct 15;44(10):1072–1078. doi: 10.1073/pnas.44.10.1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taber H. Isolation and properties of cytochrome a deficient mutants of Bacillus subtilis. J Gen Microbiol. 1974 Apr;81(2):435–444. doi: 10.1099/00221287-81-2-435. [DOI] [PubMed] [Google Scholar]
- Warren S. C. Sporulation in Bacillus subtilis. Biochemical changes. Biochem J. 1968 Oct;109(5):811–818. doi: 10.1042/bj1090811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yousten A. A., Hanson R. S. Sporulation of tricarboxylic acid cycle mutants of Bacillus subtilis. J Bacteriol. 1972 Feb;109(2):886–894. doi: 10.1128/jb.109.2.886-894.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]



